Abstract
Context
Some of the high costs of robot-assisted radical prostatectomy (RARP), intensity-modulated radiotherapy (IMRT), and proton beam therapy may be offset by better outcomes or less resource use during the treatment episode.
Objective
To systematically review the literature to identify the key economic trade-offs implicit in a particular treatment choice for prostate cancer.
Evidence acquisition
We systematically reviewed the literature according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement and protocol. We searched Medline, Embase, and Web of Science for articles published between January 2001 and July 2016, which compared the treatment costs of RARP, IMRT, or proton beam therapy to the standard treatment. We identified 37, nine, and three studies, respectively.
Evidence synthesis
RARP is costlier than radical retropubic prostatectomy for hospitals and payers. However, RARP has the potential for a moderate cost advantage for payers and society over a longer time horizon when optimal cancer and quality-of-life outcomes are achieved. IMRT is more expensive from a payer’s perspective compared with three-dimensional conformal radiotherapy, but also more cost effective when defined by an incremental cost effectiveness ratio <$50 000 per quality-adjusted life year. Proton beam therapy is costlier than IMRT and its cost effectiveness remains unclear given the limited comparative data on outcomes. Using the Grades of Recommendation, Assessment, Development and Evaluation approach, the quality of evidence was low for RARP and IMRT, and very low for proton beam therapy.
Conclusions
Treatment with new versus traditional technologies is costlier. However, given the low quality of evidence and the inconsistencies across studies, the precise difference in costs remains unclear. Attempts to estimate whether this increased cost is worth the expense are hampered by the uncertainty surrounding improvements in outcomes, such as cancer control and side effects of treatment. If the new technologies can consistently achieve better outcomes, then they may be cost effective.
Keywords: Prostate cancer, New technology, Cost, Cost effectiveness, Systematic review
1. Introduction
Since the turn of the century, new technologies have revolutionized the treatment of prostate cancer. Starting in the early 2000s, intensity-modulated radiotherapy (IMRT) was rapidly adopted by radiation oncologists, such that by 2007 the vast majority of radiation for prostate cancer was delivered using this method [1]. Around the same time, surgical treatment of prostate cancer was transformed by the rapid dissemination and adoption of robot-assisted radical prostatectomy (RARP). While in 2004 <10% of radical prostatectomy procedures were performed using a robotic approach, this proportion increased to 73% by 2012 [2]. More recently, proton beam therapy has emerged as a new radiation treatment modality. This new technology diffused more slowly than IMRT and RARP, representing only 5% of radiation treatments for prostate cancer in the USA in 2012 [3]. Yet, some centers are strong proponents of its use [4], and others will build proton beam facilities in the upcoming years [5].
Treatment with any of these new technologies—IMRT, RARP, or proton beam therapy—is associated with significant upfront costs: ~$2 million for a robotic platform and ~$150 million for a proton beam therapy cyclotron facility [6,7]. Moreover, maintenance of the equipment and disposables come with additional expenditures [8]. However, some of these costs may be offset by better outcomes or less resource use during the treatment episode. For example, decreased adverse events [9] and shorter hospital stays [10] associated with robotic surgery may offset some of the additional cost.
To date, we lack a comprehensive review of the scientific literature on the costs and health care economics associated with these new treatments for prostate cancer. For this reason, we set out to systematically review the literature to identify the key economic trade-offs implicit in a particular treatment choice for prostate cancer. Specifically, we sought to identify cost-analysis, cost-effectiveness, cost-benefit, and cost-utility studies in the English literature, comparing the cost of each of the new technologies with its more traditional counterpart. These data can inform patients and clinicians when making treatment choices for prostate cancer.
2. Evidence acquisition
We performed a systematic review of the literature according to the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) statement and the PRISMA protocol (see Appendix A for the protocol) [11,12]. We performed three separate searches, one each for RARP, IMRT, and proton beam therapy. We systematically searched Medline, Embase, and Web of Science for manuscripts that compared treatment with one of the new technologies with to its predecessor standard treatment and that reported data on cost, with cost defined as cost analysis, cost effectiveness, cost benefit, or cost utility [13]. We limited our search to manuscripts in the English language that were published between January 2001 and July 2016, and excluded editorials. Restriction to the English language was felt to be unlikely to bias our results based on a recent systematic review of empirical studies on this topic [14]. The searches were performed on July 15, 2016 (IMRT and proton beam therapy) and on July 25, 2016 (RARP). After removing duplicates, the searches yielded 362 citations for IMRT, 207 citations for proton beam therapy, and 926 citations for RARP (Fig. 1). Details about the search strategies and their results are available in Appendix B.
Fig. 1.
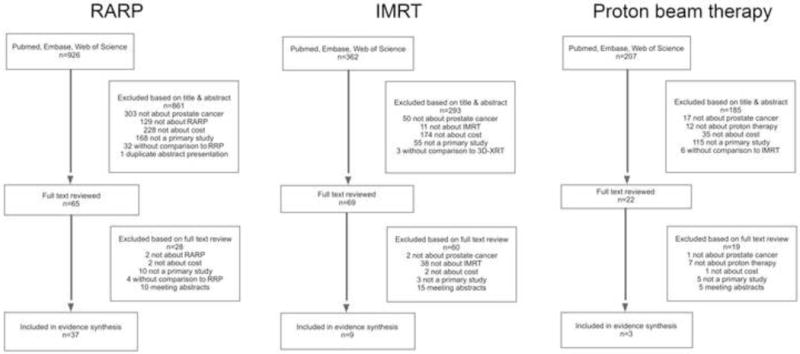
Flow diagram of the search and selection process. IMRT = intensity-modulated radiotherapy; RARP = robot-assisted radical prostatectomy; RRP = radical retropubic prostatectomy; 3D-CRT = three-dimensional conformal radiotherapy.
We excluded manuscripts that did not assess treatment of locoregional prostate cancer, did not examine the technology of interest (ie, not about RARP, IMRT, or proton beam therapy), did not include cost outcomes as defined above, were not a primary research article (eg, meeting abstract, editorial, and comment), or did not compare the technology of interest with its predecessor standard treatment (only RARP vs radical retropubic prostatectomy [RRP], IMRT vs three-dimensional conformal radiotherapy [3D-CRT], and proton beam therapy vs IMRT were included). We first excluded manuscripts based on a review of the title and abstract. Among the remaining manuscripts, we then reviewed the full text, again applying the exclusion criteria. A flow diagram of the search and selection process is shown in Figure 1.
We then systematically abstracted the evidence from the full-text manuscripts according to the protocol. We summarized the number of subjects included, type of study, comparison groups, cost definitions, perspectives (payer vs hospital vs society), and main findings. Risk of bias was assessed with a focus on selection bias, comparability of groups, and follow-up. Data were synthesized in narrative form because of the controversies surrounding methodologies to convert different types of economic outcomes published over a range of years [15]. Given the topic of this review, formal assessment of metabiases such as publication bias or selective reporting within studies was not feasible. Established methods apply mainly to quantitative meta-analyses (eg, funnel plots) or focus on comparing trial protocols with published results [12], neither of which was applicable here. Finally, we assessed the quality of the cumulative evidence according to the Grades of Recommendation, Assessment, Development and Evaluation approach [16].
3. Evidence synthesis
Many of the studies referred to in the following sections evaluated cost effectiveness by estimating the incremental cost effectiveness ratio (ICER), expressed as cost per quality-adjusted life year (QALY) gained. To do this, they compare the cost of the new technology (RARP, IMRT, or proton beam therapy) with that of the standard treatment. Next, they compare outcomes and express them as QALY gained—the arithmetic product of quantity and quality of life that allows for assessment of outcomes that affect quality of life, such as urinary or sexual side effects of treatment [17]. Lastly, they calculate the ICER as the increase in cost per QALY gained. In these analyses, an intervention with an ICER of <$50 000/QALY is generally considered cost effective, indicating that most experts agree that such interventions should be implemented [18]. However, there is considerable debate about the $50 000 threshold as it dates back to at least 1982. Some suggest that this threshold is much too low, and one may wish to implement treatments with higher ICERs depending upon one’s willingness to pay [18]. Some authors have suggested that the ICER is country specific based on the individual country’s gross domestic product, the available budget for health interventions, or the country-specific willingness to pay [19,20].
