Abstract
The intent of this NIH-sponsored study was to compare a belatacept-based immunosuppressive regimen with a maintenance regimen of tacrolimus and mycophenolate (MMF). Nineteen primary, EBV-immune renal transplant recipients with a negative cross-match were randomized to one of three groups. All patient groups received perioperative steroids and maintenance MMF. Patients in groups 1 and 2 were induced with alemtuzumab and maintained on tacrolimus or belatacept, respectively. Patients in group 3 were induced with basiliximab, received 3 months of tacrolimus, and maintained on belatacept. There was one death with a functioning allograft due to endocarditis (group 1). There were three graft losses due to vascular thrombosis (all group 2) and one graft loss due to glomerular disease (group 1). Biopsy-proven acute cellular rejection was more frequent in the belatacept treated groups, with 10 treated episodes in 7 subjects compared to 1 episode in group 1. However, eGFR was similar between groups at week 52. There were no episodes of PTLD or opportunistic infections in any group. Protocol enrollment was halted prematurely due to high rate of serious adverse events. Such negative outcomes pose challenges to clinical investigators who ultimately must weigh the risk and benefit in randomized trials.
Keywords: immunosuppression, graft loss, costimulatory blockade, rejection, trial
Introduction
Over the past three decades there has been a dramatic improvement in the 1-year graft survival of transplanted kidneys and a concomitant decrease in the incidence of acute rejection (1). While improvements in surgical technique, organ preservation, and donor management have contributed to these improvements, there can be no disputing the critical impact of improved immunosuppressive agents and regimens. However, despite significantly better short-term outcomes, long-term outcomes following kidney and extra-renal transplantation have remained relatively stagnant (2, 3). While many factors contribute to premature graft loss, including the increasing age of organ donors, the increasing medical complexity of transplant recipients, and medication non-adherence, there is widespread consensus that the very immunosuppressive agents that are responsible for the excellent short-term outcomes in transplantation contribute significantly to impaired long-term graft survival and patient morbidity and mortality.
Both calcineurin inhibitors (CNI) and corticosteroids contribute to development and severity of hypertension, new-onset diabetes after transplantation (NODAT), and dyslipidemia. These events in turn promote the development of cardio- and cerebrovascular disease, which are two of the major causes of morbidity and mortality following organ transplantation (4). In addition, CNI have known nephrotoxic effects (5). While the exact contributions of CNI to chronic injury of transplanted kidneys remains controversial (6, 7), there is no doubt that CNI are nephrotoxic and have the potential to cause significant renal injury even in the native kidneys of extra-renal transplant recipients (8).
Given these known toxicities, there has been intense interest in developing immunosuppressive regimens that avoid long-term use of CNI, but such studies have met with varying success (reviewed in (9). With the advent of costimulatory blockade agents and in light of promising results in preclinical models, there has been new interest in CNI avoidance. Early published results using belatacept regimens demonstrated safety and efficacy for the prophylaxis of acute rejection (10). Further investigation in Phase 3 studies using both standard and extended criteria donors (Benefit and Benefit-EXT) demonstrated that belatacept-treated groups had significantly higher rates of acute cellular rejection at one year (11, 12), but significantly better renal function up to 7 years post-transplant (13). Offsetting these advantages, the use of belatacept in combination with non-depleting induction therapy was associated with increased incidence and severity of acute cellular rejection, as well as PTLD, which was seen primarily in EBV seronegative recipients.
To this end, we developed a clinical trial (CTOT-10) to further understand the optimal implementation and outcomes of belatacept therapy in a population of deceased and living donor kidney transplant recipients. In order to mitigate the risk of cellular rejection, the study utilized a belatacept-based regimen incorporating immunosuppressive induction therapy with a depletional agent together with either progressively withdrawn or absent CNI. The primary outcome of interest was renal function at one year after transplantation. However, due to a high number of serious adverse events, the trial was halted early to further enrollment. These events demonstrate the responsibility of investigators to their study subjects in weighing the risk and benefit of experimental therapy.
