Abstract
Three-dimensional cell culture has the potential to revolutionize toxicology studies by allowing human-based reproduction of essential elements of organs. Beyond the study of toxicants on the most susceptible organs such as liver, kidney, skin, lung, gastrointestinal tract, testis, heart and brain, carcinogenesis research will also greatly benefit from 3D cell culture models representing any normal tissue. No tissue function can be suitably reproduced without the appropriate tissue architecture whether mimicking acini, ducts or tubes, sheets of cells or more complex cellular organizations like hepatic cords. In this review, we illustrate the fundamental characteristics of polarity that is an essential architectural feature of organs for which different 3D cell culture models are available for toxicology studies in vitro. The value of tissue polarity for the development of more accurate carcinogenesis studies is also exemplified, and the concept of using extracellular gradients of gaseous or chemical substances produced with microfluidics in 3D cell culture is discussed. Indeed such gradients-on-a-chip might bring unprecedented information to better determine permissible exposure levels. Finally, the impact of tissue architecture, established via cell-matrix interactions, on the cell nucleus is emphasized in light of the importance in toxicology of morphological and epigenetic alterations of this organelle.
Keywords: Polarity, cell nucleus, organ-on-a-chip, tight junction, basement membrane, microfluidics
1. Introduction
Organisms are exposed to a plethora of natural compounds, chemically engineered molecules and nanodevices that might exert a lasting impact on organs via their influence on genes and tissue homeostasis. Beyond well known risks of DNA aberrations, the epigenome (i.e., the collection of chemical modifications on the DNA and histones at any given time) is a primary target in toxicology, as it might be the source of chronic disorders linked to changes in gene expression, such as diabetes, obesity and cancer. However, for in vitro studies, the epigenome of a particular tissue is best mimicked using three-dimensional (3D) cell culture that places cells under physiologically relevant extracellular conditions.
The concept of 3D cell culture was developed 40 years ago, primarily via the use of floating collagen gels that helped maintain cellular differentiation and organization (Emerman and Pitelka, 1977). Importantly, a major article in this field was in toxicology, with the demonstration in vitro of hepatocellular changes induced by phenobarbital administration normally observed in vivo (Michalopoulos et al., 1976a). This early work illustrated the concept proposed by Elizabeth Hay that the extracellular matrix (ECM) controls the expression of genetic information (Hay, 1981). This concept was further developed by taking into account tissue architecture that can be defined as the recognizable features of tissue organization responsible for organ function. In summary, the ECM dictates the organization of cells and their nuclei via a continuous, but dynamic, network of connections, and thus, it controls gene expression; reciprocally, the given arrangement of the cell nucleus (notably chromatin) controls the cell’s response to extracellular stimuli (Bissell et al., 1982; Lelièvre, 2009).
The arrangement of cells into structures that resemble their organization in vivo is the basic definition of 3D cell culture, but for years the main representation of 3D cell culture was linked to making multicellular nodules or spheroids with tumors as well as with non-neoplastic cells. Many of the studies to produce tissue-like structures with non-neoplastic cells came from work with mammary epithelial cells, via the mimicry of polarized glandular structures or acini (Barcellos-Hoff et al., 1989) that, like tumor nodules, appear spheroidal in shape. It is likely that reproducing tumors and mammary acini in vitro gave the impression that 3D cell culture meant production of multicellular spheroids. Yet, beside acini or alveoli, some neuronal formations and a good portion of tumors, in vivo organs rarely contain spheroidal tissue structures. Moreover, any spheroidal structure in vivo, even a malignant tumor, has a specific organization of cells. Cellular organization reflects tissue function, itself linked to the microenvironment, notably the nature of the ECM. Achieving tissue organization requires time, as shown by the clonal nature of mammary acini in cell culture, i.e., each acinus is formed from the division of a single cell (Petersen et al., 1992). Therefore, cell culture methods that force the rapid formation of cell aggregates, without a possibility for the organization of cells and their extracellular milieu before analysis, prevent achieving proper tissue architecture.
Most 3D cell culture methods have made use of hydrogels based on collagen I or preparations from Engelbreth-Holm-Swarm (EHS) tumors that are enriched in components of the basement membrane (Grant et al., 1985), as well as newer formulations that do not include ECM components. Understandably placing cells within or on top of hydrogels favors spheroid formation; however, with certain cell types and a specific medium, ductal or tubular structures can also be formed within the appropriate ECM (Hirai et al., 1998; DesRochers et al., 2013). Also, with the correct chemistry and mechanistic property, these gels permit the establishment of proper architecture in tissues that indeed have a spheroidal organization in vivo. Such is the case for the polarized architecture of glandular epithelia in the breast, prostate, salivary gland, pancreas among other organs. Polarity is created by the asymmetrical distribution of proteins in the cell membrane, and determined by the formation of cell-cell tight junctions that separate basolateral and apical membrane domains (Wodarz and Nathke 2007). It is the backbone of proper epithelial, endothelial and liver functions. As such its reproduction is essential in 3D cell culture, and it can be obtained either in gels, when producing spheroidal multicellular structures is indicated, or by coating cell culture surfaces with ECM molecules that act as inducers of polarity for other types of multicellular structures (Inman and Bissell 2010). Other architectural elements encompass the directionality of cells (e.g., in different portions of the dermis and in the brain) and the specific distribution of one cell type relative to another cell type (e.g., support and contractile role of myoepithelial and smooth muscle cells). Importantly, architectural elements ought to be reproduced with the proper tissue geometry, like a duct, a planar structure, a network or a flexible wall. The development of organs-on-a-chip, a sophisticated form of 3D cell culture, can help approach the actual organization of certain tissues by providing a mold on which cells can reproducibly adopt a complex assembly.
Toxicological studies in vitro require the reproduction of normal tissues and portions of functional organs, as well as the mimicry of tumor formation. Normal tissues are used for assessing the impact of exogenous factors (e.g., drugs, cosmetic materials, pollutants, oxidative environments) on cellular functions and the risk of carcinogenesis (Kim et al., 2015). Whereas tumors are used to assess mechanisms of toxicity that could help improve anticancer treatments (Katt et al., 2016). In pharmacological and toxicological applications it is important to take into account polarity, an inevitable feature of the architecture of most tissues that controls differentiation. Cell-cell or cell-ECM interactions are other usual aspects of the structural organization in vivo; they control polarity and are also disturbed with loss of polarity. In this review, our main focus it to examine how the polarized architecture in normal tissues and its impact on the cell nucleus plays a central role in toxicology assays. In a first part, we briefly highlight 3D cell culture models of major organs used for toxicology assays, in which complete or partial tissue architecture has been reproduced. When reproducing polarity is not enough to obtain proper tissue function, examples are given of additional steps necessary for improved mimicry of tissue architecture. In a second part, we present the role of tissue architecture, first in the context of polarity, in the study of carcinogens before extending the notion of tissue architecture to tissue geometry. In a last part, we discuss how tissue architecture dictates the cell’s response to external stimuli via an impact on the nucleus, and how this understanding will help further develop exquisite models for toxicology in vitro.
2. Mimicry of tissue architecture in 3D cell culture for toxicology
A major challenge in toxicology is to define relevant in vitro systems for human studies. Animal models have been widely used to assess the toxicity of chemicals, but many of the models do not accurately predict the effects of chemical exposure in humans, notably due to species specificity. An additional reason for moving away from using laboratory animals is the recognition that, ethically, it is better to focus on the development of in vitro models since technological advances allow scientists to design such models. While using primary cells is appealing, when placed in 2D cultures they dedifferentiate rapidly, resulting in the loss of tissue specific phenotype and function. Therefore, chronic toxicity of chemicals that often relies on insidious effects, specifically on gene expression (Kulkarni et al., 2008), entails maintaining tissue differentiation. This endeavor requires establishing, thanks to 3D cell culture, the cell-ECM interactions and the tissue architecture necessary for tissue differentiation.