3.1. Robotic prostatectomy versus RRP
3.1.1. RARP versus RRP from the hospital’s perspective
Between 2004 and 2016, 18 studies were published comparing the cost of RARP and RRP from the hospital’s perspective (Table 1). Most studies were from the USA, with two studies from Australia and one each from Canada and Italy. The vast majority of studies (17 of 18) found an increased cost for RARP, ranging from $195 to over $6000 more per case. Only one study found a decreased cost for RARP, but did not adjust for patient differences that may have accounted for this finding [21]. In fact, many studies did not adjust cost for patient differences such as age, comorbidity, and socioeconomic factors. RARP patients tended to be younger and healthier than RRP patients; thus, the increased cost of RARP was likely underestimated in these studies. The magnitude of the increased cost for RARP varied substantially from study to study, likely because of different methodologies used. The most common methodologies included cost comparisons based on local hospital accounting data (n = 7), use of population-based data and cost-to-charge ratios (n = 5), and cost modeling including institutional data and data from the literature (n = 4). Limitations of these methodologies include the following: (1) cost comparison based on local hospital data is not generalizable; (2) cost-to-charge ratios likely do not lead to an accurate estimate of cost—especially for new technologies—because they are aggregated at the hospital level and can vary widely across hospital departments [22]; and (3) cost modeling is highly dependent on assumptions that go into the model. In addition, there was variation across studies in whether they included or did not include the cost of robot acquisition. Among the 11 studies that either included local hospital accounting data or used cost modeling, seven accounted for the cost of robot acquisition and four did not. Nevertheless, the cumulative evidence supports the fact that RARP is costlier than RRP for hospitals, but the exact difference likely varies from hospital to hospital. RARP costs appear to be lower with increasing hospital volume [23,24] and decreasing length of stay and operating room time [8,25].
Table 1. Studies comparing costs of RARP and RRP.
| Study | Patients, no. | Type of study | Data source | Comparison | Cost definition | Costs included (direct vs indirect) | Perspective | Main findings including comment on risk of bias |
|---|---|---|---|---|---|---|---|---|
| Studies from the hospital’s perspective | ||||||||
| Lotan (2004) [8] | NA | Cost modeling | Intrainstitutional costs were obtained from billing department, supplemented by data from literature | RARP versus RRP | Costs modeled in decision tree analyses | Direct and indirect costs, including purchase cost and amortization of robot | Hospital | Mean RARP cost: $7280 Mean RRP cost: $5554 Difference: $1726 (no adjustment for inflation done) Data are based on one individual hospital and may not be generalizable to other settings. Risk of bias moderate due to uncertainty of the data abstracted from the literature. |
| Scales (2005) [25] | NA | Cost modeling | Intrainstitutional costs were obtained from billing department, supplemented by data from literature | RARP versus RRP | Costs modeled in decision analytic model | Direct and indirect costs including purchase cost and amortization of robot | Hospital | RARP cost: $8929 (2004) RRP cost: $8734 (2004) Difference:$195 (2004) Above number for calculation in specialist setting. Additional findings: RARP cost mainly depended on LOS and cost of room and board. Data are based on one individual hospital and may not be generalizable to other settings. Risk of bias moderate due to uncertainty of the data abstracted from the literature. |
| Mouraviev (2007) [21] | 137 RARP 197 RRP | Retrospective cohort study | Institutional database 2002–2005 | RARP versus RRP | Total direct hospital costs and grand total hospital costs including direct, indirect expenses | Obtained direct and indirect costs from local hospital accounting (cost of robot and maintenance not included) | Hospital | Mean RARP cost: $10 047 Mean RRP cost: $10 704 Difference: -$657 (no adjustment for inflation done, statistically significant difference) Risk of bias is high. No adjusted models were constructed. RRP patients were slightly older. |
| Lotan (2010) [63] | 246 RARP 157 RRP | Retrospective cohort study | Private hospital billing and accounting data 2000–2008 | RARP versus RRP | Costs estimated based on local hospital accounting system | Total, direct, and indirect costs (cost of robot and maintenance not included) | Hospital | Comorbidities were not reported. Mean RARP cost: $10 269 Mean RRP cost: $6473 Difference: $3796 (no adjustment for inflation done) RARP not profitable for hospital. Data are based on one individual hospital and may not be generalizable to other settings. Hospital cost accounting likely differs from hospital to hospital. Risk of bias is high because patient differences are not reported and accounted for. |
| Bolenz (2010) [64] | 262 RARP 156 RRP | Retrospective cohort study | Institutional database 2003–2008 | RARP versus RRP | Direct costs obtained from the hospital billing department | Direct costs (cost of robot and maintenance not included) | Hospital | Mean RARP direct cost: $6749 (2007) Mean RRP direct cost: $4514 (2007) Difference RARP versus RRP:$2235 (2007) Risk of bias high because the above costs were not adjusted for patient factors. Study was focused on impact of BMI, not on cost comparison between the two modalities. |
| Kim (2013) [65] | 20 424 RARP 9413 open RP | Retrospective cohort study | Nationwide Inpatient Sample and American Hospital Association Annual Survey 2006–2008 | RARP versus open RP | Costs estimated based on cost-to-charge ratio | Direct and indirect costs | Hospital | Median RARP cost: $10 409 (2008) Median open RP cost: $8862 (2008) Adjusted costs $2142 (2008) were higher for RARP than for open RP (adjusted for age, race, comorbidity, insurance, income by zip code, year of surgery, hospital teaching status, location, region, and case volume). Risk of bias moderate as residual confounding may be present and cost-to-charge ratios do not completely reflect true cost. |
| Yu (2012) [66] | 11 513 RARP 9704 open RP (weighted sample sizes) | Retrospective cohort study | Nationwide Inpatient Sample 2008 | RARP versus open RP | Cost calculated by applying cost-to-charge ratios | Only charges measured | Hospital | Median adjusted RARP cost: $10 804 (2008) Median adjusted open RP cost: $9 693 (2008) Difference: $1111 (2008) Cost adjusted for measured patient and hospital characteristics. Risk of bias moderate as unmeasured confounding may have been present and charges are unlikely to reflect true cost. |
| Hall (2014) [67] | 100 RARP 100 RRP | Retrospective cohort study | Institutional data 2007–2009 | RARP versus RRP | Institutional costing reports | Probably direct and indirect costs, but not clearly described (cost of robot and maintenance likely not included) | Hospital | RARP mean cost: A$17 582 (2008) RRP mean cost: A$13 605 (2008) Difference: A$3977 (2008) Generalizability is limited outside of Royal Brisbane Hospital. Risk of bias high because costs were not adjusted for patient characteristics and the number of patients included is low. |
| Tomasze wski (2012) [68] | 115 RARP 358 RRP | Retrospective cohort study | Institutional data 2009–2010 | RARP versus RRP | Cost accounting from the hospital finance department | Direct and indirect costs including purchase and maintenance costs of the robot | Hospital | Mean RARP cost: $14 006 Mean RRP cost: $8686 Difference: $5320 (no adjustment for inflation done, statistically significant difference) Risk of bias high as cost was not adjusted for patient characteristics. Differences in patient characteristics between RARP and RRP patients were not reported. |
| Chang (2015) [69] | 150 921 RARP 338 448 non-RARP (weighted sample) | Retrospective cohort study | Premier Hospital Database, USA 2003–2010 | RARP versus non-RARP | 90-d direct hospital costs (unclear how exactly they were measured) | Direct costs | Hospital | RARP cost: $10 622 (2010) Non-RARP cost: $8681 (2010) Difference: $1941 (2010) Risk of bias high because the above costs were not adjusted for patient factors. |
| Chughtai (2015) [70] | ~850 000 RPs, breakdown of RARP versus RRP not given | Retrospective cohort study | Nationwide Inpatient Sample 2000–2011 | MIRP versus RRP | Charge data and cost-to-charge ratios | Only charges measured | Hospital | Median MIRP cost: $11 145 (2010) Median RRP cost: $8918 (2010) Difference: $2227 (2010) MIRP costs significantly higher than RRP costs when adjusting for patient demographics, comorbidities, LOS, and hospital characteristics ($ not given). Risk of bias moderate, because charges are unlikely to reflect true cost. |
| Fabbro (2014) [71] | 53 RARP 50 RRP | Retrospective cohort study | Institutional data 2009–2010 | RARP versus RRP | Costs were based on the use of consumable equipment, surgical and anesthesia equipment, staff, transfusions using internal hospital data | Direct and indirect costs were included, including purchase cost, amortization, and maintenance of robot | Hospital | RARP cost: €11 272 RRP cost: €4834 Difference: €6438 Risk of bias is high, because no adjustments for patient characteristics were made and the number of patients included is low. |
