Abstract
Background
RUNX1 haplodeficiency is associated with thrombocytopenia, platelet dysfunction and a predisposition to acute leukemia. Platelets possess three distinct types of granules and secretory processes involving dense granules (DG), α–granules and vesicles or lysosomes containing acid hydrolases (AH). DG and granule deficiencies have been reported in patients with RUNX1 mutations. Little is known regarding the secretion from acid-hydrolase containing vesicles.
Methods and Results
We studied two related patients with a RUNX1 mutation, easy bruising, and mild thrombocytopenia. Platelet aggregation and 14C serotonin in platelet-rich plasma were impaired in response to ADP, epinephrine, collagen and arachidonic acid. Contents of DG (ATP, ADP), α–granules (β-thromboglobulin), and AH-containing vesicles (β-glucuronidase, β-hexosaminidase, α-mannosidase) were normal or minimally decreased. DG secretion on stimulation of gel-filtered platelets with thrombin and divalent ionophore A23187 (4–12 μM) were diminished. β-thromboglobulin and acid hydrolase secretion was impaired in response to thrombin or A23187. We studied thromboxane-related pathways. The incorporation of 14C-arachidonic acid into phospholipids and subsequent arachidonic acid release on thrombin activation was normal. Platelet thromboxane A2 production in whole blood serum and on thrombin stimulation of platelet-rich plasma was normal, suggesting that the defective secretion was not due to impaired thromboxane production.
Conclusions
These studies provide the first evidence in patients with a RUNX1 mutation for a defect in AH (lysosomal) secretion, and for a global defect in secretion involving all three types of platelet granules that is unrelated to a granule content deficiency. They highlight the pleiotropic effects and multiple platelet defects associated with RUNX1 mutations.
Keywords: Platelets, Inherited platelet disorders, RUNX1 Runt-related transcription factor, Platelet secretion, Acid hydrolases, Dense granule secretion
INTRODUCTION
Transcription factor RUNX1 plays a major role in hematopoiesis and megakaryopoiesis, and RUNX1 mutations are associated with familial thrombocytopenia, impaired platelet function and megakaryopoiesis, and a predisposition to acute myelogenous leukemia [1–3]. The mutation in most such patients is in the conserved DNA-binding RUNT domain [1, 2, 4]. A number of platelet abnormalities have been reported in patients with RUNX1 mutations, including a deficiency of granular content (storage pool deficiency, SPD) affecting dense granules (DG) and alpha granules, impaired aggregation, DG secretion, and protein phosphorylation [2–5]. In addition, abnormalities in mechanisms that regulate secretion have been reported in some patients [2, 3, 5], including in myosin light chain and pleckstrin phosphorylation, and decreased platelet PKC-θ [6, 7]. Platelet mRNA expression profiling of a patient with RUNX1 mutation [8] and other studies [7] have shown downregulation of several genes, including MYL9, PRKQ ALOX12 and PF4, and some of these are direct transcriptional targets of RUNX1, providing evidence for aberrations in multiple platelets mechanisms, including in those regulating platelet responses to activation [2, 9–12].
Platelets possess three distinct types of granules – dense and α-granules, and acid hydrolase (AH)-bearing vesicles (lysosomes), whose contents are secreted on platelet activation [13–16]. Deficiencies of platelet granule contents affecting dense and α granules, alone or combined, are documented in patients with RUNX1 mutations [2, 3, 5]. Impaired secretion of granule contents on platelet activation may occur because of decreased granules (or their contents) or due to abnormalities in the signaling and other mechanisms that regulate exocytosis. The diverse abnormalities recognized in patients with RUNX1 mutations [2, 5] suggest that these patients have defective secretory mechanisms, as well. Little is known about the platelet secretion from AH-containing vesicles, which are distinct from dense and α-granules. These lysosomes contain several enzymes including β-N-acetylglucosaminidase, β-N-acetylgalactosaminidase, β-glucuronidase, β-galactosidase and α-mannosidase that are secreted in response to strong stimuli such as thrombin, collagen, and the divalent cationophore A23187 [13–16]. AH secretion differs from secretion from α– and dense-granules. While contents of the latter granules are completely released by strong stimuli, only 30–60% of the total acid hydrolase content is secretable [13–16]. Moreover, metabolic inhibitors inhibit secretion of acid hydrolases more effectively than secretion from α– or dense-granules [13]. Further, in individual patients with platelet dysfunction, deficiencies of the specific granule affected may differ. For example, in patients reported with the storage pool (SPD) the content of acid hydrolases were normal despite markedly diminished α-granules and DG constituents [17]. In patients with the attention deficit disorder and platelet dysfunction, we have previously reported [18] secretion of DG contents and acid hydrolases to be decreased, while secretion of α-granule contents was normal indicating that these granule-specific secretory processes may be distinct. Here, we describe detailed studies on platelet granule contents and secretion from acid hydrolases containing vesicles, and α– and dense-granules in two related patients with a RUNX1 mutation, and provide the first evidence for a defect in AH secretion and for a global defect in secretion involving all three types of platelet granules that is present separate from a deficiency of granule content. These studies highlight the pleiotropic effects and the complex platelet phenotype associated with RUNX1 mutations.