Methods
Study design
The Clinical Trials in Organ Transplant-10 (“Optimization of NULOJIX® (Belatacept) Usage As A Means of Avoiding Calcineurin Inhibitor (CNI) and Steroids in Renal Transplantation”; NCT01436305; IND 111,783) was a three-year, randomized prospective trial conducted at three transplant centers in the United States (Figure 1). Enrollment began in November 2011, and primary outcomes were pre-specified to be analyzed at 12 months post-transplant. The trial protocol and any amendments were reviewed and approved by a NIAID-sponsored DSMB and the Institutional Review Board (IRB) of each site prior to initiation of the study. The trial conduct was consistent with International Conference on Harmonization Good Clinical Practice (ICH GCP) and other applicable regulatory requirements.
Figure 1.
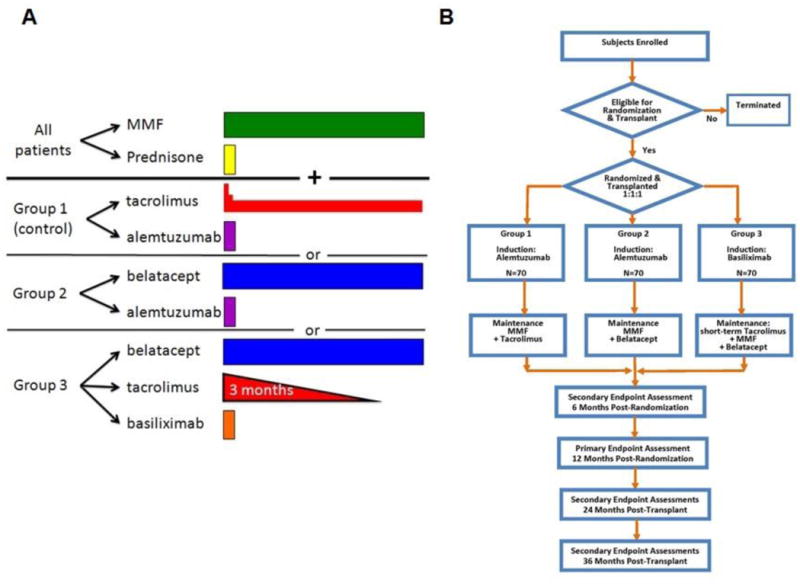
Study schema for CTOT-10 trial. (A) The study therapy as defined in each of the three treatment groups. For details of dosing see text. (B) The enrollment schema for CTOT-10.
Investigational therapy, dosing and study arms
The study consisted of three arms (Figure 1A): in group 1, recipients received alemtuzumab induction, rapid methylprednisolone taper and maintenance tacrolimus and mycophenolate mofetil. Group 2 received alemtuzumab induction, rapid methylprednisolone taper and maintenance belatacept and mycophenolate mofetil. As a control for the use of depletional induction in groups 1 and 2, non-depletional induction with basiliximab was given using standard dosing regimen, with rapid methylprednisolone taper, a 3 month course of tacrolimus, and maintenance belatacept and mycophenolate in group 3. Specific dosing and timing of therapy are shown in Table 1.
Table 1. CTOT 10 therapy and timing of administration.
| Immunosuppression Regimen | Dose and Timing | Treatment Group |
|---|---|---|
| Campath-1H (alemtuzumab) | Single IV dose of 30mg intra-operatively over a period of 2 hours. | Groups 1 and 2 |
| Simulect® (basiliximab) | The first 20 mg dose administered within 2 hours prior to transplantation surgery. The second 20 mg dose given 4 days after transplantation. | Group 3 |
| Nulojix® (belatacept) | NULOJIX® (belatacept) administered at 10mg/kg on Day 0 (day of transplant), and then at days 4, 14, 28, 56 and 84. After 84 days, subjects will receive belatacept at the maintenance dose of 5 mg/kg every 4 weeks until completion of the trial. | Group 2 and 3 |
| Prograf® (tacrolimus) or generic | Tacrolimus administered at a dose of 0.1mg/kg PO BID beginning on the day of surgery or post-operative day 1 depending upon when during the day the surgery is completed, then adjusted to target trough levels of 8-12 ng/ml during the first 24 weeks post-transplant, then adjusted to target trough levels of 5-8 ng/ml thereafter. | Group 1 |
| Prograf® (tacrolimus) or generic | Tacrolimus administered at a dose of 0.1mg/kg PO BID, beginning on the day of surgery or post-operative day 1 depending upon when during the day the surgery is completed. The dosage will be adjusted to achieve the following therapeutic trough levels: 8-12 ng/ml by day 29, 5-8 ng/ml by day 57, 3-5 ng/ml by day 85 and then stopped | Group 3 |
| CellCept® (mycophenolate mofetil), Myfortic®, or generic | CellCept® (Mycophenolate Mofetil- MMF) administered at a target dose of 1000 mg PO BID beginning on the day of surgery or post-operative day 1 (max. MMF dosing 2G per day). MMF adjusted based on clinical complications. After 1 year, dosing may be modified at the discretion of the site investigator.Myfortic® (mycophenolate sodium) may be used as a replacement for MMF, dosed at 720 mg PO BID. | All treatment groups |
| Medrol® (methylprednisolone) | Methylprednisolone administered at a target dose of 500 mg beginning on the day of transplant, and tapered to 250 mg day 1 post-transplant, 125 mg day 2 post-transplant, 60 mg day 3 post-transplant, 30 mg day 4 post-transplant and day 5 post-transplant 0 mg if therapeutic tacrolimus level achieved. | All treatment groups |
