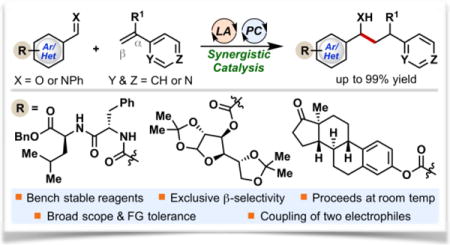
Abstract
Umpolung (polarity reversal) strategies of aldehydes and imines have dramatically expanded the scope of carbonyl and iminyl chemistry by facilitating reactions with non-nucleophilic reagents. Herein, we report the first visible light photoredox-catalyzed β-selective reductive coupling of alkenylpyridines with carbonyl or iminyl derivatives with the aid of a Lewis acid co-catalyst. Our process tolerates complex molecular scaffolds (e.g. sugar, natural product, and peptide derivatives) and is applicable to the preparation of compounds containing a broad range of heterocyclic moieties. Mechanistic investigations indicate that the key step involves single electron transfer (SET) reduction of aldehydes or imines followed by the addition of resulting ketyl or α-aminoalkyl radicals to Lewis acid-activated alkenylpyridines.
TOC image
Pyridines are important substructures of natural products,1 pharmaceuticals,2 agrochemicals,3 functional materials,4 and ligands in catalysis.5 They are often recognized as “privileged” scaffolds in medicinal chemistry for discovery and optimization of new synthetic drug molecules. Currently, over 100 marketed pharmaceuticals, including drug molecules such as Nexium (anti-acid) and Imatinib (anti-cancer) bearing at least one pyridine ring.6 Due to the prevalence of pyridines in medicinal chemistry, there is a high demand for novel synthetic methodologies that enable an access to new molecular architectures containing this important pharmacophore.
Alkenyl pyridines are versatile synthetic intermediates that have been used in the synthesis of more complex molecules with pyridine subunits.7 Due to their electrophilic nature, the reductive coupling of alkenyl pyridines with another electrophile is rare.8 Pioneering studies by Krische and coworkers in 2008 resulted in the first catalytic reductive coupling of 2-vinylpyridines to imines (Scheme 1a).8a After this seminal report, Lam et al. developed an elegant copper-catalyzed enantioselective reductive coupling of alkenylpyridines with ketones8b and imines (Scheme 1a).8c These protocols offer a useful tool for reductive C-C coupling at the α-position of the vinyl or alkenyl moiety. However, a general reaction for β-selective reductive coupling of alkenylpyridines with carbonyl/iminyl derivatives has yet to be achieved. The successful development of such a reaction would provide access to a complementary scope of synthetically useful products.
Scheme 1.
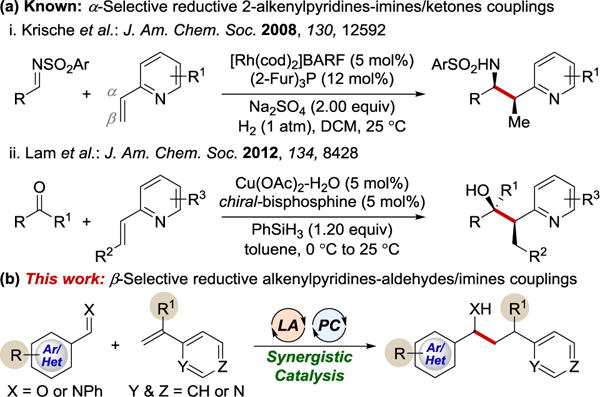
Catalytic transformations of alkenylpyridines.
We postulated that intervention of a reductive Umpolung step involving ketyl/α-aminoalkyl radicals would provide a powerful strategy to access previously unattainable pyridine-containing structures and enhanced flexibility to the design of new synthetic targets. Nevertheless, a widespread application of ketyl radicals in synthesis has been hindered by the highly negative reduction potential of aldehydes (E1/2red = −1.93 V vs SCE for benzaldehyde),9 and the requirement of employing toxic, air- and moisture-sensitive reducing agents and harsh reaction conditions to access the ketyl radical intermediate.10 Recently, however, visible light photoredox catalysis has emerged as an efficient way to generate ketyl11 and α-aminoalkyl radicals from the corresponding aldehydes and imines under mild reaction conditions.11k, 12 Nonetheless, photocatalytic intermolecular ketyl-olefin reductive coupling reactions are very rare.11l Herein we report the first β-selective intermolecular reductive coupling reaction between alkenylpyridines and carbonyl or iminyl derivatives enabled by a synergistic Lewis acid/photoredox catalytic system (Scheme 1b).