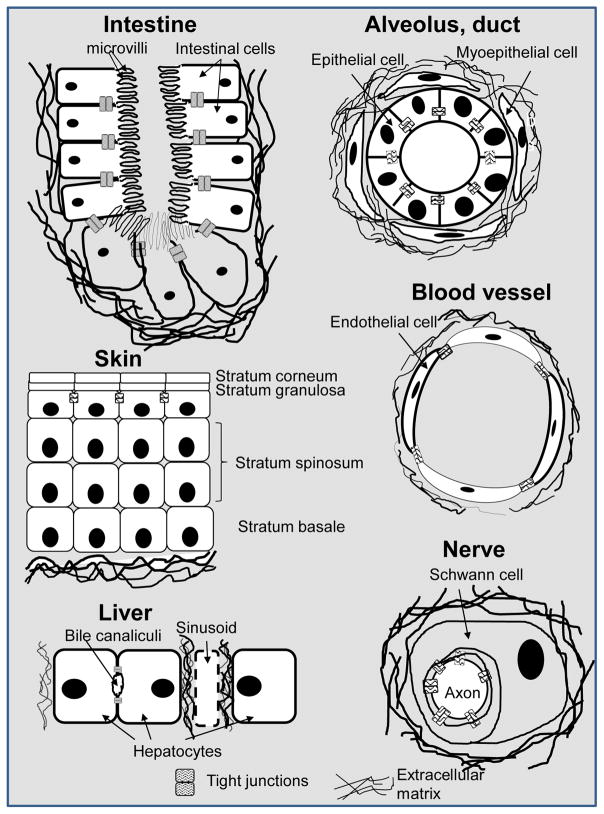
3D cell culture models are being developed for toxicology studies related to the liver, the kidney, the skin, the lungs, the gastrointestinal track, the neurological system, the testis and the heart that bear major consequences for an individual upon toxic exposure. An increasing number of studies are moving away from nonrelevant spheroids for most of these organs and are making strides to mimic the polarized architecture underlying fundamental functions. The functional unit in the liver, a hub for toxin and drug metabolism, is the hepatic lobule. It encompasses rows of hepatocytes that intermingle with Kupffer cells, stellate cells and endothelial cells, and are arranged hexagonally, radiating outward from a central vein. In the absence of a complete basement membrane type of ECM, fibronectin has been shown to be essential for hepatocyte polarity (Martinez-Hernandez and Amenta, 1993). The apical membrane of hepatocytes forms the continuous network of bile canaliculi and the basal membrane makes contact with the sinusoidal network, hence enabling hallmark endocytosis function, itself necessary to maintain basoapical polarity (Fig. 1). The collagen sandwich, using primary murine hepatocytes cultured between two layers of collagen, has been confirmed as a reliable method to reproduce basoapical polarity (Zeigerer et al., 2016). Another method encompassed the use of a cell sheet-manipulator coated with hydrogel (fibrin-coated gelatin) to create two layers of hepatocytes in sandwich between layers of endothelial cells. Here important elements of the ECM to establish polarity (collagen IV and fibronectin) were present in-between endothelial cells and hepatocytes, as were the apical locations of tight junction protein claudin-3 and apical marker multidrug-resistance protein (MRP2) between the two layers of hepatocytes. The triple layering ‘endothelial cells-hepatocytes-endothelial cells’ appeared essential to reproduce basoapical polarity (Kim et al,. 2015). Although human embryonic stem cells have been successfully used for differentiation into cholangiocytes with formation of polarized cysts and biliary ducts in 3D cell culture (Dianat et al., 2014), recapitulation of the polarized architecture of hepatic cords with human cells using the sandwich method is awaited. Ultimately, these relatively simple monoculture and coculture systems can serve as basis to include more complex parameters of liver physiology, such as a directional movement of medium to mimic sinusoidal blood flow and shear forces (Burkhardt et al., 2014; Choi et al., 2016).
Figure 1. Examples of polarity axis in various organs or functional units of organs.
Simple drawings represent the cells in which polarity is established with, at the apical pole, the presence of tight junctions at lateroapical cell-cell contacts, and at the basal pole, contacts with the ECM (most often a basement membrane type of ECM or a modified version of a basement membrane). Note: Not all cell types in a particular organ are represented.
The basic functional unit of the kidney, another organ with constant exposure to chemicals, consists of a corpuscle linked to polarized tubular segments interlaced with the renal vasculature that transforms the plasma filtrate into urine. Reproducing polarity is essential for the vectorial transport of ions and uids that is the basis for the regulation of re-absorptive and secretory renal functions. The tight junctions responsible for polarity display different patterns depending on the tubular segment, notably in terms of composition of proteins and lipids within the plasma membrane domains on each side of the tight junction barrier (Wilson, 2011). Polarity was inferred in a model of proximal tubules made from hTERT-immortalized human renal cortical cells using a cell culture insert system with a mixture of EHS and collagen I matrix; (in a cell culture insert system, cells are in contact with medium underneath as well as above the culture surface). These cells formed interconnected tubular structures within two weeks and expressed functions resembling those in vivo, including the expression of transport proteins compared to 2D culture (DesRochers et al., 2013). Importantly, this simple model showed sustained differentiation allowing drug toxicity testing over time. Given the emerging grasp of the importance of mechanics in polarity (Asnacios and Hamant, 2012) and the presence of uid-induced shear forces in renal tubules, on-a-chip systems designed to study shear stress by seeding tubular cells in micro uidic channels ought to take polarity into account (Jang et al., 2013; DesRochers et al., 2014). Indeed, fluid shear stress was proposed to improve polarization (notably via tight junction protein assessment) of primary human kidney cells cultured on basement membrane component collagen IV compared to the static cell culture insert system (Jang et al., 2013).
Skin exposure to chemicals from the environment and cosmetic applications, makes this organ a prime target for 3D cell culture. The skin is comprised of keratinocytes sitting on top of dermis layers containing a mixture of fibroblasts, Langerhans cells, Merkel cells, endothelial cells, among other cell types. In this organ the polarity axis is established along several layers of keratinocytes, with the apical boundary made of the stratum granulosum that expresses tight junctions. The remainder of the polarity axis relies on the differential expression and/or localization of polarity markers, including adhesive junctions (Muroyama and Lechler, 2012). Proper skin function is therefore dependent on a hierarchical cell population with a complex organization of polarity. Several in vitro 3D reconstructed human skin models consisting of human keratinocytes, sometime cocultured with human fibroblasts, within an ECM are currently available. One of the early models displayed the polarized multilayered differentiation of keratinocytes thanks to a series of differentiating culture media and to air exposure, i.e. the top of the culture is exposed to air and the bottom portion is resting on a collagen I-based gel in contact with the medium (Gangatirkar et al., 2007). It has also been shown that the production of a mature ECM by fibroblasts in the dermis is essential for sustained differentiation of the epithelium (Stark et al., 2004). However, establishing polarity is not enough for applications in toxicology that require absorption studies; here, including mixed populations of stromal cells seems important to recreate the strong barrier function of the skin (Mathes et al., 2014).
The airways are primary sites for air pollution and treatments with inhalants. They are lined with a layer of polarized epithelial cells, forming the air-liquid interface between the gaseous and the interstitial compartments. Polarity is essential for vectorial transport; it appears important for maintenance of a transepithelial pH gradient, notably via the degree of impermeability of the apical cell membrane to acid/base equivalents (Joseph et al., 2002). The cellular composition and function of the epithelium differ depending on the localization along the airways, such as trachea, bronchi, bronchioles and alveoli, which creates a challenge for pulmonary toxicology. Moreover, pulmonary toxicity involves different cell types such as alveolar epithelial type I and type II cells, fibroblasts, endothelial cells and other cells specific of the anatomical portion of the airway system. Polarity as indicated by the location of tight junction proteins has been described in models established in cell culture insert systems and microfluidic platforms in the presence of hydrogels with human and animal cells that reproduce different parts of the respiratory system (Nichols et al., 2014; O’Leary et al. 2016). The study of the basement membrane and ECM in general is of particular interest since composition and stiffness vary greatly depending on the region of the respiratory system. For instance, type 1 collagen and hyaluronate were used as bilayered scaffold to enable the mimicry of a tracheobronchial architecture (O’Leary et al., 2016). Microengineered lung-on-chip models have been developed for pharmacological, clinical and toxicological applications (Huh et al., 2010; Huh et al., 2013). These models include physiological flow and cyclic suction to mimic breathing movements in a system containing alveolar and capillary cells thus, adding an essential mechanical aspect of lung function. Indeed, mechanical strain enhances the response to silica nanoparticles and their cellular uptake. However, extended toxicology investigation will require the design of models with lung alveoli interconnecting with bronchial airways and blood vessels in order to reproduce the dynamic in vivo setting in the human lungs (Nichols et al., 2014).
