Abstract
Problem
Influenza infection severity may be mediated by estradiol and/or progesterone.
Method of Study
An exploratory study was designed to evaluate 17-β-estradiol and progesterone on influenza infection, and examine immune mediated response in a mouse model. Inoculation with placebo or mouse-adapted H1N1 influenza virus occurred. Treatment groups included 17-β-estradiol, progesterone, ovariectomy, and pregnancy. Mice were assessed for morbidity and mortality. Toll-like receptor gene studies and airspace cell differentials were performed.
Results
Onset of morbidity was earlier, and morbidity duration greater for progesterone. Absence of morbidity/mortality and overall survival was greater for 17-β-estradiol. Airspace cell differentials suggest improved immune cell recruitment for 17-β-estradiol. Pregnant mouse data demonstrate significant mortality during the period of increased progesterone. Select immune cell markers demonstrate patterns of regulation that may promote proper immune response to influenza infection for 17-β-estradiol.
Conclusions
Estradiol may play a protective and progesterone a detrimental role in the pathophysiology of influenza infection.
Keywords: Estradiol, IL6, IL10, influenza, progesterone, TLR9
Introduction
Throughout history, influenza pandemics have disproportionally affected the young, elderly and gravid segments of the population. Mortality and severity of illness in these groups is significantly greater in comparison to other population groups. When considering the pregnant population, in 1919 a report was published that reviewed more than 1300 cases of pandemic influenza in pregnancy during the 1918–1919 pandemic. Of these cases, approximately half of the patients developed pneumonia. Mortality of those with pneumonia was approximately 50 percent and highest in the third trimester. These findings were echoed in the 1957 Asian influenza pandemic, where a disproportionate number of pregnant women accounted for approximately 50 percent of the observed mortality for the reproductive age segment of the female population.1 Reports during the 2009 influenza pandemic revealed similar findings. In 2010, Siston et al. reported on the disproportionately high risk of mortality in pregnant women due to the 2009 influenza A (H1N1) pandemic. Nine hundred and fifty-three cases of pregnant women were reviewed and showed a striking relationship between mortality and pregnancy trimester with 4 deaths occurring in the first trimester (7.1%), 15 in the second trimester (26.8%), and 36 in the third trimester (64.3%).2 Louie et al. reviewed influenza cases from the 2009 pandemic from the state of California and reported similar findings.3
Animal models have been developed for study during pregnancy. The United States Food and Drug Administration (FDA) highlight specific parameters to consider following a public workshop convened in 2012. Ferret models are often used to study the pathobiology of influenza virus due to susceptibility to the virus, and demonstration of similar clinical signs.4 Similarly, mouse models have been used to conduct influenza virus research, with pregnancy models developed for study.5 While the clinical signs of influenza virus infection are somewhat different than in human influenza, they demonstrate signs of infectious morbidity that include cachexia, decreased activity, and ruffled fur. Additionally, robust innate immune inflammatory response is evident in mouse models that seem to parallel human findings.6 In available mouse models, increased severity of influenza infection during pregnancy has been demonstrated.7,8 Mackenzie et al. found that inoculation with influenza virus during the third gestational week in mice resulted in increased mortality rates compared to controls.9 Chan et al. demonstrated higher mortality and more severe interstitial pneumonitis in pregnant versus non-pregnant mouse controls when challenged with the same influenza viral inoculation.10
Pregnancy is classically thought of as an immuno-tolerant state to allow for the development of the fetal-placental allograft. However, overwhelming cytokine and inflammatory response to influenza has been demonstrated in mouse models with dramatic increases in morbidity and mortality during pregnancy.9,10 In a study investigating the potential role of wild-caught house mice species in avian influenza outbreaks, it was observed that female mice demonstrated higher viral replication rates compared to males. They were not known to be pregnant at the time of viral inoculation, but it is reported that several gave birth during the quarantine period.11 These mouse studies combined with observational pandemic influenza data that detail significant risk during pregnancy, with disease severity increasing by trimester, highlight the importance of studying the incompletely characterized underlying mechanism(s).7
Progesterone and estrogen are sex steroid hormones that increase incrementally during pregnancy. The relationship between estrogen and immune response to influenza infection has been studied, but remains incompletely characterized. In some studies, estradiol has been shown to be protective in regard to severe disease pathology in pregnant and non-pregnant mouse models.7,12,13 Progesterone’s influence on the immune system has been studied, but its contribution to influenza infection morbidity and mortality has only been reported in a single study that details a protective role against lethal and sublethal viral influenza A infection in an ovariectomized mouse model.14 Interestingly, Lyden et al. demonstrated that sex steroid hormones influence Coxsackievirus myocarditis susceptibility in a mouse model. When exogenous progesterone was administered to either castrate male or intact female mice, increased cardiac pathology and viremia was seen.15 The same group demonstrated that the severity of Coxsackievirus myocarditis, viral concentrations, and morbidity, worsens with the progressive rise in progesterone during mouse pregnancy.16 These inconclusive studies coupled with observational data from pandemic influenza, present an important question regarding the role of estrogen and progesterone during the immune response to influenza.
The goal of these studies is to examine the role of estrogen and progesterone during influenza infection, specifically examining the individual contribution of estradiol and progesterone to the morbidity and mortality associated with, and immune response to, influenza infection in a mouse model.
Materials and Methods
Ethics Statement
All animal work was performed in accordance with the National Institutes of Health guidelines for laboratory animals and had approval from the Institutional Animal Care and Use Committee at the University of Vermont (Protocol #10-039). The University of Vermont’s animal research program is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) and maintains Assurance through the Public Health Service (PHS) Office of Laboratory Animal Welfare (OLAW Assurance number A3301-01).