The investigational drug used in this trial was Nulojix® (belatacept) (NDC 0003-0371-13). Nulojix® is manufactured by Bristol-Myers Squibb (BMS, Princeton, NJ) and was supplied as 250 mg intravenous infusion-only vials (BMS Lot numbers: 1F65900, 1L66659, 3E77131, 4A83805, 4A82606, 4B79981). The total infusion dose of Nulojix® was based on actual body weight at the time of transplantation, and not modified over the course of the study unless there was a change in body weight greater than 10%. Participants received Nulojix® 10mg/kg on day 0 (day of transplant), and then on days 4, 14, 28, 56, and 84. After day 84, participants received a maintenance dose of 5mg/kg every 4 weeks until completion of the trial.
Subjects
Recipients age 18-65 years old of a first-time kidney transplant from a living or deceased donor were considered eligible for enrollment (see inclusion/exclusion criteria, Table 2). Key exclusion criteria included prior or multi-organ transplant, elevated panel reactive antibody over 30%, EBV seronegative recipients, and individuals at risk for recurrence of their renal disease.
Table 2. Inclusion and Exclusion Criteria for CTOT 10 Trial.
|
|
Hypothesis and Outcome Measures
The hypothesis of this trial was that steroid-free belatacept-based immunosuppressive regimens using induction with either alemtuzumab or basiliximab and tacrolimus would achieve superior renal function with comparable rates of rejection compared to a tacrolimus-based maintenance immunosuppressive regimen. The primary outcome measure was mean glomerular filtration rate (GFR) calculated for each treatment group using the Chronic Kidney Disease Epidemiology Collaboration Equation (CKD-EPI) at 52 weeks post-transplantation (14). Secondary outcome measures were histologic evidence of acute rejection with graft dysfunction in the first 24 weeks of treatment, and other measures of renal function and injury at 52, 104, and 156 weeks of treatment. Measurements of cardiovascular and metabolic parameters such as new onset diabetes or impaired fasting glucose at 52 weeks, the incidence of treated diabetes, hemoglobin A1C measurements, and fasting lipid profiles were also included in secondary endpoints.
Safety Outcome Measures
An independent data and safety monitoring board (DSMB) assessed cumulative safety and efficacy data throughout the trial and provided NIAID with recommendations about study continuation. Safety events of interest included the incidence of death or graft loss, incidence of rejection, and the incidence of all adverse events and serious adverse events (SAEs). Moreover, we focused on any infections requiring hospitalization or systemic therapy, and specifically performed surveillance for BK and CMV viremia (locally monitored) and EBV infections.
Other Secondary Endpoints
Mechanistic assays were performed on specimens collected pre-transplant, then on days 28 and 84, as well as weeks 24, 36, 52, 72, 104 and 156 post-transplant. These included measures of immune reactivity and immune function. Due to the small number of subjects enrolled, the results of these studies will not be addressed in this manuscript.
Statistical Analysis
Since the trial was terminated early for safety reasons with only 19 subjects randomized, only summary descriptive statistics are reported here. Categorical variables are summarized with counts and percentages, and continuous variables are summarized with medians and ranges. Calculated GFR is displayed graphically in dot plots at 52 weeks and longitudinally in line plots by treatment group. Kaplan-Meier plots are presented to depict graft survival and rejection-free survival to compare the treatment groups.