MATERIALS AND METHODS
Patient Information
We describe here studies in two related patients (propositus son and mother) with a mutation in RUNX1; they have a heterozygous mutation in intron 3 (G to T) at the splice acceptor site for exon 4, leading to the use of an enforced cryptic splice acceptor site in exon 4 and a frame shift with premature termination in the Runt domain [1]. The propositus, his sibling, mother, maternal aunt and grandmother have had thrombocytopenia, a bleeding diathesis, and the same mutation in RUNX1 [1]. This pedigree involves 3 generations of mixed Czechoslovakian and Hungarian descent. There was no history of consanguinity in the family. The propositus presented at age of 4 years for hypospadius repair. He had bruising since infancy with platelet counts around ~100,000/μl. The mother had a platelet count of 132,000/μl. Both the mother and the propositus have had mild thrombocytopenia, prolonged bleeding time (>15 minutes) and a mucocutaneous bleeding diathesis.
Healthy subjects were concurrently studied and were laboratory personnel (mean age 32 years, range 25–42) without known bleeding disorders and who had not taking any medications for 10 days before blood drawing. They were equally distributed between the two sexes.
This research was approved by the institutional human subjects review board and all human participants gave written informed consent.
Materials and Methods
Aggregation and 14C-serotonin uptake, retention and secretion in platelet-rich-plasma
Platelet-rich plasma (PRP) prepared from whole blood collected into one-tenth volume of 3.8% sodium citrate was incubated with 0.5 μM of 14C-serotonin and its incorporation into platelets over 30 minutes and retention over 300 minutes was studied as described [18]. The aggregation and secretion response of PRP to ADP, epinephrine, arachidonic acid and collagen were assessed [18].
Secretion studies in gel-filtered platelets
Gel-filtered platelets (GFP) were prepared using calcium-free Tyrode’s solution, containing 5 mM glucose and 0.2% human serum albumin. Secretion from DG (ATP plus ADP), α–granules (β-thromboglobulin (βTG)) and AH-containing vesicles were assessed as described [18]. For thrombin-induced secretion, aliquots of GFP (1 ml) were incubated with 100 μl of 0.15 μM NaCl or thrombin concentrations at 37°C for two minutes. The secreted ATP + ADP, βTG, and AH were measured in the supernatants (2000g, 2 minutes at room temperature) as described below. The extent of secretion was calculated as the level of the substance in the supernatant relative to the total content in cells. A23187-induced secretion was measured in supernatants of 1 ml aliquots of GFP incubated with various concentrations of agonist in DMSO for three minutes at 37°C [18].
The concentration of ATP and ADP were determined by a firefly luminescence method [19]. βTG was measured using a radioimmunoassay [20]. The activities of β-hexosaminidase, β–glucuronidase and α-mannosidase were determined with appropriate 4-methyl umbelliferone substrates, according to Danglemaier and Holmsen [21].
Studies on liberation of 14C-arachidonate from phospholipids in GFP and thromboxane A2 production
A major platelet response to activation is the production of thromboxane A2. The initial and the rate-limiting step in thromboxane production is the release from phospholipids of free arachidonic acid, which is then converted to endoperoxides and thromboxane A2. We therefore studied the incorporation of arachidonic acid into phospholipids and its release upon thrombin stimulation. Arachidonate-labeled PRP was prepared as described previously [22]. After gel-filtration of the labeled PRP, 1 ml aliquots were incubated with increasing concentrations of thrombin (0.06–5.0 units per ml) for 5 minutes at 37°C without stirring. The samples were extracted with chloroform/methanol, fractionated by thin layer chromatography and the amount of 14C-phospholipids, 14C arachidonate and oxygenation products were determined as described [22]. Thromboxane A2 production was measured in spontaneously clotted whole blood serum and in supernatants from PRP stimulated with thrombin for 5 minutes at 37°C as described [18].
RESULTS
Platelet Aggregation and 14C-Serotonin Secretion Studies
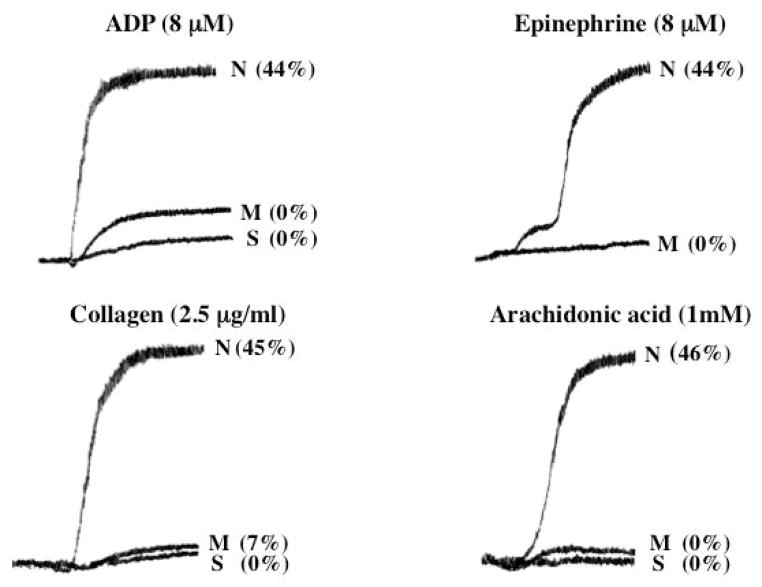
Aggregation responses in PRP in response to ADP (8 μM), epinephrine (8 μM), collagen (2.5 μg/ml) and arachidonic acid (1 mM) were markedly abnormal in the propositus and the mother (Figure 1). The release of 14C-serotonin from the DG was also markedly decreased.