Mice
Ovariectomized CD-1 adult mice were purchased from Charles River Laboratories (Wilmington, MA) and acclimated to the University of Vermont animal care facility. Under isoflurane anesthesia (3% in oxygen gas), steroid hormone pellet or sham pellet insertion procedures were performed by subcutaneous technique 14 days after ovariectomy and 3 days prior to influenza inoculation (note: the sham pellet insertion mice were labeled OVX mice). Twenty one-day continuous release steroid hormone pellets were purchased from Innovative Research of America (Sarasota, FL) at a total dose of: 17-β-estradiol (E2)=0.042 mg/pellet, or progesterone (P)=31.5mg/pellet.17 Twenty-four mice per hormone group were studied for morbidity and mortality studies. Mice were shaved behind the ear and a pellet was placed between the left ear and shoulder through a small incision using sterile surgical technique. The incision was then closed with a resorbable suture. Day 3 following insertion procedures, mice were anesthetized with low-dose intraperitoneal ketamine and xylazine (20 mg/kg and 4 mg/kg, respectively) and underwent intranasal inoculation with mouse-adapted H1N1 influenza (strain A/Puerto Rico/8/34 (PR8) generously provided by Dr. Jelly-Gibbs at the Trudeau Institute, Saranac Lake, NY) virus at 2500 egg infecting units (EIU50). For morbidity and mortality studies, mice were observed by the same examiners until death or for up to 21 days post-inoculation (dpi) for morbidity as demonstrated by weight loss, ruffled fur, or decreased activity in an unblinded fashion. Morbidity and mortality proxies were selected a priori. For Toll-like receptor (TLR) signaling pathway PCR array gene expression studies 3 animals per group were studied. On days 0, 4, and 9 post-inoculation mice were euthanized by standard animal care procedures and lung and other tissues were collected.
To perform bronchoalveolar lavage (BAL), the trachea was cannulated with 22-gauge tubing and one ml of sterile PBS was instilled into the lung. The fluid was collected and cells were pelleted and counted. Cells (50×103) were cytospun onto a glass slide. They were stained with Hema-3 (Biochemical Sciences) and slides de-identified to conceal treatment groups. Cell differential counts were performed by a single blinded examiner.
For a series of pregnant mouse studies, 49 CD-1 mice on gestational days 9 and 14 were utilized for these studies. Under low dose ketamine/xylazine (10 mg/kg and 50 mg/kg, respectively), the mice underwent intranasal inoculation (IN) with mouse-adapted H1N1 influenza (strain A/Puerto Rico/8/34 (PR8)) virus at 10,000 egg infectious units (EIU). Subsequently, the mice were observed for preterm delivery (PTD) and morbidity (determined by ruffled fur, decreased activity and/or weight loss) until death or up to 21 dpi.
Real-time quantitative PCR
Whole lung tissue and other tissues were collected at specified time points. Half of these tissues were snap frozen in liquid nitrogen for later protein analysis and half were placed in RNALater (Life Technologies, Carlsbad, CA) and stored for approximately one week at 4°C. The tissue samples were then removed and stored at −80°C until further processed. Total RNA was isolated from whole lung tissue using TRIzol reagent (Life Technologies) and further purified using the RNeasy Mini Kit (Qiagen, Frederick, MD). Genomic DNA (gDNA) was removed using TURBO DNA-free (Life Technologies). Complimentary DNA was prepared using the RT2 First Strand Kit. Quantitative PCR (qPCR) reactions were prepared with RT2 SYBR qPCR Master Mix and loaded onto the Mouse TLR Signaling Pathway RT2 Profiler PCR Array (Qiagen) that examined 84 TLR related genes. The University of Vermont’s DNA Analysis Core performed real-time analysis on an ABI Prism 7900HT Sequence Detection System (Life Technologies).
Influenza viral message was determined by quantitative RT-PCR to assess the relative amount of viral polymerase acidic protein (PA) subunit cDNA in each lung. Virus RNA message as represented by the estimated number of copies of PA gene per lung has been shown to be proportional to virus infectious particles (PFU calculation).18 Relative expression was determined and reported as fold change compared to a randomly assigned sample within each group. Actin was used as an endogenous control. Primers used were: PR8-PA-forward: GAGCCTATGTGGATGGATTC, PR8-PA-reverse: TGCAGTTCTGCCAGTACTTG, β–actin-forward: GTCCCTCAGCCTCCCAAAAG, and β-actin-reverse: GCTGCCTCAACACCTCAACCC.
Statistical Analysis
Morbidity and mortality statistical analysis was performed on SigmaPlot 11.0 (Systat Software, Inc, San Jose, CA) and Stata SE 13.1 (StataCorp, College Station, TX). Data was analyzed by descriptive statistics and reported as the mean ± standard deviation (S.D.), one-way analysis of variance (ANOVA) with pairwise comparisons for parametric data, and by Wilcoxon rank sum for cell differential comparisons. Multiple comparisons were controlled for (i.e. Scheffe’s or Dunn’s method) where applicable. Survival analysis performed by Kaplan-Meier curve and log-rank test. Gene expression data was analyzed using RT2 Profiler PCR Array Data Analysis software (Qiagen) and normalized to internal controls. P values were calculated based on a Student’s t-test of the replicate 2^(−Delta Ct) values for each gene in the control group and treatment groups. PCR array data was considered to have biologic plausibility if: 1) the average gene threshold cycle was relatively high (>30) in either the control or test sample, and reasonably low in the other sample (<30), 2) statistical significance was determined with a P-value <0.05, or 3) a 2-fold change in expression compared to that found in control (day 0) mice was observed.