| Faiena (2015) [72] | 24 636 RARP 13 590 RRP | Retrospective cohort study | Nationwide Inpatient Sample 2009–2011 | RARP versus RRP | Charge data and cost-to-charge ratios | Only charges measured | Hospital | RARP was more expensive than RRP in all regions of the USA except for the northeast. This persisted after adjusting for patient characteristics. Risk of bias moderate, because charges are unlikely to reflect true cost. |
| Yanamadala (2016) [73] | 28 301 RARP 8393 open RP | Retrospective cohort study | HCUP State Inpatient and Amubulatory Surgery Databases for CA, FL, NY 2009–2011 | RARP versus open RP | Cost calculated by applying cost-to-charge ratios | Only charges measured | Hospital | Mean RARP cost: $13 615 (2011) Mean open RP cost: $12 167 (2011) Difference: $1448 (2011) Risk of bias high, because cost not adjusted for patient-level differences and charges are unlikely to reflect true cost. |
| Leow (2016) [23] | 311 135 RARP 318 458 RRP (weighted sample) | Retrospective cohort study | Premier Hospital Database 2003–2013 | RARP versus RRP | 90-d direct hospital costs | Direct costs only | Hospital | Adjusted 90-d RARP cost: $14 897 (2014) Adjusted 90-d RRP cost: $9558 (2014) Difference: $4528 (2014) Cost difference not significant among the highest-volume surgeons (≥2 cases/wk). Risk of bias moderate as residual confounding may be present. |
| Basto (2016) [24] | 2646 MIRP 2702 RRP | Cost modeling | Victorian Admitted Episode Dataset 2010–2013 | MIRP versus RRP | Costs modeled based on DRG, LOS, indirect costs, blood transfusion costs | Direct and indirect costs, including purchase cost and maintenance of robot | Hospital | Incremental cost of RARP when LOS and blood transfusion cost offset was applied: A$3548 (2013) for da Vinci SI dual model. Cost neutrality reached with ~200 RPs per year. Risk of bias high because patient factors were not accounted for. |
| Gagnon (2014) [74] | 200 RARP 200 RRP | Retrospective cohort study | Institutional data Years from which data were pulled are not provided | RARP versus RRP | Cost estimation including additional OR time, disposable supply, depreciation, and service contracts. No additional details given on how this was calculated | Direct and indirect costs, including amortization and maintenance cost of robot | Hospital | Added cost of CAN$5629 per case for RARP. Risk of bias is high as no details on cost calculation are given in the manuscript. It does not appear as if cost was adjusted for patient characteristics. |
| Bijlani (2016) [29] | NA | Cost modeling | Review of the published literature to estimate outcomes | RARP versus RRP | Costs modeled based on care pathways and published government and other sources Reimbursements for procedure, complications, functional outcomes, and adjuvant treatment within 3 yr | Direct and indirect costs, including purchase and maintenance cost of robot | Hospital | RARP cost $341 (2014) more than RRP. |
| Payer | RARP saved $1451 (2014), mainly because of lower complication, incontinence, and sexual dysfunction costs. | |||||||
| Societal | RARP saved $1202 due to faster recovery and less lost wages. | |||||||
| Risk of bias and potential for COI is high as this study was conducted by Intuitive Surgical employees. Note: In the payer perspective analyses, no sensitivity analyses are presented, which would have been helpful as estimates around incontinence and sexual dysfunction vary a lot in the literature. |
||||||||
| Studies from the Daver’s perspective | ||||||||
| Burgess (2006) [75] | 78 RARP 16 RRP | Retrospective cohort study | Institutional database 2002–2004 | RARP versus RRP | Hospital charges obtained from patient bills | Only charges measured | Payer | Mean RARP charges: $39 315 (2003) Mean RRP charges: $31 518 (2003) Difference RARP versus RRP: $7797 (2003) Risk of bias high because the above costs were not adjusted for patient factors and because the number of included patients was low. |
| Nguyen (2011) [39] | N not given for MIRP and RRP | Retrospective cohort study | SEER-Medicare 2002–2005 | MIRP versus RRP | Medicare payments in the year after diagnosis minus those in the year prior to diagnosis | Medicare payments | Payer | Mean MIRP cost: $16 762 (2008) Mean RRP cost: $16 469 (2008) Difference: $293 (2008) Risk of bias high. No adjusted models were constructed. MIRP patients were of better socioeconomic status and had lower-risk disease. Additionally, Medicare reimbursement varies by many intentional factors other than type of treatment, including, for example, price differences based on regional wage disparities, cost of living, illness severity, and expense of caring for underinsured patients [16]. |
| Prasad (2011) [76] | 1548 MIRP 5565 RRP | Retrospective cohort study | SEER-Medicare 2002–2005 | MIRP versus RRP | 1. Total Medicare payments from day of surgery through 6 mo thereafter 2. Prostate cancer-related Medicare payments from day of surgery through 6 mo thereafter | Medicare payments | Payer | Mean MIRP cost: $14 939 Mean RRP cost: $14 301 Difference: $638 (no adjustment for inflation done, statistically significant difference) Limiting to claims with prostate cancer diagnosis code: Mean MIRP cost: $12 289 Mean RRP cost: $11 884 Difference: $405 Risk of bias is high. No adjusted models were constructed for the cost estimation. Additionally, Medicare reimbursement varies by many intentional factors other than type of treatment, including, for example, price differences based on regional wage disparities, cost of living, illness severity, and the expense of caring for underinsured patients [16]. |
| Lowrance (2012) [77] | 991 MIRP 4454 RRP | Retrospective cohort study | SEER-Medicare 2003–2005 | MIRP versus RRP | Medicare payments for all claims during the year after day of hospital admission for prostatectomy | Medicare payments | Payer | Mean MIRP cost: $16 919 (2006) Mean RRP cost: $15 692 (2006) Difference: $1227 (2006) Difference not statistically significant. In adjusted analyses, MIRP cost was approximately 2% greater, but difference was not statistically significant. Risk of bias moderate as residual confounding may have been present. Additionally, Medicare reimbursement varies by many intentional factors other than type of treatment, including, for example, price differences based on regional wage disparities, cost of living, illness severity, and the expense of caring for underinsured patients [16]. |
| Shih (2012) [31] | 3144 MIRP 7525 RRP | Retrospective cohort study | IMS Life Link Health Plan Claims Database 2003–2007 (managed care plan enrollees) | MIRP versus RRP | Payments allowed by the health plan (amount paid by the health plan plus patient copay) within the first 6 mo after surgery | Health plan payments | Payer | Mean MIRP cost: $22 837 (2010) Mean RRP cost: $21 914 (2010) Difference: $923 (2010) Adjusted 6 mo cost difference was $8 (95% CI −952 to 969). Risk of bias is moderate, as residual confounding may have been present. |
| Hofer (2013) [78] | 23 473 MIRP 118 266 RRP | Retrospective cohort study | Nationwide Inpatient Sample 2002–2008 | MIRP versus RRP | Charges directly examined | Only charges measured | Payer | MIRP mean charge: $34 000 (2008) RRP mean charge: $32 000 (2008) Difference: $2000 (2008) Charges are unlikely to be meaningful because hospitals can freely set charge master rates. While most markups fall into the 1.5–4 range relative to Medicare allowable cost, the markup may be more than 10 times that cost [19]. |
| Abdollah (2011) [26] | 1188 MIRP 3354 RRP | Population-based retrospective cohort | Florida Hospital Inpatient data 2008 | MIRP versus RRP | Total undiscounted charges | Only charges measured | Payer | Median MIRP charge: $33 234 (2008) Median RRP charge: $33 674 (2008) Difference MIRP versus RRP: -$440 (2008) Charges lower for MIRP than RRP if a high case volume, higher if a low case volume. Risk of bias high, because charges were not adjusted for patient factors (eg, age, comorbidity, SES). |
| Epstein (2013) [32] | 10 032 MIRP 13 778 RRP | Retrospective cohort study | Truven MarketScan database 2000–2009 (employer-sponsored health plans) | MIRP versus RRP | 1. Health plan expenditures on medical care, including both medical and pharmacy costs 2. Days absent from work | Expenditures were measured (presumably representing both direct and indirect costs) | Payer | MIRP spending significantly higher than that for RRP in adjusted analyses ($1350 in the 1st year after surgery, 2009). |
| Societal | Days absent from work significantly shorter after MIRP than RRP in adjusted analyses (9 d). Risk of bias is moderate, as unobserved confounding may have affected results. |
|||||||
| Cooperbe rg (2013) [35] | NA | Cost utility analyses | Review of the published literature to estimate outcomes | RARP versus RRP | Decision-analytic Markov model to evaluate QALYs, and lifetime costs. Costs for visits and procedures derived from the 2009 National Medicare Fee schedule | Direct costs and sensitivity analyses incorporating cost to patient (time off work) | Payer | Direct medical RARP cost:$8547 (2009) Direct medical RRP cost: $8056 (2009) Difference RARP versus RRP: $491 (2009) No significant difference in QALYs comparing RARP and RRP. No significant difference in mean discounted lifetime costs comparing RARP and RRP. Risk of bias low, as sensitivity analyses changing many assumptions did not change the outcome of the analyses. |