Another critical development of 3D models in toxicology has been the gastrointestinal tract, as it is the site for enterohepatic recycling of drugs and their metabolites, as well as the target of deleterious effects from toxicants. Each segment in the gastrointestinal tract, e.g. small intestine, large intestine, has its specific cellular composition and functions. In the in vitro models established with human colon adenocarcinoma cells Caco-2 polarity was reproduced using cell culture inserts, as shown by transepithelial electrical resistance and tight junction markers (Hidalgo et al., 1989; Ranaldi et al., 1992; Rothen-Rutishauser et al., 2000). Polarity is only one step in building this model. More recently on-a-chip systems have been designed allowing fluid perfusion and cyclic air suction to exert mechanical stretch on the epithelium, with shear forces on the apical surface, and the possibility of mimicking peristaltic contractions. Mechanical stimulus led to morphogenesis of intestinal villi with tight junction marker, brush borders and mucus deposition (Kim and Ingber, 2013). The complexity of the environment, with multiple cell types, lumen characteristics (e.g. pH) and mechanical peristaltic movement are challenging, but worthwhile features to integrate in 3D culture models for toxicology.
The brain and the heart are vital organs that are targets of systemic toxicity and for which 3D cell culture models are beginning to emerge although serious challenges in mimicking these organs’ structure and function need to be overcome. Polarity is an important feature of the organization and function of the brain that is present in two forms, basoapical polarity of the glial cells and the dendrite-axon polarity of the neurons. Difficulties in apprehending the best culture conditions include physiological and structural differences between the brain of mammals and other vertebrates with which most studies have been performed. Organoids in a dish have been developed to study developmental stages in the context of neurological disorders (Lancaster et al., 2013; Lancaster and Knoblich 2014). The main challenge to the existing organoid models for planar cell polarityneuronal and glial cells is that they do not organize as in vivo (Kelava and Lancaster 2016). Improvements in technical design are necessary to reproduce cell-cell interaction (for neural cells and glial cells) and cell-ECM interaction (neural cells and glial cells with proteoglycans, tenascins, laminin, collagen, etc.) necessary for polarity.
Microengineered biological models that mimic valvular and vascular microenvironment features of the heart have been created to have an insight into the physiology of cardiomyocytes in the context of drug development (Ribas et al., 2016). Proper differentiation of cardiomyocytes is critical for normal cardiac development and function, and this process is dependent at least on planar cell polarity (i.e., coordinated polarization of cells across the tissue plane) and mechanical stimulus of cardiomyocytes (Wu et al., 2011; Hirt et al., 2014). Adult cardiomyocytes appear terminally differentiated, which is an obstacle for the use of primary human cardiomyocytes for in vitro modeling. Human induced pluripotent stem cell (iPSC)-derived cardiomyocytes and other cardiac cell types are currently included in 3D culture systems to replicate physiological conditions for a specific region of the heart. In these systems, the cells reside within or on the surface of hydrogels (e.g. fibrin) or prefabricated scaffolds (e.g., polyurethane). Cells might also be cultured scaffold-free, in a cast-model or as stacked sheets (Zuppinger 2016). Critically, microfluidic 3D models with mimicry of pulsation are necessary to mimic heart functions. As an example, to provide a screening platform for cardiotoxins, human iPSC-derived cardiomyocytes were embedded in a fibrin gel on a microfluidic system, including pneumatic actuation for the production of uniaxial cyclic strains (Marsano et al., 2016). Organs can be altered during development; therefore, it is important to be able to reproduce these initial alterations in order to include function-impaired tissues in toxicity studies. Polarity dynamics needs further understanding to study the formation of congenital heart malformation, but cardiac contractility and neuregulin signaling have recently been shown to be required for cardiomyocyte apical constriction and depolarization during the developmental process of trabeculation (i.e., a process involving delamination and proliferation of cardiomyocytes necessary for the formation of the ventricular wall) (Jiménez-Amilburu et al., 2016).
In the organ-on-a-chip models described above, microfluidics is often used to mimic delivery of nutrients and gases. However, it might be necessary for the study of certain functions or toxins (Prozialeck et al., 2008; Woods et al., 2008) to recreate the vasculature alone or along with other tissues. Angiogenesis is guided by polarity of the endothelial cells (Lee and Bautch 2011), and vessel formation is regulated by the presence of angiogenetic growth factors, the ECM and mechanical shear force (McCure et al., 2006; Herbert and Stainier 2011). Polarity exists at several levels during the development of vessels including apical-basolateral polarity, planar cell polarity and migratory polarity. Several approaches have been developed for engineered tissue vascularization, for instance by embodiment of angiogenesis growth factors in polymeric scaffolds or coculture of endothelial cells with angiogenesis signaling cells (Kaully et al., 2009).
For all the portions of organs discussed above, the ECM plays a major role in promoting tissue polarity. Some cell types might make a complete basement membrane or other specific ECM once in culture (e.g., when combining myoepithelial cells and epithelial cells to make a basement membrane or combination of fibroblasts and myocardial cells), but it is also often necessary to include a naturally-derived inducer molecule of polarity and differentiation. This inducer depends on the tissue type. For instance, fibronectin needs to be present to induce polarity in hepatocytes and possibly in myocardial cells, and laminin is important to induce polarity in epithelial, endothelial and glial cells. Mixtures of these molecules [e.g., laminin and collagen IV for epithelial cells] might also be envisioned depending on the scaffold or cell type used (e.g., regular cell line or primary cells vs. iPSC). Subtypes of laminin might be different as a main basement membrane element depending on the tissue type and may be commercially available for cell culture (Pajęcka et al. 2017). The use of a coat of natural ECM molecules or inclusion of ECM molecules within synthetic hydrogels depends on the tissue to be reproduced and the chosen culture conditions. The ECM aspect of cell culture alone would deserve a separate article. Examples of processes used to include ECM molecules in complex cell culture systems are given in a recent review (Hinderer et al., 2016).
3. Importance of tissue architecture for carcinogenesis
Studying carcinogenic effects would logically require starting from phenotypically normal tissues and using long-term cultures. Theoretically, any model of normal tissue differentiation could serve the assessment of potential carcinogens, the agents that initiate cancers. The International Agency for Research on Cancer (IARC) had classified nearly 1000 compounds, both natural and synthetic, as possible, probable or known carcinogens based on in vitro, in vivo and epidemiological studies (Thun et al., 2003). Classification relying on toxicity assays in two-dimensional (2D) cell culture has been shown to underestimate the carcinogenic potential of several compounds (Stevens et al., 2009). One of the reasons for the weakness of the classification model is the lack of reproduction of the extracellular microenvironment that normally modulates cellular functions. Earlier demonstration that a matrix from cancer can lead to carcinogenesis (Bergers et al., 2000; Bourboulia et al., 2010; Buchheit et al., 2012) is a testament to the importance of the extracellular milieu for the control of cancer onset. Already in 1976, experiments with hepatocytes in floating collagen cultures had revealed that under these conditions the cells retain functions (e.g., increase in cytochromes) responsible for the activation of carcinogenic substances (Michalopoulos et al, 1976b). Currently, essential biochemical and biophysical features of the extracellular milieu such as oxidative stress and matrix stiffness are known contributors to carcinogenesis by directly (or indirectly via stromal cells) influencing the homeostasis of the organ. These extracellular features ought to be integrated in the in vitro models to study carcinogenesis.
Dietary nutrients, metabolites of alcohol and cigarette smoke, heavy metals and other toxic substances through drinking water or as an occupational hazard play a role in carcinogenesis (Lutz and Fekete, 1996). Cellular responses to environmental factors are a result of cell-stromal interaction, and toxicity testing requires a model that can capture this feature efficiently to determine the permissible exposure levels (PELs), formerly termed the threshold limit values, for any compound of interest that is to be classified as a carcinogen. Specifically, the model needs to take into account local microenvironmental features that limit, concentrate or guide the distribution of nutrients, oxygen and other molecules such as reactive oxygen species that influence cells. There is evidence that 3D culture models with non-neoplastic cells can be used to study cancer initiation, as it was shown via the activation of certain signaling pathways in the breast (Muthuswamy et al., 2001), but such models have not been traditionally focused on carcinogens. In these models, evidence of cancer onset was brought by the formation of tumors with alteration of epithelial polarity and cell proliferation. Using physiologically relevant 3D cell culture of non-neoplastic cells is conceptually superior to current carcinogenesis tests consisting of colony formation on agarose from cells usually transformed following genetic damage. Indeed the ECM and the architecture of tissues can tame cells with genetic anomalies, demonstrating the overriding impact of the microenvironment on disease onset (Weaver et al., 1997).