Results
Altered morbidity and mortality post-inoculation with influenza virus (PR8)
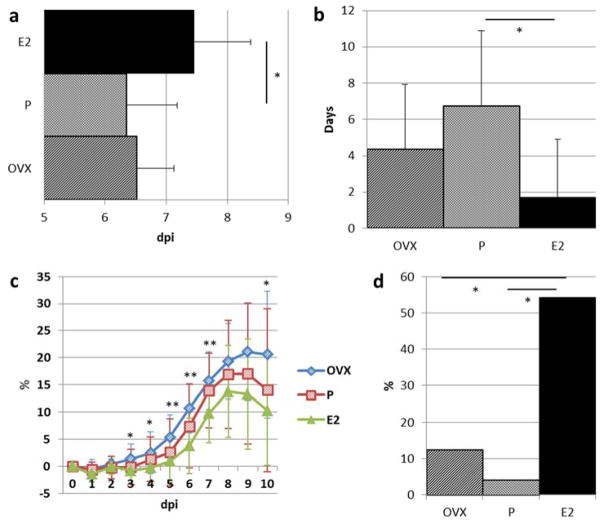
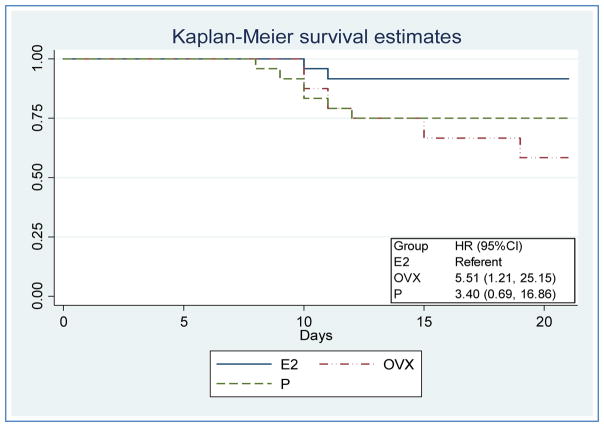
To examine the influence of P, E2, and OVX on PR8 influenza infectious morbidity and mortality, mice were monitored for 21 dpi. Onset of morbidity was earlier in P mice at 6.3±0.8 days compared to E2 at 7.5±0.9 days and OVX at 6.5±0.6 days (Figure 1a). Duration of morbidity in surviving mice was less in E2 (1.7±3.2 days) compared to P (6.7±4.2 days) and OVX (4.4±3.6 days) (Figure 1b). The mean percent weight loss for E2 was significantly less than OVX on dpi 3–7, and 10. A trend was visualized for P treated mice to have mean daily weight loss that was intermediate to E2 and OVX treated mice (dpi 1–10)(Figure 1c). The percentage of mice with absence of morbidity and mortality was greater for E2 (54.2%), compared to P (4.2%) and OVX (12.5%)(Figure 1d). With E2 as the referent group, the risk of death to 21 dpi was more likely for P, demonstrated by a hazard ratio (HR) of 3.4 (0.69, 16.86 95%CI), and OVX, with a HR of 5.51 (1.21, 25.15 95%CI) (Figure 2).
Figure 1. Altered morbidity and mortality post-inoculation with influenza virus (PR8).
(a) Days post PR8 inoculation (dpi) and the onset of morbidity (i.e. weight loss, ruffled fur, and/or decreased activity). (b) Duration of morbidity in surviving mice after influenza PR8 inoculation. (c) Daily mean percent weight loss post-inoculation. Statistical significance denoted for E2 vs. OVX pairwise comparison. Other pairwise comparisons are non-significant. (d) Absence of morbidity or mortality observations (i.e. weight loss, ruffled fur, decreased activity, and/or > 20 or 30 percent weight loss). Data in mean + S.D., n= 20–24 per treatment group, and *= P<0.05, **=P<0.01, ANOVA with pairwise comparisons. E2, 17-β-estradiol; P, progesterone; OVX, ovariectomy.
Figure 2. Survival analysis and hazard ratios (HR) post-inoculation with influenza virus (PR8).
Log-rank test of survival function: P=0.04. E2, 17-β-estradiol; P, progesterone; OVX, ovariectomy.
Pregnancy, influenza and mortality
For the pregnant mice inoculated on gestational day (gd) 9, signs of morbidity (i.e. ruffled fur, decreased activity and/or weight loss) was first observed on dpi 5 to 7 (i.e. gd 14 – 16). Among the mice in this group surviving until term, none of these mice underwent preterm deliveries (defined as delivery on or before gd 18). Among this group of mice inoculated on gd 9, the overall mortality rate was 40% (i.e. 12/30 mice); with the deaths occurring from 7 – 20 dpi (mean 10.5 ± 3.5 S.D.). In contrast, for the pregnant mice inoculated on gd14, none underwent PTD, morbidity was first observed on dpi 6 to 7 (i.e. postpartum day 1–2), and the overall mortality rate was 5% (i.e. 1/19 mice); which was significantly different from the mortality rate for the mice inoculated on gd 9 (Fisher exact test p < 0.01).
Influence of E2, P and OVX on immune cell infiltrate
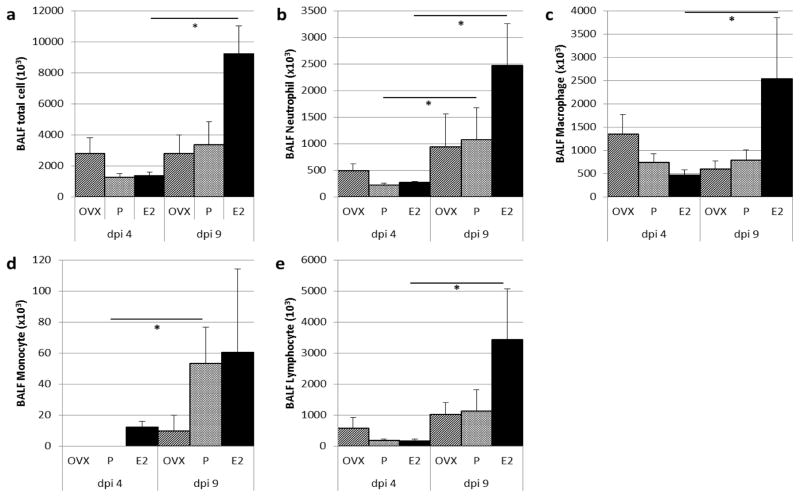
Immune cell infiltrate into the airspace was assessed for all study groups from dpi 4 to dpi 9, and demonstrated by BALF cell counts. E2 treated mice demonstrate significantly increased recruitment over this time period for total cells, neutrophils, macrophages and lymphocytes. While P treated mice demonstrate an increase in immune cell infiltrate, it is not as robust as the E2 group. OVX mice appear to weakly recruit immune cell populations (Figure 3).
Figure 3. Influence of E2, P and OVX on immune cell infiltrate in bronchoalveolar lavage fluid (BALF) 4 and 9 days post influenza virus inoculation (dpi).
(a–e) Total and differential BALF cell counts for neutrophils, macrophages, monocytes, and lymphocytes. Data in mean + S.E., n=3–4 per treatment group, and *= P<0.05, Wilcoxon rank-sum. E2, 17-β-estradiol; P, progesterone; OVX, ovariectomy.