| Anderson (2012) [79] | 12 588 RARP 8968 RRP | Population-based retrospective cohort | Nationwide Inpatient Sample 2008–2009 | RARP versus RRP | Total charges | Only charges measured | Payer | Mean RARP charge: $38 542 (2009) Mean RRP charge: $33 934 (2009) Difference RARP versus RRP: $4608 (2009) After adjustment, RARP charges remained statistically significantly higher than RRP charges (exact number not given). RARP had shorter LOS (1 d). Risk of bias moderate, because charges are unlikely to reflect true cost. |
| Gandaglia (2014) [80] | 3476 RARP 2439 open RP | Retrospective cohort study | SEER-Medicare 2008–2009 | RARP vs open RP | Medicare expenditures in the year after surgery minus those in the year prior to surgery | Medicare expenditures measured | Payer | RARP median expenditure: $13 395 (2009) Open RP median expenditure: $11 970 (2009) Difference: $1425 (2009) Risk of bias is high, because Medicare reimbursement varies by many intentional factors other than type of treatment, including, for example, price differences based on regional wage disparities, cost of living, illness severity, and the expense of caring for underinsured patients [16]. |
| Kim (2015) [33] | 8981 MIRP 8629 RRP | Retrospective cohort study | IMS LifeLink Health Plan Claims Database 2003–2010 | MIRP versus RRP | Hospital reimbursement (amount allowed by insurance) | Expenditures were measured (presumably representing both direct and indirect costs) | Payer | Median MIRP reimbursement: $16 661 (2010) Median RRP reimbursement: $14 784 (2010) Adjusted mean hospital reimbursement was $1945 (2010) more per case for MIRP versus RRP (adjusted for age, comorbidity, region, and year). Risk of bias moderate as residual confounding may be present. |
| Eldefrawy (2013) [36] | NA | Cost modeling | Primarily based on institutional data 2010 | RARP versus RRP | Markov model to evaluate the cost of complications and recurrence Inpatient costs derived from internal institutional data Costs for visits and procedures derived from the 2010 Florida Medicare reimbursement | Costs were based on Medicare reimbursement rates | Payer | 10-yr cost RARP: $22 762 (2010) 10-yr cost RRP: $15 084 (2010) Difference RARP versus RRP: $7678 (2010) Risk of bias is moderate because no sensitivity analyses were performed to test the impact of the many assumptions on the outcome of the cost model. |
| Hyams (2013) [81] | 1499 RARP 2565 RRP | Retrospective cohort study | State of Maryland Health Service Cost Review Commission discharge database 2008–2011 | RARP versus RRP | Likely charges were examined, although this is not explicitly stated | Likely charges examined | Payer | Mean RARP charges: $14 000 (2011) Mean RRP charges: $10 100 (2011) Difference: $3900 (2011) Significant difference persists after adjusting for age, race, patient complexity, LOS, ICU stay, insurance status. Risk of bias is moderate because residual confounding is likely present. Charges are unlikely to be meaningful because hospitals can freely set charge master rates. While most markups fall into the 1.5-4 range relative to Medicare allowable cost, the markup may be more than 10 times that cost [19]. |
| Niklas (2016) [27] | 122 RARP 700 RRP | Retrospective cohort study | German Statutory Health Insurance database 2009–2012 | RARP versus RRP | German Statutory Health Insurance payments over 4 yr (2009–2012) | German Statutory Health Insurance payments | Payer | Mean RARP cost: €21 674 Mean RRP cost: €24 512 Difference: −€2865 Risk of bias high: 4 yr of cost after diagnosis and treatment was examined. There could have been many reasons for why costs were lower among men undergoing RARP, including higher socioeconomic status and overall better health in this group of men, none of which were accounted for in the analyses. |
| Hughes (2016) [28] | 5858 RARP 7806 open RP | Retrospective cohort study | National UK Hospital Episode Statistics data 2008–2013 | RARP versus open RP | Costs based on National Health Service tariffs for 2013 | Includes direct and indirect costs, only after discharge from hospital | Payer | RARP mean f/u cost at 1 yr: £1679 (2013) Open RP mean f/u cost at 1 yr: £2031 (2013) Difference: −£352 (2013) Risk of bias is high. No adjusted models were constructed. Healthier patients may have chosen RARP explaining the cost savings. |
| Bijlani (2016) [29] | NA | Cost modeling | Review of the published literature to estimate outcomes | RARP versus RRP | Costs modeled based on care pathways and published government and other sources Reimbursements for procedure, complications, functional outcomes and adjuvant treatment within 3 yr | Direct and indirect costs | Hospital | RARP cost $341 (2014) more than RRP. |
| Payer | RARP saved $1451 (2014), mainly because of lower complication, incontinence, and sexual dysfunction costs. | |||||||
| Societal | RARP saved $1202 due to faster recovery and less lost wages. | |||||||
| Risk of bias and potential for COI is high as this study was conducted by Intuitive Surgical employees. Note: In the payer perspective analyses, no sensitivity analyses are presented, which would have been helpful as estimates around incontinence and sexual dysfunction vary a lot in the literature. |
||||||||
| Studies from the societal perspective | ||||||||
| O’Malley (2007) [37] | NA | Cost-utility evaluation | Local accounting data, estimation of QALY from the literature | RARP versus RRP | Actual costs from local hospital (including direct and indirect cost) 2005 | Directly obtained direct and indirect costs from local hospital accounting | Society | Cost per QALY A$24 457 (clearly below the generally accepted range) Risk of bias is deemed high: gain in QALY by robotic prostatectomy was quite optimistic, using only data from one study (Menon et al, Urology, 2005). No sensitivity analyses were performed. |
| Hohwü (2011) [38] | 77 RARP 154 RRP | Retrospective cohort study | Institutional data and data from the literature | RARP versus RRP | Economic evaluation calculating incremental cost-effectiveness ratio per successful surgery and per QALY | Includes direct and indirect costs | Societal perspective | RARP mean cost: €21 780 (2008) RRP mean cost: €16 328 (2008) Difference: €5452 (2008) ICER for successful surgery (cancer control, no incontinence, erectile function): €77 857 No QALY benefit for RARP over RRP based on SF-36. Study limited by a low number of patients included and by significant amount of uncertainty surrounding the proportion of patients treated with “successful surgery”. |
| Epstein (2013) [32] | 10 032 MIRP 13 778 RRP | Retrospective cohort study | Truven MarketScan database 2000–2009 (employer sponsored health plans) | MIRP versus RRP | 1. Health plan expenditures on medical care, including both medical and pharmacy costs | Expenditures were measured (presumably representing both direct and indirect costs) | Payer | MIRP spending significantly higher than that for RRP in adjusted analyses ($1350 in the 1st year after surgery, 2009). |
| Societal | Days absent from work significantly shorter after MIRP than RRP in adjusted analyses (9 d). Risk of bias is moderate, as unobserved confounding may have affected results. |
|||||||
| Bijlani (2016) [29] | NA | Cost modeling | Review of the published literature to estimate outcomes | RARP versus RRP | 2. Days absent from work Costs modeled based on care pathways and published government and other sources Reimbursements for procedure, complications, functional outcomes, and adjuvant treatment within 3 yr |
Direct and indirect costs | Hospital | RARP cost $341 (2014) more than RRP. |
| Payer | RARP saved $1451 (2014), mainly because of lower complication, incontinence, and sexual dysfunction costs. | |||||||
| Societal | RARP saved $1202 (2014) due to faster recovery and less lost wages. | |||||||
| Risk of bias and potential for COI is high as this study was conducted by Intuitive Surgical employees. | ||||||||
| Note: in the payer perspective analyses, no sensitivity analyses are presented, which would have been helpful as estimates around incontinence and sexual dysfunction vary a lot in the literature. | ||||||||
| Study with unclear perspective | ||||||||
| Sugihara (2014) [82] | 2126 RARP 7202 open RP | Retrospective cohort study | DPC database (Japanese inpatient administrative claims database) 2012–2013 | RARP versus open RP | Specific cost definition not provided | Unclear | Median RARP cost: $15 676 | Sugihara (2014) [82] Median open RP cost: $10 946 Difference: $4730 (no adjustment for inflation done) In adjusted model, RARP 52% more costly than open RP. Risk of bias moderate as residual confounding may have been present. Unclear what kind of cost was examined and which perspective was taken, as no details given in manuscript. |
BMI = body mass index; CI = confidence interval; COI = conflict of interest; DPC = Diagnosis Procedure Combination; DRG = diagnosis-related group; f/u = follow-up; ICER = incremental cost effectiveness ratio; ICU = intensive care unit; LOS = length of stay; MIRP = minimally invasive radical prostatectomy; NA = not applicable; OR = operating room; QALY = quality-adjusted life year; RARP = robot-assisted radical prostatectomy; RP = radical prostatectomy; SEER = Surveillance, Epidemiology, and End Results; SES = socioeconomic status; SF-36 = Short-form Health Survey 36.
MIRP was used in claims-based analyses when the codes did not allow for differentiation between RARP and pure laparoscopic prostatectomy. In these cases, the assumption was made that the vast majority of MIRPs are RARPs.