Here we give an example of how PELs might be better studied in 3D cell culture models that integrate the microenvironment. Metabolites of numerous organic and inorganic toxic compounds, reactive oxygen species are important components of the tissue microenvironment that play a role in cancer initiation (Waris et al., 2006), via the accumulation of oxidative stress inside cells (Klaunig et al., 2004). In addition to the direct oxidization of cellular components in epithelial or liver cells prone to cancer, stromal fibroblasts under oxidative stress become activated, which leads to the release of paracrine factors and an alteration of stromal composition with matrix stiffening (Jezierska-Drutel et al., 2013) conducive to cancer onset potentially in any organ. Hence, testing tools that include a metabolic product of exposure to cancer risk factors such as reactive oxygen species in the presence of a stroma might be ideal for PELs determination. In earlier studies aimed at testing drug effects on osteogenic differentiation (Jang et al., 2008), the authors pointed out that one of the limitations in their microfluidic system was the lack of possibility to monitor concentration-dependent changes in cellular activity and consequently, a concentration gradient needed to be created in the microchannels. Indeed, a gradient-capable microfluidic system would enable to identify threshold concentrations of toxins or drugs, depending on set microenvironmental conditions (e.g., matrix stiffness or shear forces), leading to phenotypic, genotypic or metabolic switches that can be monitored in cell culture (Figure 2). Microfluidic devices have been created for oxygen gradient production using spatially confined chemical reactions and successfully used in cell culture with drug treatment (Chen et al., 2011).
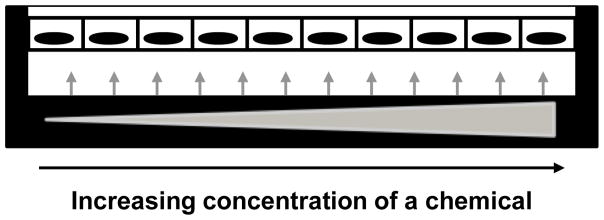
Figure 2. Applying a chemical gradient in cell culture.
A cell culture specimen receives a chemical at increasing concentrations (as displayed by the widening triangle and the arrows) depending on the location in the culture area. Here cells of an epithelium are represented by rectangles with nuclei drawn in black, in the culture platform. With such system, it is possible to create controlled heterogeneity of the presence of the chemical throughout the culture surface. It is also possible to identify concentration thresholds for the impact of the chemical on cells depending on a given variation in cell culture parameters.
There are several advantages in using microfluidics in cell culture, including microscale consumption of reagents, individual cell capture, medium control and compartmentalization of cells. Mimicking the action of capillaries in microchannels and the use of components such as polydimethyl siloxane (PDMS) that are permeable to diffusion of oxygen are essential for cell survival in long-term 3D cell cultures necessary in toxicology studies. Microfluidic platforms integrate a plethora of other substrates to allow flexibility in use such as glass, paper, natural ECM compounds and agarose (Xia et al., 1998; Jang et al., 2008; Derda et al., 2009; Webster et al., 2011). Importantly, synthetic and natural substrates provide different stiffness degrees that contribute to determining the response to microenvironmental stimuli, notably via an impact on the epigenome (Qin et al., 2010; Johnstone et al., 2010; Du et al., 2016).
The design of organs-on-a-chip involves other important aspects beyond microfluidics; each aspect of the design ought to be carefully chosen to best mimic the necessary architecture and function of tissues and organs. For instance microfabrication might be used to create culture surfaces of certain shapes. We showed that a curved geometry of microchannels in which tumors are cultured and that mimics ductal geometry in the breast had an influence on drug sensitivity (Vidi et al., 2014). This extended view of tissue architecture considers that the higher order organization of cells or the geometrical constraints for tissue expansion also influence phenotypes. This concept has been illustrated by the fact that a curved surface vs. a flat surface of culture has an impact on the morphology of cell nuclei within tumor nodules (Vidi et al., 2014). Novel microengineering and 3D bioprinting methods are being developed to design organs-on-a-chip that integrate the culture of different cell types on a single platform and assess the response of the target cell type under external stimuli, in a manner consistent with a body-on-a chip (Lee and Cho 2016). Systems are being built for easy reuse; such is the case for mimicry of the brain vasculature with PDMS-fabricated microchannels to build endothelialized microvasculature on-a-chip (Pitingolo et al., 2017). There are increasing examples of on-chip microfluidic models that reproduce mechanical features of the organs of interest. One of the latest reported devices mimics blood pressure and heart beat as in vivo. The device consists of four chambers as in the heart and an adjustable pressure based on a pumping system that can monitor changes in pressure over time (Chen et al., 2017).
4. Tissue architecture-cell nucleus relationship to control the response to external stimuli
In routine screenings for environmental risk factors, measured parameters have often included, but were not limited to chromosomal aberrations, cell proliferation and cell death (e.g., lymphocyte proliferation and death for effects on the immune systems) (Eastmond et al., 2009; Parasuraman 2011). However, these alterations correspond to late or acute effects of exposure to toxic molecules. The cell nucleus, a highly structured organelle linked to tissue architecture (Lelièvre, 2010), provides a source of recognizable alterations corresponding to early responses to toxins, notably via changes in the nucleus shape and size that can influence chromosomal arrangement and gene expression (Stein et al., 2000; Versaevel et al., 2012). Most cell behaviors are under tight control by the cell nucleus that houses the epigenome. Sensitivity to external stimuli and long-term impact of toxicants may lead to sustained gene modifications often with an obvious impact on nuclear organization. For instance, induction of oxidative stress upon metabolism of toxic compounds like heavy metals and ethanol is accompanied with altered nucleus morphology (Coluzzi et al., 2014).
The importance of changes in nuclear organization linked to cell behavior in 3D cell culture, as visualized by the redistribution of nuclear proteins, was demonstrated almost two decades ago (Lelièvre et al., 1998). The nuclear structural protein NuMA was shown to drastically change its distribution depending on the phenotype of breast epithelial cells (e.g., quiescent, proliferating, cancerous) (Lelièvre, et al., 1998; Knowles et al., 2006). An essential step to illustrate the role of protein location on cell phenotype was to demonstrate that forcing the relocation of the protein, instead of changing its expression level, was sufficient to alter the differentiation stage of cells (Lelièvre et al., 1998). Structural proteins such as CTCF, NuMA and lamins organize chromatin (Abad et al., 2007; Dechat et al., 2008; Kim et al., 2015), and by doing so they might redistribute when the phenotype of cells changes. Proteins like NuMA and splicing factor speckle SC35 have been used with high-content analysis of nuclear morphometric descriptors to identify changes in differentiation stages, notably of stem cells, before standard markers are modified (Liu et al., 2010; Vega et al. 2015; Vega et al., 2017. More simply, changes in nuclear morphology such as shape (circularity) and size (area) might be sufficient to indicate a drastic impact of the microenvironment on cell behavior. The expression level of master regulator of cell death and proliferation, p53, was found to correlate positively with nuclear circularity (Mijovic et al., 2013). We also showed that nuclear circularity correlated with different levels of drug sensitivity in cancer cells (Vidi et al., 2014; unpublished data Lelièvre laboratory). Logically, since cell phenotypes, and particularly differentiation stages, are best mimicked in 3D culture, the distribution of nuclear proteins and nuclear morphology is dramatically different when comparing physiologically relevant 3D culture and 2D culture (Lelièvre et al., 1998; Le Beyec et al., 2007; Lelièvre, 2009; Vidi et al., 2014). The plasticity of nuclear structure can be in part linked to the relationship between tissue architecture and the cell nucleus (Chandramouli et al., 2007; Lelièvre, 2010; Bray et al., 2010; Versaevel et al., 2012). For instance, we have shown that the distribution of the chromatin organizer NuMA is sensitive to the type of ECM connection, and controls the cellular response to DNA damage in the presence of functional basal polarity inducer β4-integrin (Vidi et al., 2012). Other structural proteins such as nuclear lamins and dystroglycans have been shown to be responsive to mechanochemical changes in the ECM (Swift et al., 2014; Varshney et al., 2015; Maya-Mendoza et al., 2016).