E2, P and OVX may influence immune response during influenza infection
The influence of E2, P and OVX on immune response to influenza infection was studied by evaluating whole lung tissue at different time points post-inoculation with either PR8 or saline by TLR signaling pathway PCR array. The inflammatory marker and chemo-attractant for monocytes and macrophages, chemokine (C-C motif) ligand 2 (CCL2), demonstrated the greatest initial up-regulation dpi 4 for E2 (191.9 fold), compared to P (106.7 fold), and OVX (54.2 fold). The pro-inflammatory cytokine interleukin-6 (IL6) had the greatest increase in up-regulation dpi 4 for E2 (24.6 fold), and less for P (16.5 fold) and OVX (8.8 fold). The anti-inflammatory cytokine interleukin-10 (IL10) demonstrated the least change in up-regulation for E2 (5.5 fold) later during infection (dpi 9), when compared to P (17.5 fold) and OVX (15.6 fold)(Table 1).
Table 1. Influence of E2, P and OVX on selected TLR signaling pathway immune response markers 4 and 9 days post-inoculation (dpi).
Fold regulation determined for each reference point (day 4 and 9) compared to day 0 saline control for each respective group. Immune response markers with ≥2-fold change denoted in green. E2, 17-β-estradiol; P, progesterone; OVX, ovariectomy; TLR, toll-like receptor; FR, fold-regulation.
| Immune response marker | Fold Regulation | |||||
|---|---|---|---|---|---|---|
| dpi 4 | dpi 9 | |||||
| E2 | P | OVX | E2 | P | OVX | |
| Ccl2 | 191.87 | 106.72 * | 54.18 | 83.82 | 18.43 | 91.46 |
| Il6 | 24.56 | 16.49 * | 8.78 | 4.62 | 3.62 | 7.14 |
| Il10 | 2.53 * | 8.92 * | 6.49 * | 5.48 | 17.47 | 15.61 * |
| Tlr3 | 2.84 * | 3.38 | −1.17 | −1.14 | 1.18 | −1.21 |
| Tlr7 | 2.37 * | 2.83 * | 1.13 | −1.13 | 1.33 | 2.33 * |
| Tlr9 | 13.40 | 28.12 * | 23.75 * | 4.71 * | 7.89 * | 9.05 * |
=P<0.05, Student’s t-test.
Among the TLRs most responsible for sensitivity to viral pathogens (i.e. TLR3, TLR7, and TLR9), TLR9 demonstrated the greatest overall increase in up-regulation across all treatment groups on dpi 4 and 9 (Table 1).
The full panel of 84 TLR related genes is reported in Table 2.
Table 2. Influence of E2, P and OVX on 84 TLR signaling pathway immune response markers 4 and 9 days post-inoculation (dpi).
Fold regulation determined for each reference point (day 4 and 9) compared to day 0 saline control for each respective group. Immune response markers with ≥2-fold change denoted in green, 2-fold change denoted in red. E2, 17-β-estradiol; P, progesterone; OVX, ovariectomy; TLR, toll-like receptor; FR, fold-regulation.
| Immune response marker | Fold Regulation | Description | |||||
|---|---|---|---|---|---|---|---|
| dpi 4 | dpi 9 | ||||||
| E2 | P | OVX | E2 | P | OVX | ||
| Btk | 1.23 | 2.27 * | −1.08 | −1.04 | 2.65 * | 1.38 | Bruton agammaglobulinemia tyrosine kinase |
| Casp8 | 1.05 | 1.57 | −6.30 | −1.34 | 1.30 | −1.54 * | Caspase 8 |
| Ccl2 | 191.87 * | 106.72 * | 54.18 | 83.82 | 18.43 | 91.46 | Chemokine (C-C motif) ligand 2 |
| Cd14 | 2.09 * | 1.90 | 2.01 | 1.09 | 1.02 | 1.24 | CD14 antigen |
| Cd80 | 2.11 | 2.80 * | 1.26 | 1.33 | 2.12 * | 2.08 * | CD80 antigen |
| Cd86 | 3.62 | 3.70 * | 1.62 | 1.68 * | 1.88 | 2.22 * | CD86 antigen |
| Cebpb | −1.09 | 1.14 | 1.08 | −1.94 * | −1.21 | −1.87 * | CCAAT/enhancer binding protein (C/EBP), beta |
| Chuk | −1.04 | 1.18 | −3.78 * | −1.43 * | −1.06 | −1.87 * | Conserved helix-loop-helix ubiquitous kinase |
| Clec4e | 1.72 | 1.72 | −2.17 | −1.29 | −1.38 | 1.41 | C-type lectin domain family 4, member e |
| Csf2 | 3.17 * | 4.30 | 1.91 | −2.17 | 1.05 | −4.13 * | Colony stimulating factor 2 (granulocyte-macrophage) |
| Csf3 | 7.89 * | 11.79 * | 19.90 | 2.87 | 2.36 | 5.93 | Colony stimulating factor 3 (granulocyte) |
| Cxcl10 | 589.24 * | 80.10 | 243.94 | 185.31 | 8.50 | 94.70 * | Chemokine (C-X-C motif) ligand 10 |
| Elk1 | 1.01 | 1.42 | −5.81 | −2.06 * | 1.24 | −1.65 | ELK1, member of ETS oncogene family |
| Fadd | −1.05 | 1.43 | 1.17 | −1.42 * | 1.26 | −1.34 | Fas (TNFRSF6)-associated via death domain |
| Fos | −1.47 | −2.60 | −1.60 | −1.55 | −2.13 | −1.47 | FBJ osteosarcoma oncogene |