Our initial investigation focused on the reaction between 4-fluorobenzaldehyde 1a and 4-vinylpyridine 2a (Table 1). We observed that Lewis acid plays a critical role in achieving the desired reactivity. When a solution of 1a and 2a in MeCN was irradiated with blue LED light employing Ru(bpy)3(PF6)2 as a photoredox catalyst and Hantzsch ester (HEH) as a stoichiometric reductant, no desired product (3a) was formed in the absence of Lewis acid (entry 1).13 With a sub-stoichiometric amount of Yb(OTf)3 and bipyridine ligand, 3a was formed, albeit in low yield (entry 2). The yield of 3a was drastically improved with Gd(OTf)3 (entry 3), while with La(OTf)3, 3a was generated quantitatively (entry 4). We also observed that the reaction worked equally well when Ru(bpy)3(PF6)2 was substituted with Ir(ppy)3 (entry 5), but the yield was diminished with Rhodamine 6G (entry 6). Interestingly, without any photoredox catalyst, a small amount of 3a was still observed (entry 7). Presumably, ketyl radicals were formed through direct SET between photoexcited Hantzsch ester and aldehydes.14 Yet, such SET is less efficient than that in the presence of a photoredox catalyst. Notably, the use of Hantzsch ester as a reductant is crucial. When either Hünig’s base (entry 8) or 1-benzyl-1,4-dihydronicotinamide (BNAH) (entry 9) was employed as a stoichiometric reductant in place of HEH, no 3a was obtained. Use of HEH as a reductant is beneficial because the oxidized-Hantzsch ester (Ox-HE) could be readily recycled and converted to HEH through hydrogenation.15 In the absence of bipyridine, the reaction afforded 3a in only 78% yield (entry 10), the drop likely attributed to the poor solubility of unligated La(OTf)3 in MeCN. In the control experiments, we found that both light (entry 11) and an oxygen-free atmosphere (entry 12) were necessary for the reaction to proceed.
Table 1.
Selected optimization experiments.

| ||||
|---|---|---|---|---|
|
| ||||
| Entry | Photocatalyst (1.00 mol%) |
Lewis acid (20.0 mol%) |
Reductant (2.00 equiv) |
Yield [%]a |
| 1 | Ru(bpy)3(PF6)2 | – | HEH | 0 |
| 2 | Ru(bpy)3(PF6)2 | Yb(OTf)3 | HEH | 18 |
| 3 | Ru(bpy)3(PF6)2 | Gd(OTf)3 | HEH | 85 |
| 4 | Ru(bpy)3(PF6)2 | La(OTf)3 | HEH | >99 |
| 5 | Ir(PPy)3 | La(OTf)3 | HEH | >99 |
| 6 | Rho 6G | La(OTf)3 | HEH | 47 |
| 7 | – | La(OTf)3 | HEH | 16 |
| 8 | Ru(bpy)3(PF6)2 | La(OTf)3 | DIPEA | 0 |
| 9 | Ru(bpy)3(PF6)2 | La(OTf)3 | BNAH | 0 |
| 10 | Ru(bpy)3(PF6)2 | La(OTf)3 | HFH | 78b |
| 11 | Ru(bpy)3(PF6)2 | La(OTf)3 | HEH | 0c |
| 12 | Ru(bpy)3(PF6)2 | La(OTf)3 | HEH | 0d |
Yields were determined by 19F NMR using 3-fluoronitrobenzene as the internal standard unless otherwise specified.
Without bipyridine.
Without light.
Under oxygen atmosphere.