Figure 1. Platelet Aggregation and 14C-Serotonin Secretion Studies.
Shown are the responses to ADP, epinephrine, collagen and arachidonic acid in PRP from the son (S, propositus) and his mother (M), and a healthy normal subject (N). The numbers next to the tracings show the percent 14C-serotonin secreted.
Uptake and Retention of 14C-Serotonin
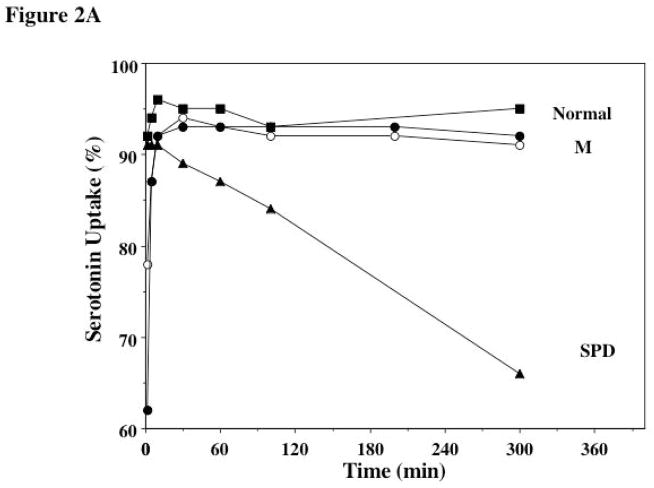
In patients with δ-SPD the retention over 4–6 hours of 14C-serotonin after uptake by platelets is impaired because of the DG defect [23]. We studied the uptake and retention over 5 hours of 14C-serotonin by platelets from the mother on 2 separate occasions and it was normal (Figure 2A). Also shown in Figure 2 for comparison are results from a patient with δ-SPD and the Hermansky-Pudlak syndrome, showing a near normal uptake of 14C-serotonin followed gradual leakage of the radioactive label from the platelets over time, indicating markedly abnormal retention.
Figure 2.
Figure 2A. Uptake and Retention of 14C-Serotonin in the Mother and a Healthy Normal Control.. PRP was incubated with 14C-serotonin and the uptake and retention was monitored over 300 minutes. Shown are the findings in a normal subject (squares), the mother (studied twice, circles) and for comparison results from a patient with the dense granule storage pool deficiency (SPD, triangles) associated with the Hermansky-Pudlak syndrome.
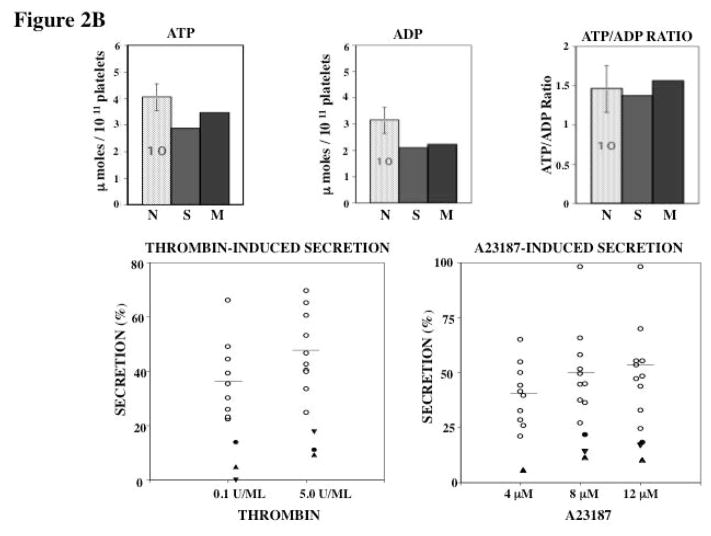
Figure 2B. Dense-Granule Contents and Secretion in Response to Thrombin and A23187. The bar diagrams show the platelet content of ATP, ADP and the ATP/ADP ratio in the propositus, his mother and 10 healthy normal controls (N) (mean ± SEM). Secretion of ATP+ADP is shown as a percentage of total platelet content released upon activation with thrombin and A23187 in the two patients, son (filled circles) and his mother (studied twice, filled triangles,) and 10 healthy controls. The horizontal line is the mean value in control subjects.