| Hmgb1 | −1.44 * | −1.31 | −3.24 * | −2.40 * | −1.60 * | −3.50 * | High mobility group box 1 |
| Hras1 | −1.10 | −1.10 | −1.02 | −1.70 * | −1.32 | −2.04 * | Harvey rat sarcoma virus oncogene 1 |
| Agfg1 | 1.04 | 1.86 | −1.82 | −1.22 | 1.39 | −1.40 * | ArfGAP with FG repeats 1 |
| Hspa1a | 7.30 | −1.30 | 5.40 | 1.25 | −4.16 | −2.46 | Heat shock protein 1A |
| Hspd1 | 1.27 | 1.40 | −1.75 * | −1.02 | 1.05 | −1.57 * | Heat shock protein 1 (chaperonin) |
| Ifnb1 | 385.51 * | 302.57 | 231.91 | 3.57 | 1.18 | 1.68 | Interferon beta 1, fibroblast |
| Ifng | 21.52 * | 33.80 * | 8.40 | 28.74 * | 16.56 | 31.71 * | Interferon gamma |
| Ikbkb | −1.13 | 1.37 | −1.33 | −1.53 * | 1.40 | −1.38 | Inhibitor of kappaB kinase beta |
| Il10 | 2.53 * | 8.92 * | 6.49 * | 5.48 | 17.47 | 15.61 * | Interleukin 10 |
| Il12a | −1.14 | 1.36 | −1.68 * | −2.51 * | 1.64 * | −3.75 * | Interleukin 12A |
| Il1a | 1.44 | 2.27 | −3.70 | −2.33 * | 1.88 | −2.99 * | Interleukin 1 alpha |
| Il1b | 1.86 | 3.52 * | −1.60 | −1.46 | 1.71 | −1.36 | Interleukin 1 beta |
| Il1r1 | −1.36 * | 1.41 | −2.98 * | −1.67 * | 1.31 | −1.46 | Interleukin 1 receptor, type I |
| Il2 | −1.02 | −1.34 | −2.25 | 1.76 | 1.38 | 2.26 * | Interleukin 2 |
| Il6 | 24.56 * | 16.49 * | 8.78 | 4.62 | 3.62 | 7.14 | Interleukin 6 |
| Il6ra | −1.25 | 1.11 | −1.53 | −2.28 * | −1.27 | −1.61 | Interleukin 6 receptor, alpha |
| Irak1 | −1.15 | 1.38 | −1.88 * | −1.35 | 1.13 | −1.85 * | Interleukin-1 receptor-associated kinase 1 |
| Irak2 | −1.03 | 1.47 | 1.06 | −1.90 * | 1.06 | −1.73 | Interleukin-1 receptor-associated kinase 2 |
| Irf1 | 3.77 * | 3.12 | 3.10 | 2.31 | 1.49 | 2.24 * | Interferon regulatory factor 1 |
| Irf3 | 1.05 | 1.07 | −1.06 | −1.38 | 1.00 | −1.58 | Interferon regulatory factor 3 |
| Jun | 1.08 | −1.66 | 1.10 | −1.32 | −1.54 | −1.80 | Jun oncogene |
| Lta | 1.90 | 2.47 | 3.35 * | 1.61 | 2.82 | 1.51 | Lymphotoxin A |
| Muc13 | 1.14 | 1.29 | 7.85 * | −1.65 | −3.81 * | −1.08 | Mucin 13, epithelial transmembrane |
| Ly86 | 2.84 * | 4.11 * | 1.87 | 1.47 | 2.68 * | 1.99 | Lymphocyte antigen 86 |
| Ly96 | 1.43 * | 1.87 * | −1.23 | −1.14 | 1.45 | 1.23 | Lymphocyte antigen 96 |
| Map2k3 | −1.12 | −1.48 | −1.04 | −1.64 * | −1.96 | −1.84 * | Mitogen-activated protein kinase kinase 3 |
| Map2k4 | −1.13 | 1.22 | −2.49 | −1.61 * | −1.06 | −1.96 * | Mitogen-activated protein kinase kinase 4 |
| Map3k1 | −1.06 | 1.72 | 1.11 | −1.10 | 1.52 | 1.14 | Mitogen-activated protein kinase kinase kinase 1 |
| Map3k7 | −1.28 * | 1.02 | −2.60 * | −1.59 * | −1.09 | −1.47 | Mitogen-activated protein kinase kinase kinase 7 |
| Mapk8 | −1.01 | 1.09 | −3.26 | −1.55 * | −1.02 | −1.60 | Mitogen-activated protein kinase 8 |
| Mapk8ip3 | −1.18 | 1.02 | −2.29 | −1.86 * | 1.02 | −2.60 * | Mitogen-activated protein kinase 8 interacting protein 3 |
| Mapk9 | −1.20 | −1.02 | −2.58 * | −1.86 * | −1.11 | −2.35 * | Mitogen-activated protein kinase 9 |
| Myd88 | 2.61 | 3.18 * | 4.20 | 1.02 | 1.69 | 2.60 | Myeloid differentiation primary response gene 88 |
| Nfkb1 | 1.17 | 1.99 | −1.01 | −1.44 * | 1.49 | −1.27 | Nf kappa light polypeptide gene enhancer in B-cells 1, p105 |
| Nfkb2 | 1.39 | 1.85 | 2.13 | −1.11 | 1.31 | −1.00 | Nf kappa light polypeptide gene enhancer in B-cells 2, p49/p100 |
| Nfkbia | −1.85 * | 1.06 | −1.13 | −3.39 * | −1.10 | −1.68 | Nf kappa light polypeptide gene enhancer in B-cells inhibitor, alpha |
| Nfkbib | −1.81 | −1.74 | −2.97 | −1.78 | −1.73 | −1.72 | Nf kappa light polypeptide gene enhancer in B-cells inhibitor, beta |
| Nfkbil1 | 1.43 | −2.85 | −3.67 | −2.00 | −1.73 | −4.09 | Nf kappa light polypeptide gene enhancer in B-cells inhibitor-like 1 |
| Nfrkb | −1.21 * | 1.24 | −1.08 | −1.37 * | 1.04 | −1.70 * | Nf related to kappa B binding protein |
| Nr2c2 | −1.32 * | −1.07 | −2.17 * | −1.81 * | −1.25 | −2.08 * | Nuclear receptor subfamily 2, group C, member 2 |
| Peli1 | 1.12 | 1.40 | −1.46 | −1.69 * | −1.06 | −1.39 | Pellino 1 |
| Pglyrp1 | 1.23 | 1.74 | 1.33 | 1.06 | 1.05 | −1.39 | Peptidoglycan recognition protein 1 |