Note: for any cost data, the year to which inflation adjustment was made is indicated in parenthesis. Studies are sorted by perspective taken and then listed in chronological order based on the years included in the study.
3.1.2. RARP versus RRP from the payer’s perspective
There were 16 studies comparing the cost of RARP and RRP from the payer’s perspective (Table 1). These studies examined data from 2002 through 2014 and came from three different countries, including the USA (n = 14), Germany (n = 1), and the UK (n = 1). Twelve of the 16 studies found an increased cost for RARP, ranging from $293 to $7797 more per case. Larger absolute differences in cost were found in (1) studies that examined charges and (2) studies that did not adjust for patient differences, contributing to this wide range of estimates. Four studies found a reduced cost for the payer when comparing RARP with RRP. Their results were likely affected by high case volumes for RARP [26], lack of adjustment for patients’ general health status and socioeconomic status [27,28], and assumptions that went into cost modeling [29].
There was substantial heterogeneity across studies, likely because of variations in the methodologies. Three of the four studies examining Medicare payments found fairly small differences in cost to the payer between RARP and RRP. All four studies did not take steps to adjust for intentional differences in Medicare payments that may have affected the results. Medicare payments vary by many intentional factors other than the type of treatment, including price differences based on regional wage disparities, cost of living, illness severity, and the expense of caring for underinsured patients [30]. Hospital reimbursement by other US health plans was examined in three studies, two of which found significantly higher reimbursement for RARP in adjusted analyses [31–33].
Five studies examined hospital charges. However, charges are unlikely to be meaningful because hospitals can freely set charge master rates. While most markups fall into the 1.5–4 range relative to Medicare allowable cost, the markup may be more than 10 times that cost [34]. Finally, three studies constructed models to estimate cost, based on data from the literature and reimbursement rates [29,35,36]. Two of these studies did not perform sensitivity analyses to test how the assumptions would affect cost from the payer’s perspective [29,36].
In summary, from the payer’s perspective, RARP is likely somewhat more expensive than RRP. However, RARP has the potential to save costs for payers when accounting for care over a longer postoperative period and when optimal cancer and quality-of-life outcomes with RARP are achieved.
3.1.3. RARP versus RRP from the societal perspective
Four studies addressed cost for RARP from the societal perspective (Table 1). These were published more recently than the other studies (2007–2016). Two studies were from the USA, and one each from Australia and Denmark. The Australian study found a cost per QALY gained of approximately A$24 000 with RARP. However, the authors used very optimistic outcome data from only one study and did not perform sensitivity analyses [37]. The study from Denmark calculated the ICER for one successful surgery comparing RARP with RRP, rather than calculating the ICER per QALY gained—a somewhat unusual approach. Successful surgery was defined as achieving cancer control without incontinence or erectile dysfunction. They estimated an ICER per extra successful surgery of ~€78 000 [38]. This study was limited by the significant amount of uncertainty surrounding the assumptions on postoperative outcomes [38]. One study found a shortened sick leave after RARP compared with RRP, but did not estimate the economic impact of that difference [32]. Finally, a comprehensive study found that RARP saved approximately $1200 per case due to faster recovery and fewer lost wages [29]. This study used a systematic review of the literature to estimate model inputs and constructed detailed care pathways and cost models, but there is a high risk of conflict of interest because it was conducted and funded by Intuitive Surgical, the manufacturer of the surgical robot [29]. Nevertheless, it is reasonable to conclude that RARP at least has the potential for a moderate cost advantage from the societal perspective if optimal outcomes are achieved, allowing patients to return to work more quickly.
3.1.4. Quality of the evidence
The studies comparing the cost of RARP and RRP were all observational in nature. There were some inconsistencies across studies, but those could largely be explained by differences in study designs. The risk of bias was mostly deemed moderate or high. Based on this, we feel that the overall quality of the evidence is low, indicating that the true cost difference between RARP and RRP may be substantially different from that reported in the reviewed studies [16].
3.2. IMRT versus 3D-CRT
3.2.1. IMRT versus 3D-CRT from the payer’s perspective
We identified nine studies comparing the cost of IMRT and 3D-CRT from the payer’s perspective, while there were no studies from the hospital or societal perspective (Table 2). The lack of studies from the hospital perspective may be due to the fact that radiotherapy is usually provided on an outpatient basis, and capturing the related equipment, overhead, and personnel costs may be more complex than in the setting of inpatient surgery with RARP. The studies spanned the years 2005–2016 and originated in five different countries, including the USA (n = 4), Canada (n = 2), Australia (n = 1), the UK (n = 1), and Hungary (n = 1). Seven of the nine studies found an increased cost for IMRT, ranging from $381 to $26 066 more per case. The wide range of cost differential was affected by the time horizon of the cost analyses (eg, incorporating costs in the 12 mo since diagnosis [39] vs the lifetime of the patient after treatment [40]), health system of the country in which the study took place, whether or not the start-up phase of IMRT was incorporated, and the specific costs being measured (eg, Medicare reimbursements only [41] vs reimbursement costs along with the costs of equipment, supplies, personnel, and overhead [40]). The study with the lowest cost difference ($381) specifically assessed a mature IMRT program [42]. When it examined a scenario in which IMRT was in a start-up phase, the incremental cost of IMRT increased by a factor of 11 to $4268 [42]. Two studies found that IMRT costs less than 3D-CRT. Their findings were likely driven by a long time horizon (10 and 20 yr [43,44]), lack of inclusion of capital costs [44], and decreased estimated need for salvage hormone therapy and chemotherapy [43]. These studies originated in Australia and Hungary, and may not be generalizable to the patient populations in other countries.
Table 2. Studies comparing cost of IMRT and 3D-CRT.
| Study | Patients, no. | Type of study | Data source | Comparison | Cost definition | Costs included (direct vs indirect) | Perspective | Main findings including comment on risk of bias |
|---|---|---|---|---|---|---|---|---|
| Studies from the payer’s perspective | ||||||||
| Konski (2005) [41] | NA | Cost modeling (10-yr timeline) | Hospital billing data, patient questionnaires | IMRT versus 3D-CRT | Costs modeled in decision tree analyses (Markov model) | Direct costs (Medicare reimbursements); did not look at copays or deductibles | Payer (Medicare) | 70 yr old with intermediate risk Mean IMRT cost: $33 837 (2004) Mean 3D-CRT cost: $21 377 (2004) Difference: $12 460 (2004) ICER: $16 182/QALY 70 yr old with good risk Mean IMRT cost: $31 950 (2004) Mean 3D-CRT cost: $19 213 (2004) Difference: $12 737 (2004) ICER: $17 448/QALY Sensitivity analysis found that a longer time horizon and younger age favorably impacted the cost-effectiveness ratio. Probabilistic sensitivity analysis was performed to address uncertainty concerning cost, transition probabilities, and utilities using a second-order Monte Carlo simulation. Risk of bias moderate due to uncertainty of the data abstracted from the literature. |
| Konski (2006) [83] | NA | Cost modeling | Literature review, patient questionnaires, or preference at institution | IMRT versus 3D-CRT | Costs modeled in decision tree analyses (Markov model) | Direct costs | Payer (Medicare) | 70 yr old with intermediate-risk prostate cancer Mean IMRT cost: $47 931 (2004) Mean 3D-CRT cost: $21 865 (2004) Difference: $26 066 (2004) ICER: $40 101/QALY IMRT has ~53% probability of being cost effective if the ICER for deciding cost effectiveness is $50 000/QALY. The results, however, are dependent on the assumptions of improved biochemical disease-free survival with fewer patients undergoing salvage therapy and improved QOL after treatment. The results are sensitive to the time horizon of the analysis and the utilities used to inform the model. Probabilistic sensitivity analysis was performed to address uncertainty concerning cost, transition probabilities, and utilities using a second-order Monte Carlo simulation. Risk of bias moderate due to uncertainty of the data abstracted from the literature. |
| Nguyen (2011) [39] | N not given for IMRT and 3D-CRT | Retrospective cohort study | SEER-Medicare 2002–2005 | IMRT versus 3D-CRT | Medicare payments in the year after diagnosis minus those in the year prior to diagnosis | Medicare payments (did not look at indirect costs) | Payer (Medicare) | Mean IMRT cost in 2002:$37 125 (2008) Mean 3D-CRT cost in 2002: $22 384 (2008) Difference: $14 741 (2008) Mean IMRT cost in 2005: $31 574 (2008) Mean 3D-CRT cost in 2005: $20 588 (2008) Difference: $10 986 (2008) Risk of bias high. No adjusted models were constructed. IMRT patients were younger, healthier, more often nonwhite; live in higher educated and more urban areas; live in areas with higher median incomes; do not live in the south or midwest; and are less likely to have higher stage disease. |