We propose that markers of nuclear organization combined with appropriate 3D cell culture models will bring a higher level of analysis for toxicology in vitro. Indeed, carcinogenesis involves drastic modifications in gene transcription and nuclear morphology (Cox et al., 1991; Zink et al., 2004). The effect of toxicants might lead to more subtle stress reaction (e.g. nucleolar stress) for which a typical redistribution of nucleolar proteins such as C23, fibrillarin or polymerase I can be recognized (James et al., 2014), as well as changes in gene transcription that could lead to the development of chronic diseases. As an example of gene expression impact, long noncoding RNAs have become of high interest due to their dysregulation associated with cancers, and Alzheimer’s, cardiovascular and autoimmune diseases, as well as to the influence of exposures to chemicals and xenobiotics on their regulation (Dempsey and Cui, 2016). Similar observations of perturbed control of microRNAs and gene-specific CpG methylation associated with the response to environmental contaminants or toxicants and increased disease risk have been made (Koturbash et al., 2012; Fry et al., 2014; Sollome et al., 2016; Martin and Fry, 2016). An impact on the integrity of DNA can be measured by the response to DNA damage with redistribution or foci-like appearance of proteins such as 53BP1 or γH2AX, as it was shown in toxicity tests of dental composite components on gingival fibroblasts (Styllou et al., 2015). As mentioned earlier, the response to DNA damage is greatly dependent on the presence of basal polarity (Vidi et al., 2012) and thus, like for epigenetic regulators, 3D cell culture that reproduces polarity is best suited to readily measure a response to exposure that involves DNA damage. In addition, an increasing number of studies are mentioning altered nuclear morphology upon toxicology tests (Swarnkar et al., 2012; Styllou et al., 2015). The consequences of changes in nuclear morphology on cell phenotype are still poorly understood, but emerging directions of investigation include an impact on higher order chromatin organization and thus, gene transcription. For instance, the testis, an organ for which 3D cell culture models from rat testicular cells are being developed (Lee et al, 2006; Harris et al., 2016), includes cells characterized by specific nuclear shapes under PARP11 control. A means to investigate chemical exposure impact on fertilization might be via measuring spermatozoid head shape, as a change in shape can be a mark of chromatin detachment (Meyer-Ficca et al., 2015).
Overall, most toxicological approaches make use of nuclear events (e.g., apoptosis, chromatin alterations, changes in nuclear morphology) that, themselves, depend on the proper organization of tissues in 3D cell culture, with adhesion and polarity as primary characteristics for proper organization when investigating noncancerous situations. Moreover, fluidic and other mechanical constraints in some of the organs of interest might also have an impact on nuclear mechanics that, in turn, modulates cell function (Lammerding 2012) and thus, cellular responses to toxicants and other detrimental exposures.
5. Conclusion
Models with cells in 3D culture are available for most of the major organs investigated in toxicology, and efforts are being made to step away from nonphysiologically relevant spheroids when appropriate and reproduce tissue architecture. Carcinogenesis is a research field where progress is lacking with 3D cell cultures because the means of evaluation greatly remain linked to colony formation upon DNA damage induction, although it is shown that events other than a direct impact on DNA and associated with alterations in tissue architecture are determinant. The urgency to understand the normal behavior of cells to improve cancer research has led to the development of 3D cell culture models of phenotypically normal differentiation for most organs. These models represent great resources for the study of carcinogenesis and for establishing PELs. Moreover, the advent of organs-on-a-chip making use of microfluidics brings novel directions to study chemical and gaseous exposures in a gradient-based manner. The concept of gradient in 3D cell culture is particularly important since the ECM and blood vessels contribute to creating heterogeneity in the spreading of chemicals and gaseous molecules (Watt et al., 2011; Shin et al., 2013).
The next frontier in 3D cell culture for toxicology in vitro will be to produce models that target specific populations based on gender, ethnicity, race and the environment at large. In contrast to patient-derived xenografts for which tumors or primary tumor cells from patients are implanted in mice, precision toxicology dealing with noncancerous tissues will likely only rely on 3D cell cultures. Several of the 3D cell culture models discussed in this review already made use of primary cells instead of transformed cell lines. But primary cells were often of animal origin. The path to a bright future with 3D cell culture models for human toxicology might be in the use of iPSC, enabling the reproduction of all cell types in the model and the use of genotypes that influence the response to toxicants. Another important aspect of toxicology in vitro will be the mimicry of extracellular conditions that might modify the impact or access of chemicals and gaseous molecules to the cells. Here organs-on-a-chip and well-defined matrices with tunable stiffness are likely to become a standard to deliver, for instance, combinations of chemicals, in desired quantity, sequence and intervals, as gradients, with embedded sensors, hence permitting the modulation of metabolic and architectural tissue characteristics that are important for carcinogenesis and the onset of other disorders (Vidi et al., 2013; Robey et al., 2016).
Highlights.
Polarity is an architectural feature necessary for normal function of most organs.
Methods to reproduce polarity are tissue specific and rely on cell-ECM interactions.
The cell nucleus-tissue polarity relationship controls tissue homeostasis.
The cell nucleus can serve as main and early readout for toxicology assessment.
Microfluidics-based chemical gradients ease permissible exposure level evaluation.
Acknowledgments
The authors acknowledge the support from the Walter Cancer Institute FDN, Inc. and the Purdue University Center for Cancer Research, NIH award (P30 CA023168). These sources of funding had no involvement regarding decisions to write and submit this manuscript for publication.
Abbreviations
- 2D
two-dimensional
- 3D
three-dimensional
- ECM
extracellular matrix
- EHS
Engelbreth-Holm-Swarm
- iPSC
induced-pluritpotent stem cell
- PDMS
polydimethyl siloxane
- PEL
permissible exposure level
Footnotes
Declaration of interest
SAL reports potential conflicts of interest. There is no conflict of interest for SC and TK.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abad PC, Mian IS, Plachot C, Nelpurackal A, Bator-Kelly C, Lelièvre SA. The C terminus of the nuclear protein NuMA: Phylogenetic distribution and structure. Protein Sci. 2004;13:2573–2577. doi: 10.1110/ps.04906804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asnacios A, Hamant O. The mechanics based cell polarity. Trends Cell Biol. 2012;22:584–591. doi: 10.1016/j.tcb.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–35. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 6.Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin Cancer Biol. 2010;20:161–168. doi: 10.1016/j.semcancer.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bray M-A, Adams WJ, Geisse NA, Feinberg AW, Sheehy SP, Parker KK. Nuclear Morphology and Deformation in Engineered Cardiac Myocytes and Tissues. Biomaterials. 2010;31:5143–5150. doi: 10.1016/j.biomaterials.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burkhardt B, Martinez-Sanchez JJ, Bachmann A, Ladurner R, Nüssler AK. Long-term culture of primary hepatocytes: new matrices and microfluidic devices. Hepatol Int. 2014;8:14–22. doi: 10.1007/s12072-013-9487-3. [DOI] [PubMed] [Google Scholar]