| Ppara | −1.09 | −1.16 | −1.64 | −2.03 | −1.26 | −3.29 * | Peroxisome proliferator activated receptor alpha |
| Eif2ak2 | 5.79 * | 5.48 * | −1.06 | 1.31 | 1.37 | 1.05 | Eukaryotic translation initiation factor 2-alpha kinase 2 |
| Ptgs2 | 2.92 * | −1.83 | 1.15 | 1.32 | −1.67 | 1.19 | Prostaglandin-endoperoxide synthase 2 |
| Rel | 1.15 | 2.14 * | −1.19 | −1.44 | 1.87 | −1.04 | Reticuloendotheliosis oncogene |
| Rela | −1.04 | 1.17 | 1.08 | −1.49 * | −1.04 | −1.77 * | V-rel reticuloendotheliosis viral oncogene homolog A (avian) |
| Ripk2 | 1.58 | 2.16 | −1.41 | −1.21 | −1.04 | −1.17 | Receptor (TNFRSF)-interacting serine-threonine kinase 2 |
| Tbk1 | 1.34 | 1.83 | −1.49 | −1.16 | 1.12 | −1.07 | TANK-binding kinase 1 |
| Ticam1 | −1.01 | −1.15 | 1.67 | 1.03 | 1.28 | −1.04 | Toll-like receptor adaptor molecule 1 |
| Ticam2 | 1.07 | 1.69 | 1.09 | −1.24 | 1.66 | 1.38 | Toll-like receptor adaptor molecule 2 |
| Tirap | 1.37 | 1.75 | −1.50 | −1.05 | 1.21 | −1.17 | Toll-interleukin 1 receptor (TIR) domain-containing adaptor protein |
| Tlr1 | 2.97 | 5.89 * | 1.24 | 2.74 | 4.25 | 3.35 * | Toll-like receptor 1 |
| Tlr2 | 1.38 | 2.26 | 2.39 | −1.45 * | 1.32 | 1.48 | Toll-like receptor 2 |
| Tlr3 | 2.84 * | 3.38 | −1.17 | −1.14 | 1.18 | −1.21 | Toll-like receptor 3 |
| Tlr4 | 1.19 * | 1.35 | −2.30 | −1.53 * | 1.12 | 1.01 | Toll-like receptor 4 |
| Tlr5 | −1.64 * | 1.31 | −3.43 * | −3.02 * | 1.53 | −3.97 * | Toll-like receptor 5 |
| Tlr6 | 1.19 | 1.68 | −1.08 | −1.00 | 1.35 | 1.76 * | Toll-like receptor 6 |
| Tlr7 | 2.37 * | 2.83 * | 1.13 | −1.13 | 1.33 | 2.33 * | Toll-like receptor 7 |
| Tlr8 | 1.05 | 1.72 | −2.98 | −1.83 * | 1.32 | 1.49 | Toll-like receptor 8 |
| Tlr9 | 13.40 * | 28.12 * | 23.75 * | 4.71 * | 7.89 * | 9.05 * | Toll-like receptor 9 |
| Tnf | 9.15 * | 7.03 * | 10.89 * | 3.94 * | 2.60 | 6.48 * | Tumor necrosis factor |
| Tnfaip3 | −1.05 | 2.06 | 1.87 | −1.79 * | 1.58 | 1.06 | Tumor necrosis factor, alpha-induced protein 3 |
| Tnfrsf1a | 1.38 | 1.63 | 1.72 | −1.07 | 1.04 | 1.01 | Tumor necrosis factor receptor superfamily, member 1a |
| Tollip | −1.13 | −1.06 | −1.50 | −1.83 * | −1.42 | −2.58 * | Toll interacting protein |
| Tradd | 1.12 | 1.49 * | −1.16 | −1.18 | 1.17 | −1.66 | TNFRSF1A-associated via death domain |
| Traf6 | −1.17 | −1.21 | −1.69 | −1.73 * | −1.21 | −1.62 * | Tnf receptor-associated factor 6 |
| Ube2n | −1.72 | −1.10 | −3.19 | −2.72 * | −1.34 | −3.33 * | Ubiquitin-conjugating enzyme E2N |
| Ube2v1 | 1.03 | 1.49 | 1.21 | −1.17 | 1.30 | −1.58 * | Ubiquitin-conjugating enzyme E2 variant 1 |
= P < 0.05, Student’s t-test.
Relative viral message
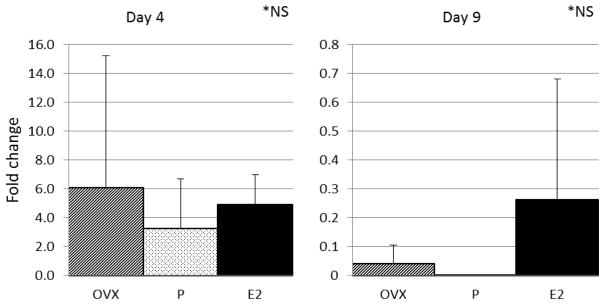
Influenza viral message in lung tissue (as assessed by the relative amount of viral PA subunit PCR product per lung) was determined and expressed as fold change within each study group. There were no statistically significant differences between the study groups on dpi 4 or dpi 9 (Figure 4).
Figure 4. Influenza viral message in lung tissue as assessed by the relative amount of viral PA subunit PCR product.
Data expressed as fold change + S.D., n= 3 per study group, NS by ANOVA. E2, 17-β-estradiol; P, progesterone; OVX, ovariectomy; NS, non-significant.
Discussion
The pregnancy-associated hormones of estradiol and progesterone were examined in the context of influenza infection in a mouse model to investigate the individual contributions of these hormones to morbidity and mortality. To our knowledge, this is the first study to directly compare the influence of estradiol and progesterone on influenza infection in a mouse model.
During gestation, progesterone levels are elevated during mid gestation and fall at term.19 Interestingly, these pregnant mouse data have confirmed significant mortality during the period of increased progesterone. Mortality is markedly reduced when progesterone levels are low. These observations provide the justification for the subsequent castration/hormone give-back studies performed in an effort to clarify the individual effects of progesterone and estradiol on the morbidity and mortality produced by influenza infection.