| Hummel (2012) [84] | NA | Cost modeling (lifetime perspective) | Data from systematic review | IMRT versus 3D-CRT | Costs modeled in decision tree analyses | Direct and indirect costs | UK National Health Service perspective | Baseline age 70 yr Mean IMRT cost: £5921 (2009) Mean 3D-CRT cost: £4799 (2009) Difference: £1122 (2009) In scenarios where survival is greater with IMRT, IMRT was cost effective (ICER <£20 000). When only a difference in late GI toxicity was assumed, the ICER was highly sensitive to uncertain model parameters. Univariate sensitivity analyses performed on key parameters, such as age, incremental cost of IMRT in comparison with 3D-CRT, and duration of late GI toxicity. Limitation: There are limited clinical data comparing IMRT and 3D-CRT. Risk of bias moderate due to uncertainty of the data abstracted from the literature. |
| Yong (2012) [40] | NA | Cost modeling (lifetime time horizon) | Data from systematic review and survey from radiation oncologists and physicists for estimated pretreatment preparation time | IMRT versus 3D-CRT | Costs estimated through activity-based costing with input from radiation oncologists, physicists, and treatment planners (Markov model) | Direct costs plus cost of equipment (capital, construction, maintenance and operating costs), cost of supplies, personnel, and overhead costs | Payer (Canadian health care system) | Baseline age 70 yr Mean IMRT cost: C$14 520 (2009) Mean 3D-CRT cost: C$13 509 (2009) Difference: C$1019 (2009) ICER: C$26 768/QALY Assumes equal biochemical survival between IMRT and 3D-CRT, but lower frequency of GI toxicity for IMRT Assumes a treatment dose of >70 Gy. Cost estimate is for a mature program. Sensitivity analyses were conducted to assess the robustness of the model results and to evaluate the cost effectiveness of IMRT in different scenarios. Limitations: Assumes the same dose. Some feel that IMRT’s advantage lies not in its technique but in the ability to give a higher dose; cost of GI toxicity based on a survey of a few radiation oncologists. Risk of bias moderate due to uncertainty of the data abstracted from the literature. |
| Carter (2014) [44] | NA | Cost modeling (20-yr timeline) | Data from systematic review, a series of component studies, and a workshop | IMRT versus 3D-CRT in the postprostatecto my setting | Markov decision model | Direct health care costs (did not include capital costs) | Australian health care system | Average patient age 65 yr Mean IMRT cost: $32 816 Mean 3D-CRT cost: $33 917 Difference: $−1101 ICER: $41 572/QALY (no year for inflation adjustment) Performed a series of one-way and probabilistic sensitivity analysis. IMRT was found to be more effective and less costly than 3D-CRT. However, the differences are small, and cost utility analyses over a long follow-up period are highly dependent on assumptions about toxicity and costs of treating these. Limitation: Heavy reliance on clinical judgment to generate inputs for the model due to a lack of published evidence. Risk of bias moderate due to uncertainty of the data abstracted from the literature. |
| Cooperbe rg (2013) [35] | NA | Cost utility analyses | Review of the published literature to estimate outcomes | IMRT versus 3D-CRT | Decision-analytic Markov model to evaluate QALYs, and lifetime costs. Costs for visits and procedures derived from the 2009 National Medicare Fee schedule | Direct costs and sensitivity analyses incorporating cost to patient (time of work) | Payer (Medicare) | Average patient age 65 yr Direct medical IMRT cost: $27 084 (2009) Direct medical 3D-CRT cost: $13 013 (2009) Difference: $14 071 (2009) IMRT was found to be significantly more effective than 3D-CRT among patients with low-risk disease (0.5 QALYs gained). No ICER calculated. Limitation: Multiple assumptions underlie the model. Risk of bias low, as sensitivity analyses changing many assumptions did not change the outcome of the analyses. |
| Yong (2016) [42] | NA | Cost modeling | Data from literature review and consulting with radiation oncologists, physicists, and radiation therapists | IMRT versus 3D-CRT | Costs estimated through activity-based costing | Direct costs plus cost of equipment (capital, construction, maintenance and operating costs), cost of supplies, personnel, and overhead costs | Payer (Canadian health care system) | Mean IMRT cost: C$12 834 (2009) Mean 3D-CRT cost: C$12 453 (2009) Difference: C$381 (2009) Tested various scenarios by varying the program maturity and the use of volumetric modulated arc therapy alongside IMRT, longer dosimetry time for IMRT, longer quality assurance for IMRT. In the start-up scenario, the incremental cost of IMRT increased by a factor of 11 ($4268 vs $381), which highlights the importance of evaluating timing. Risk of bias moderate due to cost estimates derived from two centers in Ontario. |
| Zemplényi (2016) [43] | NA | Cost modeling (10-yr time line) | Data from systematic reviews and interviews with radiation oncologists and physicists | High-dose IMRT and hypofractionated IMRT versus 3D-CRT | Costs modeled in decision tree analyses (Markov model) | Direct and indirect costs | Public payer perspective (Hungary) | Average age 70 yr Mean high-dose IMRT cost: €6831 Mean 3D-CRT cost: €7160 Difference high-dose IMRT: €−329 ICER high-dose IMRT: €−1624/QALY (no year for inflation adjustment) Significant differences in the cost structure and cost levels of hospitals in central and eastern European countries are noted compared with the USA and Canada. In Hungary, every type of EBRT is categorized into the same DRG and so has the same reimbursement. As such, they applied a microcosting method to calculate the real cost of 3D-CRT and IMRT. A series of one-way sensitivity analyses were performed for key parameters; a probabilistic sensitivity analysis was performed to assess the uncertainty in the cost-effectiveness analysis by varying model assumptions simultaneously. Limitation: The study was conducted under the assumption that higher doses can only safely be delivered with IMRT; thus, more favorable tumor control with IMRT is not linked to the therapeutic technique, but the escalated doses. Risk of bias is high as treatment costs were determined based on the data from one institution and uncertainty surrounds the assumptions about treatment effectiveness. |
GI = gastrointestinal; ICER = incremental cost effectiveness ratio; IMRT = intensity-modulated radiotherapy; QALY = quality-adjusted life year; QOL = quality of life; GI = gastrointestinal; DRG = diagnosis-related group; 3D-CRT = three-dimensional conformal radiotherapy; NA = not applicable; SEER = Surveillance, Epidemiology, and End Results.
Note: For any cost data, the year to which inflation adjustment was made is indicated in parenthesis. IMRT studies are sorted by perspective taken and listed in chronological order based on the years included in the study.
Six of the nine studies performed cost-utility analyses in which they reported an ICER. The ICERs ranged from $16 182/QALY to $41 572/QALY when comparing IMRT with 3D-CRT. This implies that even though IMRT, in many cases, was costlier than 3D-CRT, it was also more effective and—to this end—was generally thought to be a more cost-effective treatment. The caveat is that an ICER is dependent on the assumptions about the toxicity and cancer control differences between the two treatments, which varied widely between studies. However, these studies all performed a variety of sensitivity analyses to test the robustness of their findings across a range of parameters. In summary, the majority of studies found IMRT to be more expensive from a payer’s perspective, but also noted that it was still the preferred strategy from a cost-effectiveness perspective, when cost effectiveness is defined by an ICER of <$50 000/QALY. A wide range of costs were included in the analyses (eg, Medicare reimbursements only vs inclusion of capital and other costs), and assumptions on differences in toxicity and cancer control between the two treatments varied widely.
3.2.2. Quality of the evidence
All studies comparing IMRT with 3D-CRT were observational in nature with substantial inconsistencies across studies, which could be largely explained by differences in the populations examined and in study design. Thus, we rate the overall quality of evidence as low, indicating that the true cost difference between IMRT and 3D-CRT may be substantially different from that reported in the reviewed studies [16].
3.3. Proton beam therapy versus IMRT
3.3.1. Proton beam therapy versus IMRT from the payer’s perspective
Three studies compared the costs of proton beam therapy and IMRT (two from the payer’s, one from the societal, and none from the hospital’s perspective; Table 3). The two studies that examined the cost effectiveness of proton beam therapy from a payer’s perspective were both from the USA. The first study found an ICER of $63 578/QALY for a 70 yr old and $55 726/QALY for a 60 yr old [45]. The second study examined 553 Medicare beneficiaries who received proton beam therapy and 27 094 patients who received IMRT in 2008 and 2009 [4]. After matching IMRT and proton beam therapy patients based on known confounders, the study found the median sum of Medicare reimbursements in the 3 mo after treatment to be $32 428 for proton beam therapy and $18 575 for IMRT. Patients in this study who received proton beam therapy were younger, healthier, and from more affluent areas. There were no differences in the two treatments in terms of gastrointestinal and genitourinary complications at 12 mo, but proton beam therapy was significantly costlier [4].