- 9.Buchheit CL, Rayavarapu RR, Schafer ZT. The regulation of cancer cell death and metabolism by extracellular matrix attachment. Semin Cell Dev Biol. 2012;23:402–411. doi: 10.1016/j.semcdb.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Chandramouly G, Abad PC, Knowles DW, Lelièvre SA. The control of tissue architecture over nuclear organization is crucial for epithelial cell fate. J Cell Sci. 2007;120:1596–1606. doi: 10.1242/jcs.03439. [DOI] [PubMed] [Google Scholar]
- 11.Chen YA, King AD, Shih HC, Peng CC, Wu CY, Liao WH, Tung YC. Generation of oxygen gradients in microfluidic devices for cell culture using spatially confined chemical reactions. Lab Chip. 2011;11:3626–3633. doi: 10.1039/c1lc20325h. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Chan HN, Michael SA, Shen Y, Chen Y, Tian Q, Huang L, Wu H. A microfluidic circulatory system integrated with capillary-assisted pressure sensors. Lab Chip. 2017;17:653–662. doi: 10.1039/c6lc01427e. [DOI] [PubMed] [Google Scholar]
- 13.Choi YY, Kim J, Lee SH, Kim DS. Lab on a chip-based hepatic sinusoidal system simulator for optimal primary hepatocyte culture. Biomed Microdevices. 2016;18:58. doi: 10.1007/s10544-016-0079-6. [DOI] [PubMed] [Google Scholar]
- 14.Coluzzi E, Colamartino M, Cozzi R, Leone S, Meneghini C, O’Callaghan N, Sgura A. Oxidative stress induces persistent telomeric DNA damage responsible for nuclear morphology change in mammalian cells. PloS ONE. 2014;9:e110963. doi: 10.1371/journal.pone.0110963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox PM. Transcription and cancer. Br J Cancer. 1991;63:651–662. doi: 10.1038/bjc.1991.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, Goldman RD. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 2008;22:832–853. doi: 10.1101/gad.1652708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dempsey JL, Cui JY. Long non-coding RNAs: A novel paradigm for toxicology. Toxicol Sci. 2016 doi: 10.1093/toxsci/kfw203. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derda R, Laromaine A, Mammoto A. Paper-supported 3D cell culture for tissue-based bioassays. Proc Natl Acad Sci U S A. 2009;106:18457–18462. doi: 10.1073/pnas.0910666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DesRochers TM, Suter L, Roth A, Kaplan DL. Bioengineered 3D Human Kidney Tissue, a Platform for the Determination of Nephrotoxicity. PLoS ONE. 2013;8:e59219. doi: 10.1371/journal.pone.0059219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DesRochers TM, Palma E, Kaplan DL. Tissue-engineered kidney disease models. Advanced Drug Deliv Rev. 2014;69–70:67–80. doi: 10.1016/j.addr.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dianat N, Dubois-Pot-Schneider H, Steichen C, Desterke C, Leclerc P, Raveux A, Combettes L, Weber A, Corlu A, Dubart-Kuppershmitt A. Generation of functional cholangiocyte-like cells from human pluripotent stem cells and HepaRG cells. Hepatology. 2014;60:700–714. doi: 10.1002/hep.27165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du J, Zu Y, Li J, Du S, Xu Y, Zhang L, Jiang L, Wang Z, Chien S, Yang C. Extracellular matrix stiffness dictates Wnt expression through integrin pathway. Sci Rep. 2016;6:20395. doi: 10.1038/srep20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eastmond DA, Hartwig A, Anderson D, Anwarb WA, Cimino MC, Dobrev I, Douglas GR, Nohmi T, Phillips DH, Vicker C. Mutagenicity testing for chemical risk assessment: update of the WHO/IPCS harmonized scheme. Mutagenesis. 2009;24:341–349. doi: 10.1093/mutage/gep014. [DOI] [PubMed] [Google Scholar]
- 24.Emerman JT, Pitelka DR. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In vitro. 1977;13:316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- 25.Fry RC, Rager JE, Bauer R, Sebastian E, Peden DB, Jaspers I, Alexis NE. Air toxics and epigenetic effects: ozone altered microRNAs in the sputum of human subjects. Am J Physiol Lung Cell Mol Physiol. 2014;306:L1129–L1137. doi: 10.1152/ajplung.00348.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gangatirkar P, Paquet-Fifield S, Li A, Rossi R, Kaur P. Establishment of 3D organotypic cultures using human neonatal epidermal cells. Nat Protoc. 2007;2:178–186. doi: 10.1038/nprot.2006.448. [DOI] [PubMed] [Google Scholar]
- 27.Grant DS, Kleinman HK, Leblond CP, Inoue S, Chung AE, Martin GR. The basement-membrane-like matrix of the mouse EHS tumor: II. Immunohistochemical quantitation of six of its components. Am J Anat. 1985;174:387–398. doi: 10.1002/aja.1001740403. [DOI] [PubMed] [Google Scholar]
- 28.Harris S, Shubin SP, Wegner S, Van Ness K, Green F, Hong SW, Faustman EM. The presence of macrophages and inflammatory responses in an in vitro testicular co-culture model of male reproductive development enhance relevance to in vivo conditions. Toxicol In Vitro. 2016;36:210–215. doi: 10.1016/j.tiv.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hay ED. Extracellular matrix. J Cell Biol. 1981;91:205–223. doi: 10.1083/jcb.91.3.205s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herbert SP, Stainier YR. Molecular control of endothelial cell behavior during blood vessel miorphogenesis. Nat Rev Mol Cell Biol. 2011;23:551–564. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96:736–749. [PubMed] [Google Scholar]
- 32.Hinderer S, Layland SL, Schenke-Layland K. ECM and ECM-like materials –Biomaterials for applications in regenerative medicine and cancer therapy. Adv Drug Deliv Rev. 2016;97:260–269. doi: 10.1016/j.addr.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 33.Hirai Y, Lochter A, Galosy S, Koshida S, Niwa S, Bissell MJ. Epimorphin functions as a key morphoregulator for mammary epithelial cells. J Cell Biol. 1998;140:159–169. doi: 10.1083/jcb.140.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirt MN, Boeddinghaus J, Mitchell A, Schaaf S, Börnchen C, Müller C, Schulz H, Hubner N, Stenzig J, Stoehr A, Neuber C, Eder A, Luther PK, Hansen A, Eschenhagen T. Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation. J Mol Cell Cardiol. 2014;74:151–161. doi: 10.1016/j.yjmcc.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 35.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huh D, Kim HJ, Fraser JP, Shea DE, Khan M, Bahinski A, Hamilton GA, Ingber DE. Microfabrication of human organs-on-chips. Nat Prot. 2013;8:2135–2157. doi: 10.1038/nprot.2013.137. [DOI] [PubMed] [Google Scholar]
- 37.Inman JL, Bissell MJ. Apical polarity in three-dimensional cell culture systems: where to now? J Biol. 2010;9:2. doi: 10.1186/jbiol213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.James A, Wang Y, Raje H, Rosby R, DiMario P. Nucleolar stress with and without p53. Nucleus. 2014;5:402–426. doi: 10.4161/nucl.32235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jang K, Sato K, Igawa K, Chung UI, Kitamori T. Development of an osteoblast-based 3D continuous-perfusion microfluidic system for drug screening. Anal Bioanal Chem. 2008;390:825–832. doi: 10.1007/s00216-007-1752-7. [DOI] [PubMed] [Google Scholar]
- 40.Jang KJ, Mehr AP, Hamilton GA, McPartlin LA, Chung S, Suh KY, Ingber DE. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr Biol. 2013;5:1119–1129. doi: 10.1039/c3ib40049b. [DOI] [PubMed] [Google Scholar]
- 41.Jezierska-Drutel A, Rosenzweig SA, Neumann CA. Role of oxidative stress and the microenvironment in breast cancer development and progression. Advances in Cancer Research. 2013;119:107–125. doi: 10.1016/B978-0-12-407190-2.00003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiménez-Amilburu V, Rasouli SJ, Staudt DW, Nakajima H, Chiba A, Mochizuki N, Stainier DY. In Vivo Visualization of Cardiomyocyte Apicobasal Polarity Reveals Epithelial to Mesenchymal-like Transition during Cardiac Trabeculation. Cell Rep. 2016;17:2687–2699. doi: 10.1016/j.celrep.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 43.Johnstone SE, Baylin SB. Stress and the epigenetic landscape: a link to the pathobiology of human diseases? Nat Rev Genet. 2010;11:806–812. doi: 10.1038/nrg2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joseph D, Tirmizi O, Zhang X, Crandall ED, Lubman RL. Polarity of alveolar epithelial cell acid-base permeability. Am J Physiol Lung Cell Mol Physiol. 2002;282:L675–683. doi: 10.1152/ajplung.00330.2001. [DOI] [PubMed] [Google Scholar]