Dense Granule Contents and Secretion
Total platelet ATP and ADP were in the normal range in both subjects (Figure 2B). A greater proportion of platelet ADP is in the DG when compared to that of platelet ATP, and DG deficiency is characterized by an increase in ATP to ADP ratio [14]. The ATP/ADP ratio was normal in both subjects. Together, these findings and the normal retention of 14C-serotonin (Figure 2A) indicate that there is not a major deficiency of DG contents. Secretion of ATP + ADP was examined following platelet activation with thrombin and A23187 (Figure 2B). Secretion of ATP-ADP, expressed as percent of total content, in the propositus and the mother, studied twice, was decreased in both subjects in response to thrombin at the lower (0.1 U/ml) and the higher (5.0 U/ml) concentrations. Similarly, nucleotide secretion in response to the A23187 was also impaired in both subjects at all 3 concentrations (4, 8, and 12 μM) tested. The marked decrease in secretion in these subjects despite presence of the granule contents indicates that these platelets have a defect in exocytosis.
α-Granule βTG Content and Secretion
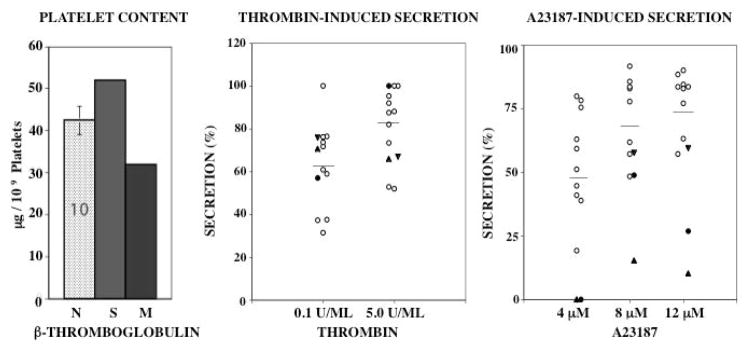
Platelet βTG level was in the normal range in both subjects (Figure 3). Thrombin-induced secretion of α-granule βTG was normal in both subjects (Figure 3). However, it was decreased in response to 4 μM A23187 in both subjects. At higher ionophore concentrations, βTG secretion in the propositus was decreased or at the lower limits of normal range. In the mother it was clearly decreased on one occasion and at the lower end of the values on another (Figure 3). Overall, the findings of normal βTG secretion with thrombin suggest that the impaired release with A23187 is due to defective release mechanisms rather than deficient granule stores.
Figure 3. α-Granule βTG Content and Secretion.
Shown is platelet content of βTG in propositus, his mother and 10 normal subjects (N), and secretion of βTG upon activation with thrombin and A23187 in the two subjects, son (filled circles), his mother (filled triangles, studied twice) and 10 healthy controls.
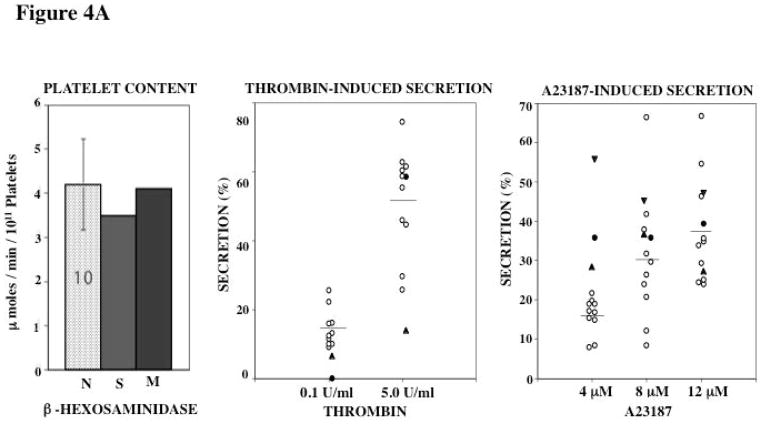
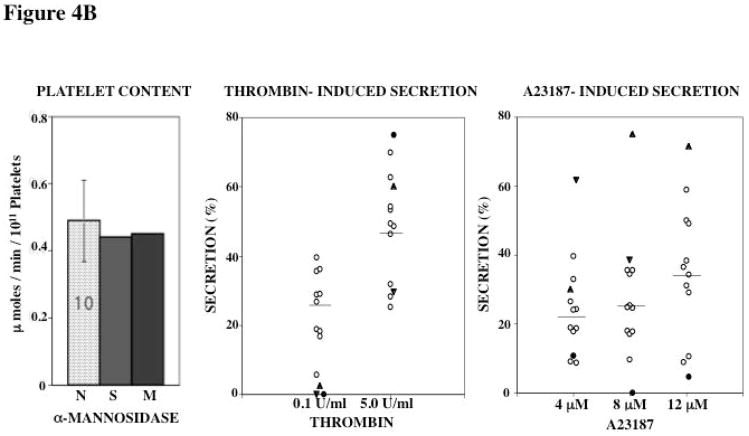
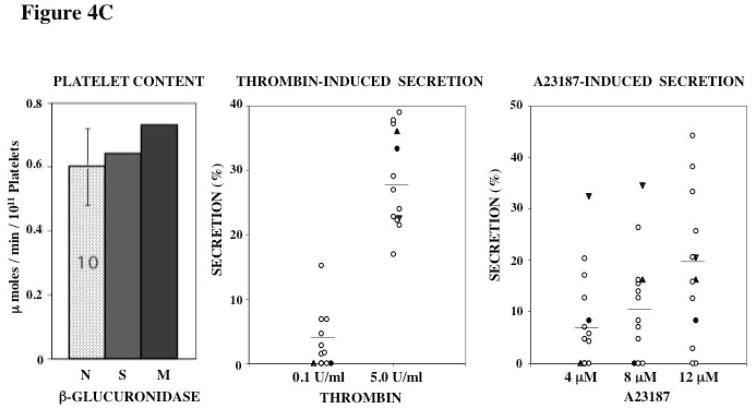
Platelet Content of Acid Hydrolases and Secretion
Platelet levels of 3 acid hydrolases β-hexosaminidase, β-glucuronidase and α-mannosidase were in the normal range in both subjects (Figures 4A–C). Thrombin activated release of β-hexosaminidase was decreased at the lower thrombin concentration (0.1 U/ml) in both subjects; at the higher concentration tested it was abnormal in the mother and normal in the propositus (Figure 4A). With A23187 the release of hexosaminidase was normal in both at all concentrations (Figure 4A).