Results
A total of 19 subjects were enrolled in the study and their demographic characteristics are outlined in Table 3. There were 6 subjects enrolled each in groups 1 and 2, and 7 in group 3. The majority were living donor recipients (68.4%) and 42% were African American, representative of the Southeast predominance of the study centers. Groups were fairly balanced demographically. Median follow-up was 1091 days (range 23-1177).
Table 3. Demographics of the study population.
| Control N=6 | Group 2 N=6 | Group 3 N=7 | |
|---|---|---|---|
| Median Recipient Age (range in years) | 45 (30-63) | 40 (35-60) | 51 (37-63) |
| Gender (M/F) | 4 / 2 | 3 / 3 | 6 / 1 |
| Race | 4W / 2AA | 4W / 1A / 1AA | 2W / 5AA |
| Donor Type | 5 LD / 1 DD | 4 LD / 2 DD | 4 LD / 3DD |
| Median Donor Age (range in years) | 43 (28-58) | 32 (29-58) | 43 (25-61) |
| Dialysis Dependent (n) | 4 | 5 | 7 |
| Median Time on dialysis (range in months) | 29 (1-64) | 30 (7-84) | 29 (4-132) |
| HLA Mismatches (A/B/DR) Median (range) | 4 (2,6) | 3 (2,6) | 6 (1,6) |
Kidney graft survival is shown in Figure 2. There were 5 graft losses overall. In group 1, there was one death with a functioning allograft due to staphylococcal bacteremia and endocarditis and one allograft loss due to collapsing glomerulopathy on day 382. There was no conclusive evidence that viral infection mediated the glomerular injury. There were 3 allograft losses in group 2, all attributed to vascular thrombosis and technical injury. In one case, dissection of the recipient's external iliac artery, attributed to a proximal vascular clamp injury, was detected at 1.5 weeks after an uneventful living related kidney transplant. This subject was taken to the operating room where the kidney was explanted and flushed, a Gortex interposition graft placed and the kidney re-implanted. While persistent perfusion was documented by Doppler ultrasound, the graft never regained function. In the second case, a living related transplant was performed pre-emptively. Intraoperatively there was low-grade fever and mild hypotension that persisted postoperatively. Doppler ultrasound of the allograft on post-transplant day 1 showed absent flow in the renal vein consistent with a renal vein thrombosis. Re-exploration confirmed vascular thrombosis but failed to reveal a technical cause. Despite thrombectomy and anticoagulation, meaningful flow could not be reestablished, and the graft was removed. The third subject, who had ESRD due to polycystic kidney disease, received a standard criteria deceased donor allograft, with significant intraoperative fever and hypotension. Doppler ultrasound demonstrated no flow in the immediate post-operative period. Re-exploration demonstrated patent vessels but with poor perfusion secondary to an arterial platelet plug. Despite thrombectomy and anticoagulation, a nephrectomy was ultimately performed and intraoperative assessment revealed renal vein thrombosis with compression of the inferior vena cava due to a large polycystic kidney. Following native nephrectomy to relieve IVC compression, the patient was subsequently successfully re-transplanted. Finally, there was one intraoperative thrombosis in control group 1, and this was managed successfully with intraoperative thrombectomy and heparinization, with excellent graft function post-operatively.
Figure 2.