Our give-back studies found that estradiol treated mice demonstrated decreased cachexia, shorter duration of illness, later onset of morbidity and overall less morbidity and mortality than progesterone treated and no hormone replaced (OVX). Progesterone treated mice had increased cachexia, longer duration of illness, earlier onset of disease and overall increased morbidity and mortality. These steroid hormone effects are very consistent with mortality observations in our pregnant mouse studies inoculated with influenza.20 Based on all of these mouse studies, estradiol and progesterone may contribute to disease severity observed in gravid women, with estradiol providing a protective effect and progesterone being detrimental.
To investigate immune response during our OVX/hormone replacement studies, we assessed the expression of several TLR signaling pathway markers using a TLR pathway qPCR array. Among the TLRs most responsible for sensing viral pathogens, TLR9 demonstrated the greatest overall increase in up-regulation, suggesting that it might play an important role in mediating the innate immune response to influenza.
Additional studies are necessary to fully understand and detail the underlying mechanism(s) responsible for the increased morbidity and mortality observed with influenza infection during pregnancy. However, results from our mouse study help to begin to determine the contributions of estradiol and progesterone during influenza infection. These hormones play an important role during pregnancy, with pregnancy highly influenced by progesterone as gestation advances. TLR signaling pathway marker and immune cell infiltrate findings suggest that estradiol treated mice may have an appropriate balance of pro-inflammatory and a tempered anti-inflammatory response that might promote overall improved outcomes. Interestingly, 17-β-estradiol has been described to restore antibody responses to an influenza vaccine in a postmenopausal mouse model.21 In contrast, the progesterone treated mice have an initial pro-inflammatory response that is present but to a lesser degree than estradiol, followed by possible dominance of an anti-inflammatory response (IL10) that may ultimately promote poorer outcomes. The OVX mice seem to have an initial weaker pro-inflammatory response followed by an anti-inflammatory response that may influence an overall weak immune response with associated poorer outcomes. IL6 has been described as an essential cytokine during the initial immune response to influenza and is necessary for protection against the H1N1 virus by promoting lung neutrophil survival.22 Excessive IL10 production has been described as an important mediator of enhanced susceptibility to secondary pneumonia and reduced neutrophil function in the lungs after influenza infection.23
Progesterone is a key component of modern contraception, with modern methods defined by the Population Division of the Department of Economic and Social Affairs, United Nations, to include oral hormonal pills, the intra-uterinedevice (IUD), injectables, implants, and emergency contraception.24 The worldwide median estimate of modern method contraceptive prevalence was cited as 57.8 percent in 2016.25 Even with an increasing prevalence of modern contraceptive use, study of the effect of progesterone on immunity is limited beyond the acquisition and transmission of sexually transmitted infections, such as HIV-1. Recent studies suggest that some progestins may modulate the reproductive tract environment and may be associated with an increased risk for transmission and acquisition of sexually transmitted infections.26–28 With regard to pathogen acquisition beyond the reproductive tract, publications are limited. Hall et al. report a protective role against lethal and sublethal viral influenza A infection with progesterone giveback to an ovariectomized progesterone-deplete mouse model, but do not include a comparative estradiol model.14 Our study includes comparative progesterone and estradiol giveback models and suggests that progesterone may have superior immunomodulatory effects when compared to the hormone-deplete group. However, when we compare estradiol giveback to progesterone and the hormone-deplete groups, estradiol dominates as the hormone to more significantly reduce mortality and mediate a more robust response to influenza infection. The global use of progestin compounds, the prevalence of pregnancy and the composite results from these studies support the importance of further investigation to understand the underlying mechanism(s) responsible for the above findings.
Additional investigation should include histological characterization of pulmonary tissue changes associated with influenza, as influenced by hormone add-back. Larger studies should be performed to confirm gene expression findings and include correlation to lung cytokine protein levels.
This study is limited by small sample size in the airspace cell differentials and TLR signaling pathway gene expression studies. This limitation is recognized and these findings are meant to be exploratory in nature to generate additional research questions and areas of study. Additionally, whole lung was used for TLR qPCR array studies; therefore, it is unclear which cell populations most significantly impact findings and how variations in their relative proportions affect the measured results. This study was designed to specifically evaluate the contributions of estradiol and progesterone on influenza infection by a hormonal add-back approach, and therefore the hormone deplete OVX group findings cannot be extrapolated to a non-pregnant reproductive age group.
In summary, estradiol may play a protective and progesterone a comparatively detrimental role in the pathophysiology of influenza infection. Influences from these pregnancy-associated hormones may underlie disease severity observed in gravid women during influenza infection.
Acknowledgments
This work was supported by the National Institutes of Health [NIH R21HD065396]. We would like to thank Oliver Dienz, PhD, Vermont Center for Immunobiology and Infectious Disease, The University of Vermont, Burlington, VT for technical assistance in cell differential slide preparation and performance of virus real-time qRT-PCR, funded by NIGMS P20 GM103496-07.
Footnotes
All work performed at the University of Vermont, Burlington, VT.
Declaration of Conflicting Interests: The authors declare that there is no conflict of interest.