- 45.Katt ME, Placone AL, Wong AD, Xu ZS, Searson PC. In vitro tumor models: advantages, disadvantages, variables and selecting the right platform. Front Bioeng Biotechnol. 2016;4:12. doi: 10.3389/fbioe.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaully T, Kaufman-Francis K, Lesman A, Levenberg S. Vacularization: The conduit to viable engineered tissues. Adv Tissue Eng: Angio Tissue Eng PartB Rev. 15:159–169. doi: 10.1089/ten.teb.2008.0193. [DOI] [PubMed] [Google Scholar]
- 47.Kelava I, Lancaster MA. Stem cell models of human brain development. Cell Stem Cell. 2016;18:736–748. doi: 10.1016/j.stem.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 48.Kim HJ, Ingber DE. Gut-on-a-Chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr Biol. 2013;5:1130–40. doi: 10.1039/c3ib40126j. [DOI] [PubMed] [Google Scholar]
- 49.Kim K, Utoh R, Ohashi K, Kikuchi T, Okano T. Fabrication of functional 3D hepatic tissues with polarized hepatocytes by stacking endothelial cell sheets in vitro. J Tissue Eng Regen Med. 2015 doi: 10.1002/term.2102. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 50.Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 51.Knowles DW, Sudar D, Bator-Kelly C, Bissell MJ, Lelièvre SA. Automated local bright feature image analysis of nuclear protein distribution identifies changes in tissue phenotype. Proc Natl Acad Sci USA. 2006;103:4445–4450. doi: 10.1073/pnas.0509944102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koturbash I, Beland FA, Pogribney IP. Role of microRNAs in the regulation of drug metabolizing and transporting genes and the response to environmental toxicants. Expert Opin Drug Metab Toxicol. 2012;8:597–606. doi: 10.1517/17425255.2012.673587. [DOI] [PubMed] [Google Scholar]
- 53.Kulkarni K, Larsen P, Linninger AA. Assessing chronic liver toxicity based on relative gene expression data. J Theor Biol. 2008;254:308–318. doi: 10.1016/j.jtbi.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 54.Lammerding J. Mechanics of the Nucleus. Compr Physiol. 2011;1:783–807. doi: 10.1002/cphy.c100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345:1247125. doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- 56.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Le Beyec J, Xu R, Lee S-Y, Nelson CM, Rizki A, Alcaraz J, Bissell MJ. Cell shape regulates global histone acetylation in human mammary epithelial cells. Exp Cell Res. 2007;313:3066–3075. doi: 10.1016/j.yexcr.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee CY, Bautch VL. Ups and downs of guided vessel sprouting:the role of polarity. Physiology. 2011;26:326–333. doi: 10.1152/physiol.00018.2011. [DOI] [PubMed] [Google Scholar]
- 59.Lee H, Cho DW. One-step fabrication of an organ-on-a-chip with spatial heterogeneity using a 3D bioprinting technology. Lab Chip. 2016;16:2618–2625. doi: 10.1039/c6lc00450d. [DOI] [PubMed] [Google Scholar]
- 60.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, Beck LN, Brennan LV, Oktay K. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 61.Lelièvre SA. Contributions of extracellular matrix signaling and tissue architecture to nuclear mechanisms and spatial organization of gene expression control. Biochimica et Biophysica Acta. 2009;1790:925–935. doi: 10.1016/j.bbagen.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lelièvre SA. Tissue polarity-dependent control of mammary epithelial homeostasis and cancer development: an epigenetic perspective. J Mammary Gland Biol Neoplasia. 2010;15:49–63. doi: 10.1007/s10911-010-9168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lelièvre SA, Weaver VM, Nickerson JA, Larabell CA, Bhaumik A, Petersen OW, Bissell MJ. Tissue phenotype depends on reciprocal interactions between the extracellular matrix and the structural organization of the nucleus. Proc Natl Acad Sci. 1998;95:14711–14716. doi: 10.1073/pnas.95.25.14711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu E, Gordonov S, Treiser MD, Moghe PV. Parsing the early cytoskeletal and nuclear organizational cues that demarcate stem cell lineages. Cell Cycle. 2010;9:2108–2117. doi: 10.4161/cc.9.11.11864. [DOI] [PubMed] [Google Scholar]
- 65.Lutz WK, Fekete T. Endogenous and exogenous factors in carcinogenesis: limits to cancer prevention. Int Arch Occup Environ Health. 1996;68:120–125. doi: 10.1007/BF00381244. [DOI] [PubMed] [Google Scholar]
- 66.Marsano A, Conficconi C, Lemme M, Occhetta P, Gaudiello E, Votta E, Cerino G, Redaelli A, Rasponi M. Beating heart on a chip: a novel microfluidic platform to generate functional 3D cardiac microtissues. Lab Chip. 2016;16:599–610. doi: 10.1039/c5lc01356a. [DOI] [PubMed] [Google Scholar]
- 67.Martin EM, Fry RC. A cross-study analysis of prenatal exposures to environmental contaminants and the epigenome: support for stress-responsive transcription factor occupancy as a mediator of gene-specific CpG methylation patterning. Environ Epigenet. 2016;2:dvv011. doi: 10.1093/eep/dvv011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martinez-Hernandez A, Amenta PS. The hepatic extracellular matrix. II. Ontogenesis, regeneration and cirrhosis. Virchows Arch A Pathol Anat Histopathol. 1993;423:77–84. doi: 10.1007/BF01606580. [DOI] [PubMed] [Google Scholar]
- 69.Mathes SH, Ruffner H, Graf-Hausner U. The use of skin models in drug development. Adv Drug Deliv Rev. 2014;69–70:81–102. doi: 10.1016/j.addr.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 70.Maya-Mendoza A, Bartek J, Jackson DA, Streuli CH. Cellular microenvironment controls the nuclear architecture of breast epithelia through β1-integrin. Cell Cycle. 2016;15:345–356. doi: 10.1080/15384101.2015.1121354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCue S, Dainowiec D, Xu F, Zhang M, Jackson MR, Langille L. Shear stress regulates forward and reverse planar cell polarity of vascular endothelium in vivo and in vitro. Circ Res. 2006;98:939–946. doi: 10.1161/01.RES.0000216595.15868.55. [DOI] [PubMed] [Google Scholar]
- 72.Meyer-Ficca ML, Ihara M, Bader JJ, Leu NA, Beneke S, Meyer RG. Spermatid Head Elongation with Normal Nuclear Shaping Requires ADP-Ribosyltransferase PARP11 (ARTD11) in Mice. Biol Reprod. 2015;92:80. doi: 10.1095/biolreprod.114.123661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Michalopoulos G, Sattler CA, Sattler GL, Pitot HC. Cytochrome P-450 induction by phenobarbital and 3-methylcholanthrene in primary cultures of hepatocytes. Science. 1976;193:907–909. doi: 10.1126/science.948753. [DOI] [PubMed] [Google Scholar]
- 74.Michalopoulos G, Sattler G, Sattler C, Pitot HC. Interaction of chemical carcinogens and drug-metabolizing enzymes in primary cultures of hepatic cells from the rat. Am J Pathol. 1976b;85:755–72. [PMC free article] [PubMed] [Google Scholar]
- 75.Mijovic Z, Kostov M, Mihailovic D, Zivkovic N, Stojanovic M, Zdravkovic M. Correlation of nuclear morphometry of primary melanoma of the skin with clinicopathological parameters and expression of tumor suppressor proteins (p53 and p16(INK4a)) and bcl-2 oncoprotein. J BUON. 2013;18:471–6. [PubMed] [Google Scholar]
- 76.Muroyama A, Lechler T. Polarity and Stratification of the Epidermis. Seminars in Cell & Developmental Biology. 2012;23:890–896. doi: 10.1016/j.semcdb.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muthuswamy SK, Li D, Lelièvre S, Bissell MJ, Brugge JS. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol. 2001;3:785–792. doi: 10.1038/ncb0901-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nichols JE, Niles JA, Vega SP, Argueta LB, Eastaway A, Cortiella J. Modeling the lung: Design and development of tissue engineered macro- and micro-physiologic lung models for research use. Exp Biol Med. 2014;239:1135–1169. doi: 10.1177/1535370214536679. [DOI] [PubMed] [Google Scholar]
- 79.O’Leary C, Cavanagh B, Unger RE, Kirkpatrick CJ, O’Dea S, O’Brien FJ, Cryan SA. The development of a tissue-engineered tracheobronchial epithelial model using a bilayered collagen-hyaluronate scaffold. Biomaterials. 2016;85:111–127. doi: 10.1016/j.biomaterials.2016.01.065. [Epub 2016 Feb1] [DOI] [PubMed] [Google Scholar]
- 80.Pajęcka K, Nielsen MN, Hansen TK, Williams JM. The formation of quiescent glomerular endothelial cell monolayer in vitro is strongly dependent on the choice of extracellular matrix coating. Exp Cell Res. 2017 Feb 22; doi: 10.1016/j.yexcr.2017.02.039. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 81.Parasuraman S. Toxicological screening. J Pharmacol Pharmacother. 2011;2:74–79. doi: 10.4103/0976-500X.81895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pitingolo G, Vecchione R, Falanga AP, Guarnieri D, Netti PO. Fabrication of a modular hybrid chip to mimic endothelial lined microvessels in flow conditions. J Micromech Microeng. 2017;27:035014. [Google Scholar]