Figure 4. Platelet Content and Secretion of β-Hexosaminidase (A), α-Mannosidase (B), and β-Glucuronidase (C) in the propositus and his mother.
In each panel the bar diagram shows the platelet content of the acid hydrolase in the two patients and 10 normal subjects (N, mean ± SE). Platelet secretion is shown for each acid hydrolase in the propositus (filled circles), his mother (filled triangles, studied twice) and 10 healthy controls upon activation with thrombin and A23187.
Platelet content of α-mannosidase was normal in both subjects (Figure 4B). Thrombin-induced secretion of α-mannosidase was decreased in both subjects with 0.1 U/ml thrombin and normal with 5 U/ml thrombin stimulation (Figure 4B); the latter indicating that the impaired release at the lower concentration is not due to decreased stores. With A23187 α-mannosidase secretion was decreased in the propositus, but normal in the mother (Figure 4B).
β-glucuronidase levels were normal in both subjects (Figure 4C). In healthy controls the secretion of this enzyme on activation was relatively lower than of the other enzymes, suggesting differences in the release of individual enzymes, as previously noted [13, 22]. In response to 0.1 U/ml thrombin, no secretion was noted in both patients, but this was observed in some healthy subjects as well. At higher thrombin concentration (5 U/ml) it was normal in both. With A23187, secretion of β-glucuronidase in the propositus was at the lower levels of the values in controls, but as with thrombin, some of the controls showed little secretion, as previously observed [13, 22].
Studies on Release of Arachidonic Acid from Phospholipids and Thromboxane Synthesis
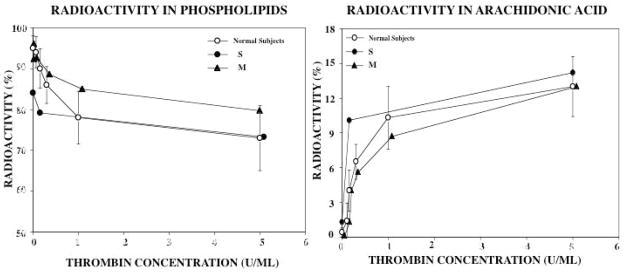
Platelet activation results in the release of free arachidonic acid from phospholipids, which is converted to thromboxane A2. The release of free arachidonic acid on activation is the rate-limiting step in thromboxane synthesis on platelet activation. Therefore, we studied this response on thrombin activation. Moreover, our studies have shown that 12-lipoxygenase (ALOX12), which converts arachidonic acid to 12-hydroxyeicosanoids, is down regulated in RUNX1 haplodeficiency with decreased platelet 12-HETE production [12]. These studies provide information on the platelet handling of arachidonic acid and its release on platelet activation. Platelets were labeled with 14C-arachidonate, which results in its incorporation into membrane phospholipids (predominantly phosphatidylcholine), and stimulated with increasing thrombin concentrations, which induces the release of free-labeled arachidonic acid. The 14C-radioactivity in phospholipids and free arachidonate were assessed. In platelets from healthy controls, there was a decrease in the radioactivity in phospholipid fraction on thrombin activation reflecting the release and oxygenation of free arachidonic acid (Figure 5). The incorporation of the labeled arachidonic acid into phospholipids, and the subsequent reduction upon thrombin activation was normal in both subjects (Figure 5). The increase in the radioactivity in free arachidonic acid fraction on thrombin activation, indicating the mobilization of arachidonic acid from phospholipids was normal in both subjects (Figure 5). Thus, the incorporation of arachidonic acid into the platelet membranes and its mobilization on platelet activation are normal.
Figure 5. Studies on Release of Arachidonic Acid from Phospholipids.