References
- 1.Beigi RH. Pandemic influenza and pregnancy: a call for preparedness planning. Obstet Gynecol. 2007;109(5):1193–1196. doi: 10.1097/01.AOG.0000262051.71925.ac. [DOI] [PubMed] [Google Scholar]
- 2.Siston AM, Rasmussen SA, Honein MA, et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303(15):1517–1525. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louie JK, Acosta M, Jamieson DJ, Honein MA. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362(1):27–35. doi: 10.1056/NEJMoa0910444. [DOI] [PubMed] [Google Scholar]
- 4.Williams D, Basavarajappa MS, Rasmussen SA, Morris S, Mattison D Animal Models of Pregnancy Working G. Highlights from the United States Food and Drug Administration’s public workshop on the development of animal models of pregnancy to address medical countermeasures in an “at-risk” population of pregnant women: Influenza as a case study. Birth Defects Res A Clin Mol Teratol. 2014;100(10):806–810. doi: 10.1002/bdra.23319. [DOI] [PubMed] [Google Scholar]
- 5.Raj RS, Bonney EA, Phillippe M. Influenza, immune system, and pregnancy. Reprod Sci. 2014;21(12):1434–1451. doi: 10.1177/1933719114537720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thangavel RR, Bouvier NM. Animal models for influenza virus pathogenesis, transmission, and immunology. J Immunol Methods. 2014;410:60–79. doi: 10.1016/j.jim.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pazos MA, Kraus TA, Munoz-Fontela C, Moran TM. Estrogen mediates innate and adaptive immune alterations to influenza infection in pregnant mice. PLoS One. 2012;7(7):e40502. doi: 10.1371/journal.pone.0040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcelin G, Aldridge JR, Duan S, et al. Fatal outcome of pandemic H1N1 2009 influenza virus infection is associated with immunopathology and impaired lung repair, not enhanced viral burden, in pregnant mice. J Virol. 2011;85(21):11208–11219. doi: 10.1128/JVI.00654-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams K, Mackenzie JS. Influenza infections during pregnancy in the mouse. J Hyg (Lond) 1977;79(2):249–257. doi: 10.1017/s0022172400053067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan KH, Zhang AJ, To KK, et al. Wild type and mutant 2009 pandemic influenza A (H1N1) viruses cause more severe disease and higher mortality in pregnant BALB/c mice. PLoS One. 2010;5(10):e13757. doi: 10.1371/journal.pone.0013757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shriner SA, VanDalen KK, Mooers NL, et al. Low-pathogenic avian influenza viruses in wild house mice. PLoS One. 2012;7(6):e39206. doi: 10.1371/journal.pone.0039206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson DP, Lorenzo ME, Jian W, Klein SL. Elevated 17beta-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathog. 2011;7(7):e1002149. doi: 10.1371/journal.ppat.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson DP, Hall OJ, Nilles TL, Bream JH, Klein SL. 17beta-estradiol protects females against influenza by recruiting neutrophils and increasing virus-specific CD8 T cell responses in the lungs. J Virol. 2014;88(9):4711–4720. doi: 10.1128/JVI.02081-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall OJ, Limjunyawong N, Vermillion MS, et al. Progesterone-Based Therapy Protects Against Influenza by Promoting Lung Repair and Recovery in Females. PLoS Pathog. 2016;12(9):e1005840. doi: 10.1371/journal.ppat.1005840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyden DC, Olszewski J, Feran M, Job LP, Huber SA. Coxsackievirus B-3-induced myocarditis. Effect of sex steroids on viremia and infectivity of cardiocytes. Am J Pathol. 1987;126(3):432–438. [PMC free article] [PubMed] [Google Scholar]
- 16.Lyden DC, Huber SA. Aggravation of coxsackievirus, group B, type 3-induced myocarditis and increase in cellular immunity to myocyte antigens in pregnant Balb/c mice and animals treated with progesterone. Cell Immunol. 1984;87(2):462–472. doi: 10.1016/0008-8749(84)90015-7. [DOI] [PubMed] [Google Scholar]
- 17.Lu S, Becker KA, Hagen MJ, et al. Transcriptional responses to estrogen and progesterone in mammary gland identify networks regulating p53 activity. Endocrinology. 2008;149(10):4809–4820. doi: 10.1210/en.2008-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jelley-Gibbs DM, Dibble JP, Brown DM, Strutt TM, McKinstry KK, Swain SL. Persistent depots of influenza antigen fail to induce a cytotoxic CD8 T cell response. Journal of immunology. 2007;178(12):7563–7570. doi: 10.4049/jimmunol.178.12.7563. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto H, Eto T, Endo K, et al. Comparative study of doses of exogenous progesterone administration needed to delay parturition in Jcl:MCH(ICR) mice. Exp Anim. 2010;59(4):521–524. doi: 10.1538/expanim.59.521. [DOI] [PubMed] [Google Scholar]
- 20.Phillippe M, Davis SM, Sweet L. Synergistic effects of bacterial PAMPs on influenza infection during pregnancy. Reprod Sci. 2012;19(3 Suppl):279A. [Google Scholar]
- 21.Nguyen DC, Masseoud F, Lu X, Scinicariello F, Sambhara S, Attanasio R. 17beta-Estradiol restores antibody responses to an influenza vaccine in a postmenopausal mouse model. Vaccine. 2011;29(14):2515–2518. doi: 10.1016/j.vaccine.2011.01.080. [DOI] [PubMed] [Google Scholar]
- 22.Dienz O, Rud JG, Eaton SM, et al. Essential role of IL-6 in protection against H1N1 influenza virus by promoting neutrophil survival in the lung. Mucosal Immunol. 2012;5(3):258–266. doi: 10.1038/mi.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Sluijs KF, van Elden LJ, Nijhuis M, et al. IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. Journal of immunology. 2004;172(12):7603–7609. doi: 10.4049/jimmunol.172.12.7603. [DOI] [PubMed] [Google Scholar]
- 24.United Nations, Department of Economic and Social Affairs, Population Division. Trends in Contraceptive Use Worldwide 2015 (ST/ESA/SER.A/349) 2015. [Google Scholar]
- 25.United Nations, Department of Economic and Social Affairs, Population Division. Model-based Estimates and Projections of Family Planning Indicators 2016. New York: United Nations; 2016. [Google Scholar]
- 26.Quispe Calla NE, Ghonime MG, Cherpes TL, Vicetti Miguel RD. Medroxyprogesterone acetate impairs human dendritic cell activation and function. Hum Reprod. 2015;30(5):1169–1177. doi: 10.1093/humrep/dev035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michel KG, Huijbregts RP, Gleason JL, Richter HE, Hel Z. Effect of hormonal contraception on the function of plasmacytoid dendritic cells and distribution of immune cell populations in the female reproductive tract. J Acquir Immune Defic Syndr. 2015;68(5):511–518. doi: 10.1097/QAI.0000000000000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huijbregts RP, Michel KG, Hel Z. Effect of progestins on immunity: medroxyprogesterone but not norethisterone or levonorgestrel suppresses the function of T cells and pDCs. Contraception. 2014;90(2):123–129. doi: 10.1016/j.contraception.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]