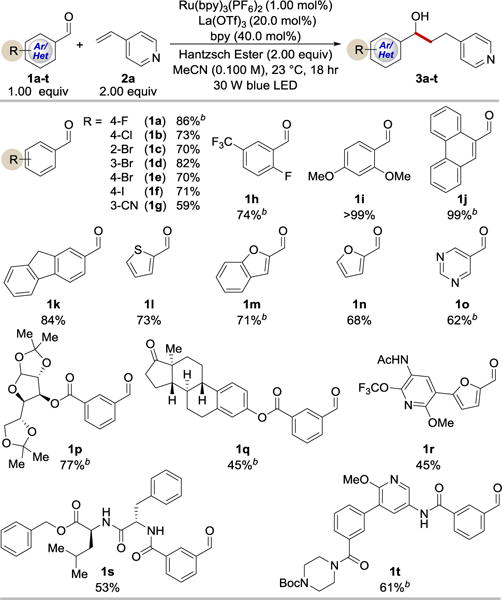
With the optimized reaction conditions in hand, we then examined the generality of this transformation with respect to the aldehyde component. As shown in Table 2, the coupling reactions of both electron-deficient and electron-rich benzaldehydes proceeded with high efficiency (up to quantitative yield). It is noteworthy that halogen functionalities (1a-f, 1h), in particular Br and I, remain intact after the reaction, providing easy handles for further synthetic elaborations. In addition, the ortho-, meta-, and para-substituents show little influence on the reaction efficiency (1c–1e). Moreover, the mild reaction conditions are compatible with a wide range of other functional groups including ester (1p, 1q, 1s), ketone (1q), amide (1r–1t), nitrile (1g), ether (1i, 1p, 1r, 1t), the OCF3 group (1r), and the CF3 group (1h). Given the importance of heterocyclic structures in the synthesis of biologically important molecules, we were pleased to find that heteroaryl aldehydes (1l–1o) and aryl aldehydes bearing heteroarene substituents (1t) readily participated in the coupling reaction. To further demonstrate the synthetic utility of the reaction, we tested more complex substrates, including sugar (diacetone-D-glucose, 1p), natural product (estrone, 1q), and peptide (1s) derivatives. To our delight, they all afforded the desired products in synthetically useful yields. Notably, only one diastereomer was obtained in these cases, which indicates that existing chiral centers on aldehydes have a strong influence on the stereochemical outcome of the transformation.
Table 2.
Selected examples of the coupling reaction between aldehydes and 4-vinylpyridine.a
|
Cited yields are of isolated material following chromatography.
Reactions performed with 2 equiv of aldehyde and 1 equiv of 4-vinylpyridine.
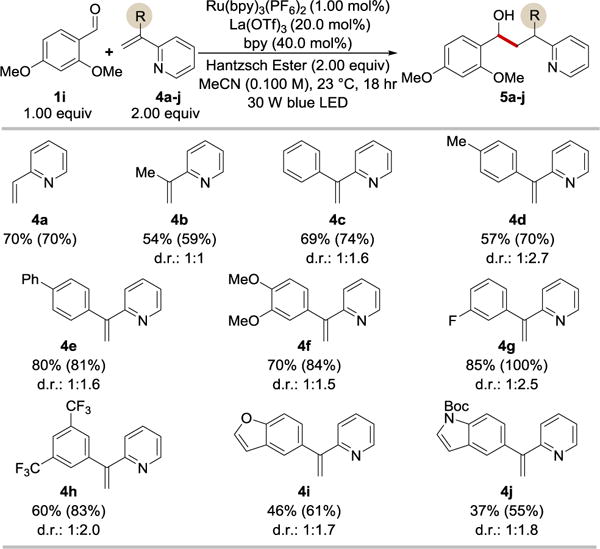
Next, we directed our attention to delineating the scope of alkenylpyridine coupling partners (Table 3). Gratifyingly, 2-vinylpyridine (4a), 2-(1-methylalkenyl)pyridine (4b), and a range of 2-(1-arylalkenyl)pyridines (4c–4j) were converted into the corresponding products 5 in good to excellent yields and with modest diastereoselectivities. The reaction tolerated substrates bearing electron-neutral (4c), electron-rich (4d, 4e, 4f) and electron poor (4g, 4h) arene rings, as well as heterocyclic structures such as benzofuran (4i) and indole (4j). The coupling reaction between aldehydes and 2-(1-arylalkenyl)pyridines described here provides the first general route to compounds 5c–5j, which prior to our studies remained unknown. Our Lewis acid/photoredox catalytic protocol enables a straightforward synthesis of this novel molecular scaffold and its exploration in the context of medicinal chemistry.