Table 3. Studies comparing cost of proton beam therapy and IMRT.
| Study | Patients, no. | Type of study | Data source | Comparison | Cost definition | Costs included (direct vs indirect) | Perspective | Main findings including comment on risk of bias |
|---|---|---|---|---|---|---|---|---|
| Studies from the payer’s perspective | ||||||||
| Konski (2007) [45] | NA | Cost modeling | Data from literature and patient interviews | Proton versus IMRT | Costs modeled in decision tree analyses (Markov model) | Direct costs | Payer’s (Medicare) perspective | 70 yr old (15-yr cost) Proton cost: $63 511 (2005) IMRT cost: $36 808 (2005) Difference: $26 703 (2005) ICER: $63 578/QALY 60 yr old (15-yr cost) Proton cost: $64 989 (2005) IMRT cost: $39 355 (2005) Difference: $25 634 (2005) ICER: $55 726/QALY Model calculated cost effectiveness based on a third-party payer (Medicare) and did not include upfront costs or yearly operational costs. Sensitivity analyses included how many years the model was run, patient’s age, probability of freedom from biochemical failure for proton and IMRT, utility of patients treated with salvage hormone therapy, and treatment costs. Risk of bias high due to uncertainty of the data abstracted from the literature. |
| Yu (2013) [4] | 553 Proton 27 094 IMRT | Retrospective cohort study | Retrospective study of Medicare beneficiaries from 2008 to 2009 (Medicare data) | Proton versus IMRT | Medicare payments | Direct costs; did not account for indirect costs, such as long travel distances for proton patients | Payer | Median Medicare reimbursement Proton: $32 428 IMRT (matched group): $18 575 Difference: $13 853 Cost of Proton and IMRT was calculated using the sum of Medicare reimbursements for all outpatient and physician claims with HCPCS codes indicative of radiotherapy, including treatment planning, management, and delivery, in the 3 mo following initiation of radiation. No adjustments for inflation, although only a 2-yr study. Used Mahalanobis matching to account for known confounders; could not use propensity score or instrumental variable analysis due to small proton numbers. Risk of bias moderate as residual confounding may be present. |
| Study from the societal perspective | ||||||||
| Lundkvist (2005) [46] | NA | Cost modeling | Data from literature | Proton versus conventional (IMRT) | Costs modeled in decision tree analyses | Direct and indirect costs, including purchase cost of proton and IMRT facilities, amortization of facilities, transportation and hotel accommodations for proton patients | Societal (Sweden) | Mean proton cost: €13 491 (2002) Mean IMRT cost: €5477 (2002) Difference: €8014 (2002) The cost effectiveness of a proton facility depends on the total patient population treated (and hence the number of patients treated with different types of cancers (eg, prostate, breast, head and neck, childhood medulloblastoma). This article assumed that 300 prostate cancer patients were treated per year. The cost per QALY was about €26 776. Limitations: Information about the clinical effects of proton therapy was very limited; also lack of information on health economic data (ie, costs and QOL in patients treated with radiotherapy); as a consequence, the estimates used in the assessment had to be based on more or less uncertain assumptions. Assessment based on an assumed lifetime of 30 yr for proton facility. This could be shortened by the introduction of other new technologies or lengthened by improvements in the facilities. Risk of bias high due to uncertainty of the data abstracted from the literature. |
ICER = incremental cost effectiveness ratio; IMRT = intensity-modulated radiotherapy; NA = not applicable; QALY = quality-adjusted life year; QOL = quality of life.
Note: for any cost data, the year to which inflation adjustment was made is indicated in parenthesis. Studies are listed in chronological order based on the years included in the study.
3.3.2. Proton beam therapy versus IMRT from the societal perspective
A Swedish study examined the costs of proton beam therapy and IMRT from a societal perspective [46]. They used a Markov model to assess cost effectiveness and found that the mean cost of proton beam therapy was €13 491 compared with €5477 for IMRT, resulting in an increased cost per QALY of €26 776. They accounted for both direct and indirect costs, including the purchasing costs of proton beam and IMRT facilities, amortization of these facilities, and transportation and hotel accommodation. The authors concluded that proton beam therapy may be cost effective if appropriate patients are selected, such as those with a higher risk of death from cancer [46]. However, their analyses were hindered by limited clinical effectiveness data, leading to uncertainty in their assumptions [46]. In summary, there is little doubt that proton beam therapy is costlier than IMRT. However, it remains unclear whether this increased cost is worth it because the comparative data on important outcomes such as cancer control and quality of life remain very limited in the literature.
3.3.3. Quality of the evidence
All studies were observational and found a higher cost for proton beam therapy. There were inconsistencies in the point estimates that could be explained by different populations and different time horizons. Two studies relied on cost modeling. One study included patient-specific data, but only 553 proton beam therapy patients were included, increasing the risk for imprecise estimates. Thus, the quality of the evidence on cost of proton therapy versus IMRT is very low, indicating that the true effect is likely substantially different from the estimates of the included studies [16].
3.4. Uncertainties surrounding the benefits of new technologies
Studies addressing the cost utility or cost benefit from the payer’s or societal perspective depend on whether outcomes are improved by the use of new technology and on the magnitude of that improvement. The main outcomes of interest include cancer control, urinary function, and sexual function. There have been several systematic reviews of the literature addressing these outcomes for RARP. Regarding cancer control, a meta-analysis performed in 2012 showed clinically and statistically equivalent positive margin rates with RARP and RRP (95% confidence interval [CI] for difference ranging from −1.9% to 2.4% after propensity score adjustment) [9]. Similar positive surgical margin rates were also found in a recent randomized trial from Australia (90% CI for difference ranging from −1% to 11%) [47]. Biochemical recurrence rates have largely been equivalent between these two surgical approaches [48].
In a meta-analysis of observational studies, RARP appears to have a statistically significant albeit small advantage over RRP when comparing urinary function, with 12-mo incontinence rates of 7.5% compared with 11.3% (absolute risk reduction 3.8%, p = 0.03) [49]. However, these cumulative data were driven by results from only two studies with only one of them using a validated questionnaire to assess urinary continence [49]. Regarding erectile function, patients undergoing RARP appear to have an increased likelihood to recover potency at 12 mo based on a cumulative analysis of six observational studies (recovery rate of 75.8% for RARP vs 52.2% for RRP, p = 0.002) [50]. However, the quality of these six observational studies was limited, as they defined potency as an erection sufficient for intercourse, which is neither a very objective nor a reproducible definition [50]. More recently, a randomized trial from Australia compared functional outcomes between RARP and RRP at 12 wk and found no significant differences between the two techniques [47].
One meta-analysis that included 23 studies compared outcomes after IMRT and 3D-CRT. IMRT was associated with decreased acute (risk ratio 0.59; 95% CI 0.44–0.78) and late gastrointestinal toxicity (risk ratio 0.54; 95% CI 0.38–0.78), improved biochemical control (risk ratio 1.17; 95% CI 1.08–1.27), and no change in late genitourinary toxicity or overall survival [51]. IMRT was associated with a slight increase in acute genitourinary toxicity (risk ratio 1.08; 95% CI 1.00–1.17) [51]. The authors concluded that IMRT may be a better choice, but cautioned that their analysis included heterogeneous studies and that the potentially increased risk of secondary malignancies with IMRT needed further evaluation [51,52]. The comparative effectiveness of IMRT and proton beam therapy is unclear. There are no completed randomized clinical trials between these two modalities, although there is one ongoing randomized trial examining gastrointestinal toxicity among patients with low and intermediate-risk disease (NCT01617161) [53]. Several retrospective studies have shown that proton beam therapy likely has similar rates of urinary and bowel toxicity to IMRT [54–57], while another study showed more gastrointestinal toxicity with proton beam therapy [58]. In short, more clinical information is needed before the true effectiveness of proton beam therapy is known.
In summary, given the overall low level of evidence with new technologies, there is significant uncertainty whether the increased treatment costs are associated with better outcomes. If ideal outcomes can be achieved on a population level, RARP and IMRT have the potential to be cost effective, but this is difficult to assess given the many patient-, physician-, and system-level factors that affect outcomes after prostate cancer treatment.
4. Conclusions
In conclusion, treatment with new technologies is costlier than treatment with traditional technologies. However, given the overall low quality of evidence and the inconsistencies across studies, precise differences in costs remain unclear. Moreover, attempts to estimate whether this increased cost is worth it are hampered by the uncertainty surrounding improvements in outcomes, such as cancer control and side effects of treatment. However, understanding the value of treatment with new technologies will become increasingly important as society and policy makers are moving toward accountable care organizations, bundled payments, and value-based reimbursement [59]. Since value is defined as outcomes relative to cost [60], we will need more accurate ways of capturing cost and outcomes of treatment using approaches such as time-driven activity-based costing [61] and prospective population-based prostate cancer registries [62]. We encourage clinicians and patients to participate in such registries whenever possible.
Supplementary Material
Patient summary.
We review the cost and cost effectiveness of robot-assisted radical prostatectomy, intensity-modulated radiotherapy, and proton beam therapy in prostate cancer treatment. These technologies are costlier than their traditional counterparts. It remains unclear whether their use is associated with improved cure and reduced morbidity, and whether the increased cost is worth the expense.