Platelets from the two patients (son S, filled circles, and mother M, filled triangles) and 10 healthy normal subjects (open circles, shown mean ± SEM) were labeled with 14C-arachidonate, and the incorporation of arachidonic acid into phospholipids and the subsequent release of free arachidonic acid on thrombin activation were studied. The left panel shows the radioactivity in the phospholipids fraction before and after platelet activation with increasing thrombin concentration. The right panel shows the increase in the radioactivity in free-arachidonic acid, reflecting the release of free-arachidonic acid upon platelet activation.
Thromboxane A2 production appeared substantial in the patients as assessed in spontaneously clotted whole blood serum (propositus 490 pmoles/108 platelets, mother 106; healthy controls, mean 223, range 94–407; n=12), and in the mother upon platelet activation of PRP with thrombin (5 U/ml) (mother 114 pmoles/108 platelets; healthy controls, mean 179, range 54–318; n=23).
DISCUSSION
Platelets possess three distinct types of granules whose contents are secreted upon platelet activation. In the patients studied here with a RUNX1 mutation, the contents of the DGs, α-granules and the AH-containing vesicles are present in normal amounts. However, secretion of DG contents (Figure 2), β-thromboglobulin (Figure 3) and acid hydrolases β-hexosaminidase and α-mannosidase (Figure 4) were decreased on activation with thrombin and/or A23187. With β-glucuronidase it was difficult to demonstrate this because even in normal subjects the fraction of total content secreted is low, which has been previously noted [13, 22]. Our present studies provide the first evidence for a global defect in exocytosis across the different types of granules, including AH-containing vesicles, in RUNX1 haplodeficiency. AH secretion has features and roles that are distinct from that of dense and α-granules [13, 14, 16, 22]. An abnormality in AH secretion has hitherto not been described in patients with RUNX1 mutations These findings further advance the understanding of the aberrations in platelets associated with RUNX1 haplodeficiency.
Deficiencies of both DG and α-granule contents have been previously documented in some patients with RUNX1 mutations [2, 3]. We demonstrate also that the decreased secretion in the patients studied in the present report with RUNX1 haplodeficiency is unrelated to a deficiency of granule contents. The retention of the 14C-serotonin over 4 hours is markedly abnormal in patients with marked δ-SPD [23]; this was normal in our patients (Figure 2), suggesting adequacy of DGs in the patients studied. Our studies establish another facet of the platelet dysfunction associated with RUNX1 mutations – that abnormalities in the secretory mechanisms constitute an important component of the platelet dysfunction. We postulate that even in other reported patients with RUNX1 mutations the overall decrease in the granule release on platelets activation is an integration of two processes – deficiency of granule contents and compromised secretory mechanisms.
Our previous studies provide a cogent mechanistic basis for the global defect in secretion across multiple distinct granules in RUNX1 haplodeficiency. In an unrelated patient with impaired platelet function but with the same mutation in RUNX 1 [7], we have documented abnormalities or deficiencies in processes that regulate platelet responses upon activation, including secretion – in protein kinase C-and phosphorylation of pleckstrin [7], myosin light chain phosphorylation [7], and 12-lipoxygenase activity [12], and in mechanisms leading to activation of the GPIIb-IIIa complex [7]. Moreover, our expression profiling studies in the same patient showed that a wide array of genes involved in platelet function and formation are downregulated [8]. We have shown that several relevant genes including PRKQ [9], MYL9 [10], ALOX12 [12], PLDN [24] and PCTP [25] are direct transcriptional targets of RUNX1. Thus, we postulate that the secretory abnormalities result from aberrations in common pathways critical to the general process of exocytosis, for example, involving vesicle transport mechanisms, Rab proteins, and SNARE proteins [16, 26]. Alternatively, and more likely, there may be specific abnormalities that distinctly impact secretion from each granule type, and that are agonist and concentration - dependent. For example, there is evidence that DG and α-granule formation and secretion are mediated by distinct regulators [16, 26]. In our studies in patients with the attention deficit disorder [18], we have reported secretion of DG and AH contents to be decreased while α-granule secretion was intact; these abnormalities were also agonist and dose dependent. Of note, the extent of secretion of various acid hydrolases is different, and this is noted in our control subjects with percent secretion of β-glucuronidase being much less than for the other two measured (Figure 4). Interestingly, the aggregation response to some of the agonists was markedly impaired in our subjects (Figure 1). While the decreased secretion, especially from the DG, may be contributory, it is relevant to note that our previous studies in RUNX1 haplodeficiency revealed [6, 7] defective receptor mediated activation of the GPIIb-IIIa complexes, which are present in normal numbers. In that patient also, aggregation responses were markedly diminished in response to multiple agonists [6]. This provides a mechanism for the markedly decreased aggregation responses.