Table 3.
Selected examples of the coupling reaction between 2,4-dimethoxybenzaldehyde and alkenylpyridines.a
|
Cited yields are of isolated material following chromatography. NMR yield in the parenthesis and d.r. were determined on the crude reaction sample using 1H-NMR.
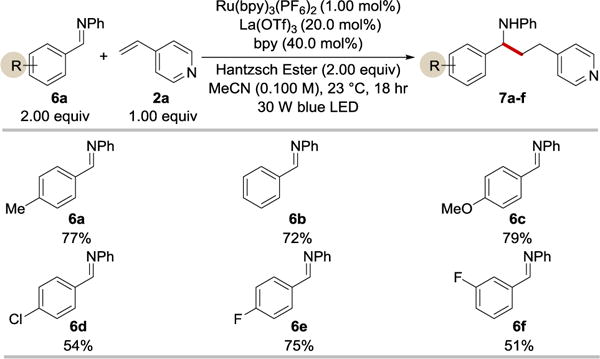
Encouraged by the results of the aldehyde-alkenylpyridine coupling, we tested imines as potential coupling partners in the reaction with 4-vinylpyridine (Table 4). Prior to this report, intermolecular reductive imine-olefin coupling reactions under photoredox conditions were rare.11l,12c This is unfortunate considering the great importance of amines in drug design and development. We were pleased to find that α-aminoalkyl radicals generated from aromatic imines upon a SET can indeed undergo the coupling reaction with 4-vinylpyride. Importantly, the reaction proceeded well regardless of the electronic of the iminyl aromatic rings.
Table 4.
Selected examples of the coupling reaction between imines and 4-vinylpyridine.a
|
Cited yields are of isolated material following chromatography.
The proposed mechanistic details of our transformation are depicted in Figure 1. Irradiation of Ru(bpy)32+ with visible light produces a long-lived (1.1 μs)16 photoexcited state, *Ru(bpy)32+. A Stern–Volmer experiment showed no measurable luminescence quenching of *Ru(bpy)32+ (E1/2red = +0.77 V vs SCE)17 by Hantzsch ester (HEH) (E1/2red = + 0.887 V vs SCE)18 in MeCN. In addition, neither benzaldehyde nor 4-vinylpyridine quenches the excited photocatalyst (both in the absence and presence of Lewis acid). In order to exclude the possibility of excited HEH (*HEH) (λmax = 362 nm in MeCN) being the quencher of *Ru(bpy)32+, we ran the reaction using a laser line filter (CWL = 488 ± 2 nm, FWHM = 10 ± 2 nm) and obtained the desired product. Based on these observations, we postulated that the excited photocatalyst is likely quenched by the catalytically generated intermediate Hantzsch ester radical (HEH•).19 Since HEH• is absent at the beginning of the reaction, it is initially generated via an alternative pathway that involves photoexcitation of HEH (*HEH), followed by a SET from *HEH to an aldehyde with a concomitant proton transfer. Reduction of *Ru(bpy)32+ by HEH• (E1/2red = −0.76 V vs SCE)18 affords a strongly reducing ruthenium (I) species, Ru(bpy)3+, and pyridinium ion PyH+. Ru(bpy)3+ (E1/2red = −1.33 V vs SCE)17 engages in a SET with aldehydes activated by hydrogen bonding with PyH+ to regenerate the Ru(bpy)32+ catalyst and afford ketyl radical intermediate I. Addition of I to Lewis acid-activated 4-vinylpyridine (IIa) forms radical IIb, which can abstract hydrogen atom directly from HEH, thus generating the Lewis acid-bounded product (IIc) and another molecule of a reductant (HEH•) capable of reducing *Ru(bpy)32+. Complex IIc undergoes ligand exchange with 4-vinylpyridine to liberate the desired coupling product and complete the catalytic cycle.