- 84.Prozialeck WC, Edwards JR, Nebert DW, Woods JM, Barchowsky A, Atchison WD. The vascular system as a target of metal toxicity. Toxicol Sci. 2008;102:207–218. doi: 10.1093/toxsci/kfm263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qin L, Han Y-P. Epigenetic Repression of Matrix Metalloproteinases in Myofibroblastic Hepatic Stellate Cells through Histone Deacetylases 4: Implication in tissue fibrosis. Am J Pathol. 2010;177:1915–1928. doi: 10.2353/ajpath.2010.100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ranaldi G, Islam K, Sambuy Y. Epithelial cells in culture as a model for the intestinal transport of antimicrobial agents. Antimicrobial Agents and Chemotherapy. 1992;36:1374–1381. doi: 10.1128/aac.36.7.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ribas J, Sadeghi H, Manbachi A, Leijten A, Brinegar K, Zhang YS, Ferriera L, Khademhosseini A. Cardiovascualr organ-on-a-chip platforms for drug discovery and development. App In vitro Toxicol. 2016;2:82–96. doi: 10.1089/aivt.2016.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Robey RB, Weisz J, Kuemmerle NB, Salzberg AC, Berg A, Brown DG, Kubik L, Palorini R, Al-Mulla F, Al-Temaimi R, Colacci A, Mondello C, Raju J, Woodrick J, Scovassi AI, Singh N, Vaccari M, Roy R, Forte S, Memeo L, Salem HK, Amedei A, Hamid RA, Williams GP, Lowe L, Meyer J, Martin FL, Bisson WH, Chiaradonna F, Ryan EP. Metabolic reprogramming and dysregulated metabolism: cause, consequence and/or enabler of environmental carcinogenesis? Carcinogenesis. 2015;36:S203–S231. doi: 10.1093/carcin/bgv037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rothen-Rutishauser B, Braun A, Günthert M, Wunderli-Allenspach H. Formation of Multilayers in the Caco-2 cell culture model; a Confocal Laser Scanning Microscopy Study. Pharm Res. 2000;17:460–465. doi: 10.1023/a:1007585105753. [DOI] [PubMed] [Google Scholar]
- 90.Shin Y, Kim H, Han S, Won J, Lee E-S, Kamm RD, Chung S. Extracellular matrix heterogeneity regulates three-dimensional morphologies of breast adenocarcinoma cell invasion. Advanced Healthcare Materials. 2013;2:790–794. doi: 10.1002/adhm.201200320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sollome J, Martin E, Sethupathy P, Fry RC. Environmental Contaminants and microRNA Regulation: Transcription Factors as Regulators of Toxicant-Altered microRNA Expression. Toxicol Appl Pharmacol. 2016;312:61–66. doi: 10.1016/j.taap.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stark HJ, Willhauck MJ, Mirancea N, Boehnke K, Nord I, Breitkreutz D, Pavesio A, Boukamp P, Fusenig NE. Authentic fibroblast matrix in dermal equivalents normalises epidermal histogenesis and dermoepidermal junction in organotypic co-culture. Eur Cell Biol. 2004;83:631–45. doi: 10.1078/0171-9335-00435. [DOI] [PubMed] [Google Scholar]
- 93.Stein GS, van Wijnen AJ, Stein JL, Lian JB, Montecino M, Choi J, Zaidi K, Javed A. Intranuclear trafficking of transcription factors: implications for biological control. J Cell Sci. 2000;113:2527–2533. doi: 10.1242/jcs.113.14.2527. [DOI] [PubMed] [Google Scholar]
- 94.Stevens MM. Toxicology: Testing in the third dimension. Nat Nanotech. 2009;4:342–343. doi: 10.1038/nnano.2009.136. [DOI] [PubMed] [Google Scholar]
- 95.Styllou M, Reichl FX, Styllou P, Urcan E, Rothmund L, Hickel R, Hogg C, Scherthan H. Dental composite components induce DNA-damage and altered nuclear morphology in gingiva fibroblasts. Dent Mater. 2015;31:1335–1344. doi: 10.1016/j.dental.2015.08.156. [DOI] [PubMed] [Google Scholar]
- 96.Swarnkar S, Goswami P, Kamat PK, Gupta S, Patro IK, Singh S, Nath C. Rotenone-induced apoptosis and role of calcium: a study on Neuro-2a cells. Arch Toxicol. 2012;86:1387–1397. doi: 10.1007/s00204-012-0853-z. [DOI] [PubMed] [Google Scholar]
- 97.Swift J, Discher DE. The nuclear lamina is mechano-responsive to ECM elasticity in mature tissue. J Cell Sci. 2014;127:3005–3015. doi: 10.1242/jcs.149203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thun MJ, Jemal A. IARC Classification of Carcinogens. In: Kufe DW, Pollock RE, Weichselbaum RR, et al., editors. Holland-Frei Cancer Medicine. 6. Hamilton Cox(ON): BC Decker; 2003. [Google Scholar]
- 99.Varshney S, Hunter DD, Brunken WJ. Extracellular Matrix Components Regulate Cellular Polarity and Tissue Structure in the Developing and Mature Retina. J Ophthalmic Vis Res. 2015;10:329–339. doi: 10.4103/2008-322X.170354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vega SL, Dhaliwal A, Arvind V, Patel PJ, Beijer NRM, de Boer J, Murthy NS, Kohn J, Moghe PV. Organizational Metrics of Interchromatin Speckle Factor Domains: Integrative Classifier for Stem Cell Adhesion & Lineage Signaling. Integr Biol. 2015;7:435–446. doi: 10.1039/c4ib00281d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vega SL, Liu E, Arvind V, Bushman J, Sung H, Becker ML, Lelièvre SA, Kohn J, Vidi PA, Moghe PJ. High-content image informatics of the structural nuclear protein NuMA parses trajectories for stem/progenitor cell lineages and oncogenic transformation. Exp Cell Res. 2017;351:11–23. doi: 10.1016/j.yexcr.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Versaevel M, Grevesse T, Gabriele S. Spatial coordination between cell and nuclear shape within micropatterned endothelial cells. Nat Commun. 2012;3:671. doi: 10.1038/ncomms1668. [DOI] [PubMed] [Google Scholar]
- 103.Vidi PA, Chandramouly G, Gray M, Wang L, Liu E, Kim JJ, Roukos V, Bissell MJ, Moghe PV, Lelièvre SA. Interconnected contribution of tissue morphogenesis and the nuclear protein NuMA to the DNA damage response. J Cell Sci. 2012;125:350–361. doi: 10.1242/jcs.089177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vidi PA, Leary JF, Lelièvre SA. Building risk-on-a-chip models to improve breast cancer risk assessment and prevention. Integr Biol. 2013;5:1110–1118. doi: 10.1039/c3ib40053k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vidi PA, Maleki T, Ochoa M, Wang L, Clark SM, Leary JF, Lelièvre SA. Disease-on-a-chip: mimicry of tumor growth in mammary ducts. Lab chip. 2014;14:172–177. doi: 10.1039/c3lc50819f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. Journal of Carcinogenesis. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Watt FM, Fujiwara H. Cell-Extracellular Matrix Interactions in Normal and Diseased Skin. Cold Spring Harbor Perspectives in Biology. 2011;3:a005124. doi: 10.1101/cshperspect.a005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Webster A, Greenman J, Haswell SJ. Development of microfluidic devices for biomedical and clinical application. J Chem Technol Biotechnol. 2011;86:10–17. [Google Scholar]
- 110.Wilson PD. Apico-basal polarity in polycystic kidney disease epithelia. Biochim Biophys Acta. 2011;1812:1239–1248. doi: 10.1016/j.bbadis.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 111.Wodarz A, Näthke I. Cell polarity in development and cancer. Nat Cell Biol. 2007;9:1016–1024. doi: 10.1038/ncb433. [DOI] [PubMed] [Google Scholar]
- 112.Woods JM, Leone M, Klosowska K, Lamar PC, Shaknovsky TJ, Prozialeck WC. Direct antiangiogenic actions of cadmium on human vascular endothelial cells. Toxicol In Vitro. 2008;22:643–651. doi: 10.1016/j.tiv.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu G, Ge J, Huang X, Hua Y, Mu D. Planar cell polarity signaling pathway in congenital heart diseases. J Biomed Biotech. 2011;2011:1–8. doi: 10.1155/2011/589414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xia YN, Whitesides GM. Soft lithography. Annu Rev Mater Sci. 1998;28:153–184. [Google Scholar]
- 115.Ziegerer A, Wuttke A, Marsico G, Seifert S, Kalaidzidis Y, Zerial M. Functional properties of hepatocytes in vitro are correlated with cell polarity maintenance. Exp Cell Res. 2016;350:242–252. doi: 10.1016/j.yexcr.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 116.Zink D, Fischer AH, Nickerson JA. Nuclear structure in cancer cells. Nat Rev Cancer. 2004;4:677–687. doi: 10.1038/nrc1430. [DOI] [PubMed] [Google Scholar]
- 117.Zuppinger C. 3D culture of cardiac cells. Biochim Biphys Acta. 2016;1863:1873–1881. doi: 10.1016/j.bbamcr.2015.11.036. [DOI] [PubMed] [Google Scholar]