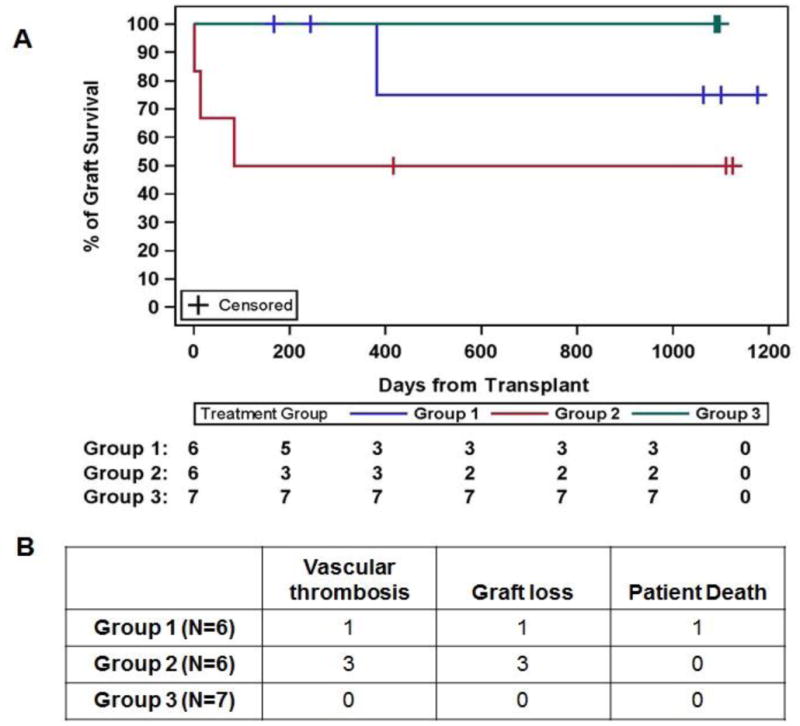
Death censored kidney graft survival in study subjects. (A) Kaplan Meier analysis of graft survival in the three study groups. (B) Graft losses and thromboses in each study arm. There was one death with a functioning graft in group 1. The 3 remaining graft losses occurred due to vascular thrombosis, all in group 2. There were no graft losses in group 3. For a full discussion, see the text.
In the control arm group 1, three subjects had allograft biopsies for allograft dysfunction; only borderline inflammation was detected, and only one of these episodes was treated with additional immunosuppression (Table 4). In contrast, there were seven subjects in groups 2 and 3 that developed rejection (53.8%), with ten episodes of rejection (2 subjects in group 2 and 5 subjects in group 3). In these Belatacept-treated groups, one rejection episode was considered borderline grade, while Banff grade 2A and 2B grades of rejection were more frequently seen. These episodes responded to treatment with pulse corticosteroids (2 cases group 2; 3 cases group 3) or rabbit ATG in combination with pulse corticosteroids (1 case group 2; 3 cases group 3). In one group 2 subject with Banff 1A cellular rejection on day 224, there were findings suspicious for antibody mediated rejection, but without detectable donor specific antibody (DSA). This subject was treated with pulse steroids, a change in the maintenance immunosuppression regimen to tacrolimus with increased mycophenolate mofetil and a prednisone taper. One episode of rejection was not treated as the biopsy findings were assessed as residual signs of rejection from prior treatment episode. As shown in Figure 3, two of five rejection episodes occurred within 21d of weaning tacrolimus (group 3) with another occurring >1-year post weaning. Following anti-rejection treatment, there were no substantial falls in eGFR in those subjects with rejection who retained their grafts (Figure 3B) and there were no cases of graft loss due to acute rejection. DSA were not detected in any study subject during the follow-up period. Finally, rejection episodes were not related to immunosuppressive reductions in the setting of opportunistic infections (data not shown).
Table 4. Rejection episodes in treatment groups.
| Group (enrollment n) | Banff Rejection Grade # Subjects, # Episodes | Subjects with Rejection n (%) | Treated Rejection Episodes (n) | Days Post-Transplant to Rejection (Mean±SD) | De Novo DSA | |||
|---|---|---|---|---|---|---|---|---|
| Borderline | 1A | 2A | 2B | |||||
| Control (n=6) | 3, 4 | 0, 0 | 0, 0 | 0, 0 | 3 (50) | 1 | 305±146 | None |
| Group 2 (n=6) | 0, 0 | 2, 2 | 0, 0 | 1, 1 | 2 (33) | 4 | 147±67 | None |
| Group 3 (n=7) | 1, 1 | 1, 2 | 2, 3 | 1, 1 | 5 (71) | 6 | 241±326 | None |
Figure 3.