Figure 1.
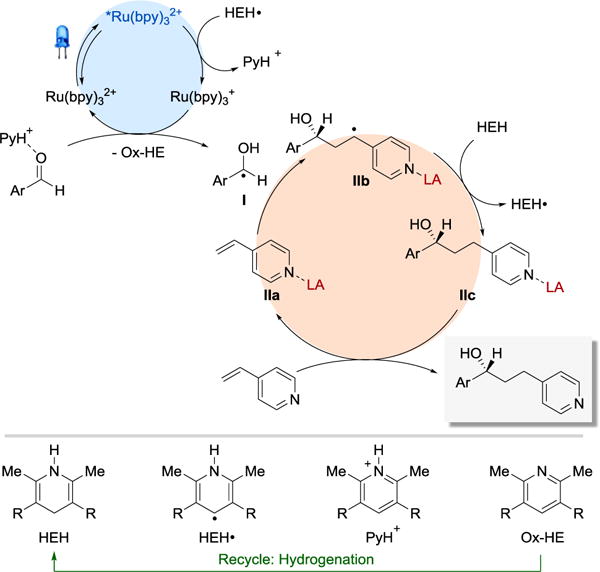
Proposed reaction mechanism.
In conclusion, we have developed the first photocatalytic reaction for the coupling between alkenylpyridines and carbonyl or iminyl derivatives with a Lewis acid co-catalysis. The unique features of our synergistic catalytic system include: (i) mild reaction conditions that tolerate a wide scope of functional groups and complex molecular architectures such as sugar, natural product, and peptide derivatives; (ii) the ability to forge alcohols and amines using a catalytic protocol that couples two bench stable electrophiles, carbonyl/iminyl derivatives and alkenylpyridines, provides an attractive alternative to methods that use stoichiometric amounts of reactive, unstable intermediates; (iii) access to a broad range of “privileged” and novel heterocyclic structures, which could provide a powerful new strategy for the synthesis of versatile small molecules of potential pharmaceutical relevance; (iv) the exclusive β-selectivity of our protocol offers a complementary method to the existing α-selective reductive coupling reactions of alkenylpyridines; (v) mechanistic studies offer valuable insight into the key step that involves addition of ketyl or α-aminoalkyl radicals to Lewis acid-activated alkenylpyridines. We anticipate that this reaction will serve as the basis for the development of broadly useful coupling reactions of two electrophiles. Further efforts will be directed towards the development of an asymmetric coupling reaction with the aid of a chiral ligand/Lewis acid system. In addition, we will continue working on new visible light photoredox-catalyzed transformations involving ketyl radical intermediates.
Supplementary Material
Acknowledgments
This work was partially supported by National Institute of General Medical Sciences (R35GM119652) and start-up funds from Stony Brook University. K.N.L. received the graduate fellowship from the NIH Chemical-Biology training grant (T32GM092714). We thank James Herbort, a NSF REU student (CHE-1358959), for the synthesis of some alkenylpyridines. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health and National Science Foundation. This paper is dedicated to Prof. Barry M. Trost on the occasion of his 75th birthday.
Footnotes
Supporting Information.
Experimental details and characterization data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Notes
The authors declare no competing financial interests.