Impaired thromboxane production compromises responses to platelet agonists. Therefore, we studied the pathway leading to thromboxane production. Our studies show that arachidonic acid incorporation into phospholipids and its subsequent release upon platelet activation were normal (Figure 5). These findings are relevant because our studies showed [25] that PCTP (phosphatidylcholine transfer protein) gene is a direct RUNX1 target and downregulated in an unrelated patient with RUNX1 mutation. PCTP encodes a protein that regulates the intermembrane transfer of phosphatidylcholine [27], a major source of AA released on platelet activation. Most important, in the present study the platelets produced substantial thromboxane A2, making it less likely that impaired thromboxane production was a major factor in the defective secretion.
Lastly, our studies highlight the multiple functional abnormalities associated with RUNX1 mutations and the complex platelet phenotype. Because RUNX1 is a transcription factor, the deficiencies associated with RUNX1 mutations are pleiotropic, as also seen with mutations of GATA1 and FOG1 [28]. The differences in the abnormalities noted among patients could be due to the degree of loss of RUNX1 expression and secondary polymorphisms, such that some pathways are more severely affected in particular individuals. These patients constitute a unique reservoir of new information into platelet and megakaryocyte biology.
Acknowledgments
The authors gratefully acknowledge the contribution of Suma Janapati, M.B.B.S. for her assistance in preparing the figures, and thank the members of the laboratory who participated in the platelet measurements. This study was supported by research funding from NIH (NHLBI) R01HL109568 and R01HL085422 to AKR
This study was supported by research funding from NIH (NHLBI) R01HL109568 and R01HL085422 to AKR.
Footnotes
AUTHORSHIP AND CONFLICT OF INTEREST
AKR designed the study, performed the research and wrote the manuscript. MP referred the patients and assisted in the drafting and review of the manuscript. The authors state that they had no interests, which might be perceived as posing a conflict or bias.
References
- 1.Song WJ, Sullivan MG, Legare RD, Hutchings S, Tan X, Kufrin D, Ratajczak J, Resende IC, Haworth C, Hock R, Loh M, Felix C, Roy DC, Busque L, Kurnit D, Willman C, Gewirtz AM, Speck NA, Bushweller JH, Li FP, et al. Haploinsufficiency of CBFA2 causes familial thrombocytopenia with propensity to develop acute myelogenous leukaemia. Nat Genet. 1999;23:166–75. doi: 10.1038/13793. [DOI] [PubMed] [Google Scholar]
- 2.Songdej N, Rao AK. Hematopoietic transcription factor mutations and inherited platelet dysfunction. F1000prime reports. 2015;7:66. doi: 10.12703/P7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Songdej N, Rao AK. Inherited platelet dysfunction and hematopoietic transcription factor mutations. Platelets. 2016:1–7. doi: 10.1080/09537104.2016.1203400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michaud J, Wu F, Osato M, Cottles GM, Yanagida M, Asou N, Shigesada K, Ito Y, Benson KF, Raskind WH, Rossier C, Antonarakis SE, Israels S, McNicol A, Weiss H, Horwitz M, Scott HS. In vitro analyses of known and novel RUNX1/AML1 mutations in dominant familial platelet disorder with predisposition to acute myelogenous leukemia: implications for mechanisms of pathogenesis. Blood. 2002;99:1364–72. doi: 10.1182/blood.v99.4.1364. [DOI] [PubMed] [Google Scholar]
- 5.Rao AK. Inherited platelet function disorders: overview and disorders of granules, secretion, and signal transduction. Hematol Oncol Clin North Am. 2013;27:585–611. doi: 10.1016/j.hoc.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Gabbeta J, Yang X, Sun L, McLane MA, Niewiarowski S, Rao AK. Abnormal inside-out signal transduction-dependent activation of glycoprotein IIb-IIIa in a patient with impaired pleckstrin phosphorylation. Blood. 1996;87:1368–76. [PubMed] [Google Scholar]
- 7.Sun L, Mao G, Rao AK. Association of CBFA2 mutation with decreased platelet PKC-θ and impaired receptor-mediated activation of GPIIb-IIIa and pleckstrin phosphorylation: proteins regulated by CBFA2 play a role in GPIIb-IIIa activation. Blood. 2004;103:948–54. doi: 10.1182/blood-2003-07-2299. [DOI] [PubMed] [Google Scholar]
- 8.Sun L, Gorospe JR, Hoffman EP, Rao AK. Decreased platelet expression of myosin regulatory light chain polypeptide (MYL9) and other genes with platelet dysfunction and CBFA2/RUNX1 mutation: insights from platelet expression profiling. J Thromb Haemost. 2007;5:146–54. doi: 10.1111/j.1538-7836.2006.02271.x. [DOI] [PubMed] [Google Scholar]