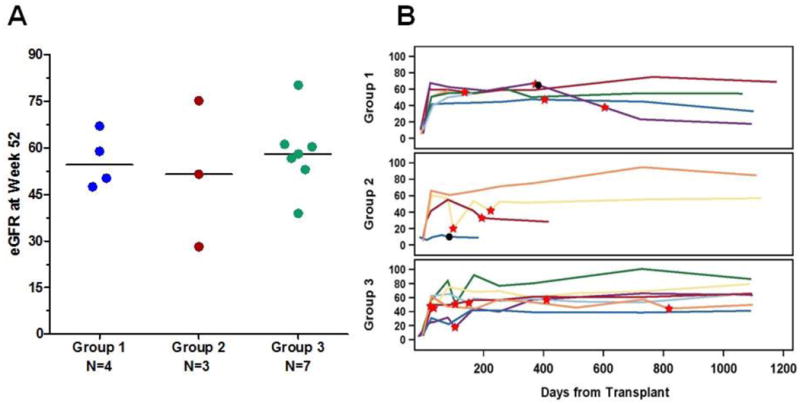
Renal function in study subjects as measured by estimated glomerular filtration rate (eGFR). (A) eGFR is shown for each subject in the group, at 52 weeks after transplantation. (B) eGFR depicted in each subject through the course of the study with a median follow-up of 1,099 days. The red stars indicate borderline or higher T-cell mediated rejection. Black dots indicate graft loss. Two subjects in group 2 who experienced graft loss within the first 2 weeks post-transplant did not have any GFR data available for inclusion in the figure.
Renal function, censored for graft loss at one year, was excellent (Figure 3A). In group 1, eGFR was 55.9±8.9 mL/min, and similar in groups 2 (51.6±23.5mL/min) and 3 (58.3±12.2 mL/min) (p=ns). Further, over the course of the study, there were no significant decreases in eGFR in any group (Figure 3B), with the exception of the single group 1 subject who experienced graft failure on day 382 due to collapsing glomerulopathy. In subjects in the belatacept groups with rejection, eGFR was similar to that in non-rejecters (data not shown). There were no cases of post-transplant lymphoproliferative disorder or progressive multifocal leukoencephalopathy. BK viremia was seen more frequently in group 3 (3 cases) with 1 case each in groups 1 and 2. CMV was infrequent and present in 2 subjects, 1 in group 1 and 1 in group 3. There were no cases of BK polyomavirus nephropathy. Other common adverse events were similar across all treatment groups.
On March 29, 2012, the study team paused enrollment in Study Arm 2, due to thrombotic serious adverse events. On April 20, 2012, the study team, with the concurrence of the DSMB, halted further enrollment in the study due to the number of rejection events in arms 2 and 3. All active study subjects continued to be followed for safety on their current therapy, as outlined in the original study protocol.
Discussion
The optimization of long-term transplant outcomes remains a challenge. Critical to late graft loss are the contributions of both chronic kidney injury in about half of recipients and death with a functioning graft (15). In the latter, cardiovascular disease is the most common cause of death, followed by infection and malignancy. The identification of immunosuppressive therapies that 1) mitigate rejection episodes that can lead to late kidney allograft failure, 2) suppress potent donor-specific antibody responses and 3) do not contribute to kidney injury and cardiovascular disease remains challenging. The goal of this study was to optimize the use of a promising agent that appeared, in early phase studies, to provide an opportunity for CNI-free therapy. While the investigators fully recognize that due to the limited enrollment it is not possible to draw conclusions about the safety of efficacy of the alternative treatment regimens, they felt an obligation to inform those working in the field of the issues encountered in the conduct of this trial.
An unexpected occurrence was the frequency of graft thrombosis in recipients randomized to arm 2. A clear concern was that the infusion of two protein compounds (alemtuzumab and belatacept) in close temporal proximity during reperfusion might have engendered a pro-thrombotic state. While we cannot exclude this, a review of the events indicated that technical issues contributed to at least two of the cases (distal dissection related to a vascular clamp, and renal vein thrombosis related to compression of the IVC). The thromboembolic events were dissimilar enough to each other that there may have been no unifying explanation. Furthermore, prior reports of treatment with either rabbit ATG (16), or alemtuzumab (17) in combination with belatacept have not identified an association with allograft thrombosis, although in both instances, the agents were temporally separated by 24 hours. Although technical issues were strongly implicated in two of the three vascular thrombotic events, the extremely high incidence of perioperative adverse vascular events understandably influenced the DSMB's decision to halt enrollment. This decision exemplifies the valuable role of the interaction between DSMB, study monitors and investigators in conducting safe and effective clinical trials, when a high incidence of adverse events makes it unlikely that an experimental intervention can emerge from statistical and clinical analysis without major clinical uncertainties or reservations (18).