References
- 1.Majumdar KC, Chattopadhyay SK. Heterocycles in Natural Product Synthesis. Wiley-VCH Verlag; Weinheim: 2011. [Google Scholar]
- 2.(a) Chaubey A, Pandeya SN. Asian J Pharm Clin Res. 2011;4:5. [Google Scholar]; (b) Li JJ. Heterocyclic Chemistry in Drug Discovery. John Wiley & Sons; Hoboken, N.J.: 2013. [Google Scholar]
- 3.Guan AY, Liu CL, Sun XF, Xie Y, Wang MA. Bioorg Med Chem. 2016;24:342. doi: 10.1016/j.bmc.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 4.Frechet JMJ, Demeftahi MV. Brit Polym J. 1984;16:193. [Google Scholar]
- 5.Zafar MN, Atif AH, Nazar MF, Sumrra SH, Gul-E-Saba, Paracha R. Russ J Coord Chem. 2016;42:1. [Google Scholar]
- 6.Goetz AE, Garg NK. Nat Chem. 2013;5:54. doi: 10.1038/nchem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Boger DL, Ichikawa S, Jiang HJ. J Am Chem Soc. 2000;122:12169. [Google Scholar]; (b) Faul MM, Ratz AM, Sullivan KA, Trankle WG, Winneroski LL. J Org Chem. 2001;66:5772. doi: 10.1021/jo0103064. [DOI] [PubMed] [Google Scholar]; (c) Waser J, Gaspar B, Nambu H, Carreira EM. J Am Chem Soc. 2006;128:11693. doi: 10.1021/ja062355+. [DOI] [PubMed] [Google Scholar]; (d) Rupnicki L, Saxena A, Lam HW. J Am Chem Soc. 2009;131:10386. doi: 10.1021/ja904365h. [DOI] [PubMed] [Google Scholar]; (e) Qi M, Gross A, Jeschke G, Godt A, Drescher M. J Am Chem Soc. 2014;136:15366. doi: 10.1021/ja508274d. [DOI] [PubMed] [Google Scholar]; (f) Best D, Lam HW. J Org Chem. 2014;79:831. doi: 10.1021/jo402414k. [DOI] [PubMed] [Google Scholar]; (g) Chen J, Li JJ, Wang JZ, Li H, Wang W, Guo YW. Org Lett. 2015;17:2214. doi: 10.1021/acs.orglett.5b00811. [DOI] [PubMed] [Google Scholar]; (h) Wang SA, Li XM, Liu HW, Xu L, Zhuang JC, Li J, Li H, Wang W. J Am Chem Soc. 2015;137:2303. doi: 10.1021/ja511143b. [DOI] [PubMed] [Google Scholar]
- 8.(a) Komanduri V, Grant CD, Krische MJ. J Am Chem Soc. 2008;130:12592. doi: 10.1021/ja805056g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Saxena A, Choi B, Lam HW. J Am Chem Soc. 2012;134:8428. doi: 10.1021/ja3036916. [DOI] [PubMed] [Google Scholar]; (c) Choi B, Saxenaa A, Smith JJ, Churchill GH, Lam HW. Synlett. 2015;26:350. [Google Scholar]; (d) Nguyen KD, Park BY, Luong T, Sato H, Garza VJ, Krische MJ. Science. 2016;354:300. doi: 10.1126/science.aah5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roth HG, Romero NA, Nicewicz DA. Synlett. 2016;27:714. [Google Scholar]
- 10.(a) Pradhan SK, Kadam SR, Kolhe JN, Radhakrishnan TV, Sohani SV, Thaker VB. J Org Chem. 1981;46:2622. [Google Scholar]; (b) Ikeda T, Yue S, Hutchinson CR. J Org Chem. 1985;50:5193. [Google Scholar]; (c) Molander GA, Kenny C. Tetrahedron Lett. 1987;28:4367. [Google Scholar]; (d) Shono T, Kashimura S, Mori Y, Hayashi T, Soejima T, Yamaguchi Y. J Org Chem. 1989;54:6001. [Google Scholar]; (e) Hays DS, Fu GC. J Org Chem. 1996;61:4. doi: 10.1021/jo960846e. [DOI] [PubMed] [Google Scholar]; (f) Estévez RE, Oller-López JL, Robles R, Melgarejo CR, Gansäuer A, Cuerva JM, Oltra JE. Org Lett. 2006;8:5433. doi: 10.1021/ol0620390. [DOI] [PubMed] [Google Scholar]; (g) Cossy J, Belotti D. Tetrahedron. 2006;62:6459. [Google Scholar]; (h) Yeh CH, Korivi RP, Cheng CH. Adv Synth Catal. 2013;355:1338. [Google Scholar]