- 9.Jalagadugula G, Mao G, Kaur G, Dhanasekaran DN, Rao AK. Platelet PKC-θ deficiency with human RUNX1 mutation: PRKCQ is a transcriptional target of RUNX1. Arterioscler Thromb Vasc Biol. 2011;31:921–7. doi: 10.1161/ATVBAHA.110.221879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jalagadugula G, Mao G, Kaur G, Goldfinger LE, Dhanasekaran DN, Rao AK. Regulation of platelet myosin light chain (MYL9) by RUNX1: implications for thrombocytopenia and platelet dysfunction in RUNX1 haplodeficiency. Blood. 2010;116:6037–45. doi: 10.1182/blood-2010-06-289850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aneja K, Jalagadugula G, Mao G, Rao AK. Mechanism of platelet factor (PF4) deficiency with RUNX1 mutations: RUNX1 is a transcriptional regulator of PF4. Blood. 2009;114:98–9. doi: 10.1111/j.1538-7836.2010.04154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaur G, Jalagadugula G, Rao AK. CBFA2/RUNX1 regulates human platelet 12-lipoxygenase: studies in runx1 haplodeficiency. Blood. 2007;118:1066A. doi: 10.1182/blood-2009-04-214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmsen H, Kaplan KL, Dangelmaier CA. Differential energy requirements for platelet responses. A simultaneous study of aggregation, three secretory processes, arachidonate liberation, phosphatidylinositol breakdown and phosphatidate production. Biochem J. 1982;208:9–18. doi: 10.1042/bj2080009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmsen H, Weiss H. Secretable storage pools in platelets. Annu Rev Med. 1979;30:119–34. doi: 10.1146/annurev.me.30.020179.001003. [DOI] [PubMed] [Google Scholar]
- 15.Holmsen H. Platelet secretion. In: Colman RW, Hirsh J, Marder VJ, Salzman EW, editors. Hemostasis and Thrombosis. Philadelphia: Lippincott; 1987. pp. 606–18. [Google Scholar]
- 16.Heijnen H, van der Sluijs P. Platelet secretory behaviour: as diverse as the granules … or not? J Thromb Haemost. 2015;13:2141–51. doi: 10.1111/jth.13147. [DOI] [PubMed] [Google Scholar]
- 17.Weiss HJ, Witte LD, Kaplan KL, Lages BA, Chernoff A, Nossel HL, Goodman DS, Baumgartner HR. Heterogeneity in storage pool deficiency: studies on granule-bound substances in 18 patients including variants deficient in alpha-granules, platelet factor 4, beta-thromboglobulin, and platelet-derived growth factor. Blood. 1979;54:1296–319. [PubMed] [Google Scholar]
- 18.Koike K, Rao AK, Holmsen H, Mueller PS. Platelet secretion defect in patients with the attention deficit disorder and easy bruising. Blood. 1984;63:427–33. [PubMed] [Google Scholar]
- 19.Holmsen H, Storm E, Day HJ. Determination of ATP and ADP in blood platelets: A modification of the firefly luciferase assay for plasma. Anal Biochem. 1972;46:489–501. doi: 10.1016/0003-2697(72)90323-5. [DOI] [PubMed] [Google Scholar]
- 20.Rucinski B, Niewiarowski S, James P, Walz DA, Budzynski AZ. Antiheparin proteins secreted by human platelets. purification, characterization, and radioimmunoassay. Blood. 1979;53:47–62. [PubMed] [Google Scholar]
- 21.Dangelmaier CA, Holmsen H. Determination of acid hydrolases in human platelets. Anal Biochem. 1980;104:182–91. doi: 10.1016/0003-2697(80)90296-1. [DOI] [PubMed] [Google Scholar]
- 22.Holmsen H, Dangelmaier CA, Holmsen HK. Thrombin-induced platelet responses differ in requirement for receptor occupancy. J Biol Chem. 1981;256:9393–6. [PubMed] [Google Scholar]
- 23.Weiss HJ, Tschopp TB, Rogers J, Brand H. Studies of platelet 5-hydroxytryptamine (serotonin) in storage pool disease and albinism. J Clin Invest. 1974;54:421–33. doi: 10.1172/JCI107778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mao GF, Goldfinger LE, Fan DC, Lambert MP, Jalagadugula G, Freishtat R, Rao AK. Dysregulation of PLDN (Pallidin) is a mechanism for platelet dense granule deficiency in RUNX1 haplodeficiency. J Thromb Haemost. 2017 doi: 10.1111/jth.13619. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Songdej N, Mao G, Voora D, Goldfinger LE, Del Carpio-Cano F, Myers R, Rao AK. PCTP (Phosphatidylcholine Transfer Protein) is regulated by RUNX1 in platelets/megakaryocytes and is associated with adverse cardiovascular events. Blood. 2016;128:365. (Manuscript under Review Circulation) [Google Scholar]
- 26.Ye F, Whiteheart SW. Molecular basis of platelets secretion. In: Marder VJ, Aird WC, Bennett JS, Schulman S, White GC, editors. Hemostasis and Thrombosis Basic Principles and Clinical Practice. 6. Philadelphia, PA: Lippincott, Williams and Wilkins; 2013. pp. 441–8. [Google Scholar]
- 27.Kang HW, Wei J, Cohen DE. PC-TP/StARD2: Of membranes and metabolism. Trends Endocrinol Metab. 2010;21:449–56. doi: 10.1016/j.tem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crispino JD, Weiss MJ. Erythro-megakaryocytic transcription factors associated with hereditary anemia. Blood. 2014;123:3080–8. doi: 10.1182/blood-2014-01-453167. [DOI] [PMC free article] [PubMed] [Google Scholar]