There was a high rate of acute cellular rejection in the belatacept groups, and while high-grade rejections were seen, these were reversed with steroids or rabbit ATG without subsequent graft failure. Such findings suggest that a more prolonged wean may be necessary in the first year post-transplantation as well as close monitoring of those individuals. The current ongoing Belatacept based Early Steroid Withdrawal Trial includes more than 200 kidney transplant recipients, aggregate cellular rejection rates were not apparently elevated when combining belatacept with lymphocyte depletional agents (19). Ultimately, it would be critical to identify treatment-specific risk factors for rejection, as the subjects of this study would be considered of low immunological risk, except for African American race, although all subjects had relatively low cPRA and had no DSA at the time of transplantation. Recent work by Espinosa et al (20) has identified a novel population of CD4 memory T cells that are present prior to transplantation and appear to predict an increased risk of rejection in patients maintained on a belatacept-based regimen. This work requires additional validation but may indicate a patient population at increased risk for rejection when using belatacept in place of CNI.
Concerns about the increased incidence and severity of acute cellular rejection have limited the enthusiasm for belatacept when used according to the product label. For some, these events overshadow the potential benefits of this agent with respect to renal function and the cardiovascular risk profile. While our numbers preclude any concrete conclusions, larger clinical trials have documented a persistent improvement in recipient allograft function over years in belatacept-treated individuals compared to those treated with cyclosporine (21), as well as a lack of donor-specific HLA antibody (13). While it is conceivable that the improved adherence resulting from an infused immunosuppressive agent and possibly greater exposure to MPA compared to cyclosporine-based maintenance regimens, further preclinical and recent clinical studies have demonstrated a potent inhibitory effect of blockade of CD28/CD80/CD86 pathway on B cell function (22). These features of this agent are of utmost clinical relevance as long-term antibody injury is a critical contributor to late kidney allograft failure. To this end, a new study of belatacept in renal transplant recipients designed to address both the potential thrombotic complications and the increased rates of acute cellular rejection is underway (CTOT-16, Clinicaltrials.gov identifier NCT01856257).
Acknowledgments
The authors wish to thank the participating centers, research pharmacist, and staff of the Clinical Trials in Organ Transplantation-10 Consortium: Emory University: Rivka Elbein, Elizabeth Ferry, Sue Mead, , Susan Rogers, Dasia Webster; University of California San Francisco: Yelena Belkin Koplowicz, Alissa Danford, Scott Fields; University of Alabama at Birmingham: Jill Andringa, Tina Ayer, Jianguo Chen, Zahra Davis, Tena Hailey, Rebecca Quinn, Anna Zmijewska; and Rho: Michele Cosgrove, Ann Nguyen, Onysym Silivra.
Disclosures: This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number U01-AI84150 (KN). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The Nulojix® (belatacept) was donated by Bristol-Myers Squibb (Princeton, NJ).
References
- 1.Zand MS. Immunosuppression and immune monitoring after renal transplantation. Semin Dial. 2005;18(6):511–9. doi: 10.1111/j.1525-139X.2005.00098.x. Epub 2006/01/10. [DOI] [PubMed] [Google Scholar]
- 2.Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transplant. 2011;11(3):450–62. doi: 10.1111/j.1600-6143.2010.03283.x. Epub 2010/10/27. [DOI] [PubMed] [Google Scholar]
- 3.Lodhi SA, Lamb KE, Meier-Kriesche HU. Improving long-term outcomes for transplant patients: making the case for long-term disease-specific and multidisciplinary research. Am J Transplant. 2011;11(10):2264–5. doi: 10.1111/j.1600-6143.2011.03713.x. Epub 2011/10/01. [DOI] [PubMed] [Google Scholar]
- 4.Transplantation. United States Renal Data System. 2012 [Google Scholar]