- 11.(a) Ishitani O, Pac C, Sakurai H. J Org Chem. 1983;48:2941. [Google Scholar]; (b) Fukuzumi S, Ishikawa K, Hironaka K, Tanaka T. J Chem Soc Perk T 2. 1987;751 [Google Scholar]; (c) Ischay MA, Anzovino ME, Du J, Yoon TP. J Am Chem Soc. 2008;130:12886. doi: 10.1021/ja805387f. [DOI] [PubMed] [Google Scholar]; (d) Du JN, Espelt LR, Guzei IA, Yoon TP. Chem Sci. 2011;2:2115. doi: 10.1039/C1SC00357G. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Larraufie MH, Pellet R, Fensterbank L, Goddard JP, Lacote E, Malacria M, Ollivier C. Angew Chem Int Ed. 2011;50:4463. doi: 10.1002/anie.201007571. [DOI] [PubMed] [Google Scholar]; (f) Petronijevic FR, Nappi M, MacMillan DWC. J Am Chem Soc. 2013;135:18323. doi: 10.1021/ja410478a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Rono LJ, Yayla HG, Wang DY, Armstrong MF, Knowles RR. J Am Chem Soc. 2013;135:17735. doi: 10.1021/ja4100595. [DOI] [PubMed] [Google Scholar]; (h) Tarantino KT, Liu P, Knowles RR. J Am Chem Soc. 2013;135:10022. doi: 10.1021/ja404342j. [DOI] [PubMed] [Google Scholar]; (i) Nakajima M, Fava E, Loescher S, Jiang Z, Rueping M. Angew Chem Int Ed. 2015;54:8828. doi: 10.1002/anie.201501556. [DOI] [PubMed] [Google Scholar]; (j) Amador AG, Sherbrook EM, Yoon TP. J Am Chem Soc. 2016;138:4722. doi: 10.1021/jacs.6b01728. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Fava E, Millet A, Nakajima M, Loescher S, Rueping M. Angew Chem Int Ed. 2016;55:6776. doi: 10.1002/anie.201511235. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Qi L, Chen YY. Angew Chem Int Ed. 2016;55:13312. doi: 10.1002/anie.201607813. [DOI] [PubMed] [Google Scholar]; (m) Wang CY, Qin J, Shen XD, Riedel R, Harms K, Meggers E. Angew Chem Int Ed. 2016;55:685. doi: 10.1002/anie.201509524. [DOI] [PubMed] [Google Scholar]
- 12.(a) Jeffrey JL, Petronijević FR, MacMillan DWC. J Am Chem Soc. 2015;137:8404. doi: 10.1021/jacs.5b05376. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Uraguchi D, Kinoshita N, Kizu T, Ooi T. J Am Chem Soc. 2015;137:13768. doi: 10.1021/jacs.5b09329. [DOI] [PubMed] [Google Scholar]; (c) Fuentes de Arriba AL, Urbitsch F, Dixon DJ. Chem Commun. 2016;52:14434. doi: 10.1039/c6cc09172e. [DOI] [PubMed] [Google Scholar]
- 13.We observed the formation of pinacol coupling product under these reaction conditions.
- 14.Jung J, Kim J, Park G, You Y, Cho EJ. Adv Synth Catal. 2016;358:74. [Google Scholar]
- 15.Chen QA, Chen MW, Yu CB, Shi L, Wang DS, Yang Y, Zhou YG. J Am Chem Soc. 2011;133:16432. doi: 10.1021/ja208073w. [DOI] [PubMed] [Google Scholar]
- 16.Juris A, Balzani V, Belser P, von Zelewsky A. Helv Chim Acta. 1981;64:2175. [Google Scholar]
- 17.Kalyanasundaram K. Coord Chem Rev. 1982;46:159. [Google Scholar]
- 18.The reduction potential was calculated by using the conversion factor described in ref. 18(a) and values reported in ref. 18(b).; (a) Pavlishchuk VV, Addison AW. Inorg Chim Acta. 2000;298:97. [Google Scholar]; (b) Zhu XQ, Li HR, Li Q, Ai T, Lu JY, Yang Y, Cheng JP. Chem Eur J. 2003;9:871. doi: 10.1002/chem.200390108. [DOI] [PubMed] [Google Scholar]
- 19.See SI for detailed mechanistic studies and discussions.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