- 5.Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349(24):2326–33. doi: 10.1056/NEJMoa020009. Epub 2003/12/12. [DOI] [PubMed] [Google Scholar]
- 6.Chapman JR. Chronic calcineurin inhibitor use is nephrotoxic. Clin Pharmacol Ther. 2011;90(2):207–9. doi: 10.1038/clpt.2011.117. Epub 2011/07/21. [DOI] [PubMed] [Google Scholar]
- 7.Matas AJ. Calcineurin inhibitors: short-term friend, long-term foe? Clin Pharmacol Ther. 2011;90(2):209–11. doi: 10.1038/clpt.2011.77. Epub 2011/07/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349(10):931–40. doi: 10.1056/NEJMoa021744. Epub 2003/09/05. [DOI] [PubMed] [Google Scholar]
- 9.Casey MJ, Meier-Kriesche HU. Calcineurin inhibitors in kidney transplantation: friend or foe? Curr Opin Nephrol Hypertens. 2011;20(6):610–5. doi: 10.1097/MNH.0b013e32834b4343. [DOI] [PubMed] [Google Scholar]
- 10.Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353(8):770–81. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 11.Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study) Am J Transplant. 2010;10(3):535–46. doi: 10.1111/j.1600-6143.2009.03005.x. Epub 2010/04/27. [DOI] [PubMed] [Google Scholar]
- 12.Durrbach A, Pestana JM, Pearson T, Vincenti F, Garcia VD, Campistol J, et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study) Am J Transplant. 2010;10(3):547–57. doi: 10.1111/j.1600-6143.2010.03016.x. [DOI] [PubMed] [Google Scholar]
- 13.Vincenti F, Rostaing L, Grinyo J, Rice K, Steinberg S, Gaite L, et al. Belatacept and Long-Term Outcomes in Kidney Transplantation. N Engl J Med. 2016;374(4):333–43. doi: 10.1056/NEJMoa1506027. [DOI] [PubMed] [Google Scholar]
- 14.Levey As Fau, Stevens LA, Stevens La Fau, Schmid CH, Schmid Ch Fau, Zhang YL, Zhang Yl Fau, Castro AF, 3rd, Castro Af Fau, 3rd, Feldman HI, Feldman Hi Fau, Kusek JW, et al. A new equation to estimate glomerular filtration rate. (1539-3704(Electronic)) [Google Scholar]
- 15.El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Amer H, Gloor JM, et al. Identifying specific causes of kidney allograft loss. Am J Transplant. 2009;9(3):527–35. doi: 10.1111/j.1600-6143.2008.02519.x. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson R, Grinyo J, Vincenti F, Kaufman DB, Woodle ES, Marder BA, et al. Immunosuppression with belatacept-based, corticosteroid-avoiding regimens in de novo kidney transplant recipients. Am J Transplant. 2011;11(1):66–76. doi: 10.1111/j.1600-6143.2010.03338.x. Epub 2010/12/01. [DOI] [PubMed] [Google Scholar]
- 17.Kirk AD, Guasch A, Xu H, Cheeseman J, Mead SI, Ghali A, et al. Renal transplantation using belatacept without maintenance steroids or calcineurin inhibitors. Am J Transplant. 2014;14(5):1142–51. doi: 10.1111/ajt.12712. Epub 2014/04/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleming TR, Hennekens CH, Pfeffer MA, DeMets DL. Enhancing trial integrity by protecting the independence of data monitoring committees in clinical trials. Journal of biopharmaceutical statistics. 2014;24(5):968–75. doi: 10.1080/10543406.2014.925719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.W ES, Kaufman D, Shields A, Leone J, Matas A, Wiseman A, et al., editors. The BEST Trial: A Prospective Randomized Trial of Belatacept-Based, CNI- and Corticosteroid-Free Immunosuppression. American Transplant Congress. American Journal of Transplantation 2016 [Google Scholar]
- 20.Espinosa J, Herr F, Tharp G, Bosinger S, Song M, Farris AB, 3rd, et al. CD57(+) CD4 T Cells Underlie Belatacept-Resistant Allograft Rejection. Am J Transplant. 2016;16(4):1102–12. doi: 10.1111/ajt.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charpentier B, Medina Pestana JO, Del CRM, Rostaing L, Grinyo J, Vanrenterghem Y, et al. Long-term exposure to belatacept in recipients of extended criteria donor kidneys. Am J Transplant. 2013;13(11):2884–91. doi: 10.1111/ajt.12459. Epub 2013/10/10. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Wang Q, Yin D, Vu V, Sciammas R, Chong AS. Cutting Edge: CTLA-4Ig Inhibits Memory B Cell Responses and Promotes Allograft Survival in Sensitized Recipients. J Immunol. 2015;195(9):4069–73. doi: 10.4049/jimmunol.1500940. Epub 2015/09/30. [DOI] [PMC free article] [PubMed] [Google Scholar]