Robot-assisted radical prostatectomy, intensity-modulated radiotherapy, and proton beam therapy for prostate cancer cost more than their traditional counterparts. Uncertainty surrounding improvements in outcomes limits our ability to estimate cost effectiveness. If the new technologies can consistently achieve better outcomes, then they may be cost effective.
Acknowledgments
We would like to acknowledge research librarians Loretta M. Grikis and Heather B. Blunt for their outstanding assistance with the systematic literature search.
Funding/Support and role of the sponsor: Florian Schroeck is supported in part by the Department of Veterans Affairs, Veterans Health Administration, VISN1 Career Development Award, the Dow-Crichlow Career Development Award in Surgery, Department of Surgery, Dartmouth-Hitchcock Medical Center, and the Conquer Cancer Foundation Career Development Award. Bruce Jacobs is supported in part by the National Institutes of Health Institutional KL2 award (KL2TR001856), the GEMSSTAR award (R03AG048091), and the Jahnigen career development award. Opinions expressed in this manuscript are those of the authors and do not constitute official positions of the U.S. Federal Government or the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Florian Rudolf Schroeck had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Schroeck, Jacobs.
Acquisition of data: Schroeck.
Analysis and interpretation of data: Schroeck, Jacobs.
Drafting of the manuscript: Schroeck, Jacobs.
Critical revision of the manuscript for important intellectual content: Bhayani, Nguyen, Penson, Hu.
Statistical analysis: None.
Obtaining funding: Schroeck, Jacobs.
Administrative, technical, or material support: Schroeck.
Supervision: Schroeck, Hu.
Other: None.
Financial disclosures: Florian Rudolf Schroeck certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Florian Schroeck is site principal investigator (without compensation) for a clinical trial sponsored by Eleven Biotherapeutics/Viventia. Bruce Jacobs is a consultant for ViaOncology. Paul Nguyen has consulted for Nanobiotix and was an unpaid consultant to Augmenix.
References
- 1.Jacobs BL, Zhang Y, Skolarus TA, Hollenbeck BK. Growth of high-cost intensity-modulated radiotherapy for prostate cancer raises concerns about overuse. Health Aff (Millwood) 2012;31:750–9. doi: 10.1377/hlthaff.2011.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu JC, O’Malley P, Chughtai B, et al. Comparative effectiveness of cancer control and survival after robot-assisted versus open radical prostatectomy. J Urol. 2017;197:115–21. doi: 10.1016/j.juro.2016.09.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahal BA, Chen Y-W, Efstathiou JA, et al. National trends and determinants of proton therapy use for prostate cancer: a National Cancer Data Base study. Cancer. 2016;122:1505–12. doi: 10.1002/cncr.29960. [DOI] [PubMed] [Google Scholar]
- 4.Yu JB, Soulos PR, Herrin J, et al. Proton versus intensity-modulated radiotherapy for prostate cancer: patterns of care and early toxicity. J Natl Cancer Inst. 2013;105:25–32. doi: 10.1093/jnci/djs463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pollack A. Hospitals look to nuclear tool to fight cancer. New York Times; 2007. [Google Scholar]
- 6.Barbash GI, Glied SA. New technology and health care costs—the case of robot-assisted surgery. N Engl J Med. 2010;363:701–4. doi: 10.1056/NEJMp1006602. [DOI] [PubMed] [Google Scholar]
- 7.Efstathiou JA, Gray PJ, Zietman AL. Proton beam therapy and localised prostate cancer: current status and controversies. Br J Cancer. 2013;108:1225–30. doi: 10.1038/bjc.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lotan Y, Cadeddu J, Gettman M. The new economics of radical prostatectomy: cost comparison of open, laparoscopic and robot assisted techniques. J Urol. 2004;172:1431–5. doi: 10.1097/01.ju.0000139714.09832.47. [DOI] [PubMed] [Google Scholar]
- 9.Tewari A, Sooriakumaran P, Bloch DA, Seshadri-Kreaden U, Hebert AE, Wiklund P. Positive surgical margin and perioperative complication rates of primary surgical treatments for prostate cancer: a systematic review and meta-analysis comparing retropubic, laparoscopic, and robotic prostatectomy. Eur Urol. 2012;62:1–15. doi: 10.1016/j.eururo.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 10.Hu JC, Gu X, Lipsitz SR, et al. Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA. 2009;302:1557–64. doi: 10.1001/jama.2009.1451. [DOI] [PubMed] [Google Scholar]
- 11.] Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shamseer L, Moher D, Clarke M, et al. Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 13.Carande-Kulis VG, Maciosek MV, Briss PA, et al. Methods for systematic reviews of economic evaluations for the guide to community preventive services. Am J Prev Med. 2000;18:75–91. doi: 10.1016/s0749-3797(99)00120-8. [DOI] [PubMed] [Google Scholar]
- 14.Morrison A, Polisena J, Husereau D, et al. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28:138–44. doi: 10.1017/S0266462312000086. [DOI] [PubMed] [Google Scholar]
- 15.Centre for Reviews and Dissemination. Systematic reviews: CRD’s guidance for undertaking reviews in healthcare. Centre for Reviews and Dissemination, York University York NHS; 2009. [Google Scholar]
- 16.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–6. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Kind P, Lafata JE, Matuszewski K, Raisch D. The use of QALYs in clinical and patient decision-making: issues and prospects. Value Health. 2009;12:S27–30. doi: 10.1111/j.1524-4733.2009.00519.x. [DOI] [PubMed] [Google Scholar]
- 18.Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn’t it increase at the rate of inflation? Arch Intern Med. 2003;163:1637. doi: 10.1001/archinte.163.14.1637. [DOI] [PubMed] [Google Scholar]
- 19.Marseille E, Larson B, Kazi DS, et al. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93:118–24. doi: 10.2471/BLT.14.138206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiroiwa T, Sung Y-K, Fukuda T, Lang H-C, Bae S-C, Tsutani K. International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ. 2010;19:422–37. doi: 10.1002/hec.1481. [DOI] [PubMed] [Google Scholar]
- 21.Mouraviev V, Nosnik I, Sun L, et al. Financial comparative analysis of minimally invasive surgery to open surgery for localized prostate cancer: a single-institution experience. Urology. 2007;69:311–4. doi: 10.1016/j.urology.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 22.Riley GF. Administrative and claims records as sources of health care cost data. Med Care. 2009;47:S51–5. doi: 10.1097/MLR.0b013e31819c95aa. [DOI] [PubMed] [Google Scholar]
- 23.Leow JJ, Chang SL, Meyer CP, et al. Robot-assisted versus open radical prostatectomy: a contemporary analysis of an all-payer discharge database. Eur Urol. 2016;70:837–45. doi: 10.1016/j.eururo.2016.01.044. [DOI] [PubMed] [Google Scholar]
- 24.Basto M, Sathianathen N, TeMarvelde L, et al. Patterns-of-care and health economic analysis of robot-assisted radical prostatectomy in the Australian public health system. BJU Int. 2016;117:930–9. doi: 10.1111/bju.13317. [DOI] [PubMed] [Google Scholar]
- 25.Scales CD, Jr, Jones PJ, Eisenstein EL, Preminger GM, Albala DM. Local cost structures and the economics of robot assisted radical prostatectomy. J Urol. 2005;174:2323–9. doi: 10.1097/01.ju.0000181830.43340.e7. [DOI] [PubMed] [Google Scholar]
- 26.Abdollah F, Budäus L, Sun M, et al. Impact of caseload on total hospital charges: a direct comparison between minimally invasive and open radical prostatectomy—a population based study. J Urol. 2011;185:855–61. doi: 10.1016/j.juro.2010.10.051. [DOI] [PubMed] [Google Scholar]
- 27.Niklas C, Saar M, Berg B, et al. da Vinci and open radical prostatectomy: comparison of clinical outcomes and analysis of insurance costs. Urol Int. 2016;96:287–94. doi: 10.1159/000431104. [DOI] [PubMed] [Google Scholar]
- 28.Hughes D, Camp C, O’Hara J, Adshead J. Health resource use after robot-assisted surgery vs open and conventional laparoscopic techniques in oncology: analysis of English secondary care data for radical prostatectomy and partial nephrectomy. BJU Int. 2016;117:940–7. doi: 10.1111/bju.13401. [DOI] [PubMed] [Google Scholar]
- 29.Bijlani A, Hebert AE, Davitian M, et al. A multidimensional analysis of prostate surgery costs in the united states: robotic-assisted versus retropubic radical prostatectomy. Value Health. 2016;19:391–403. doi: 10.1016/j.jval.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 30.Miller DC, Gust C, Dimick JB, Birkmeyer N, Skinner J, Birkmeyer JD. Large variations in Medicare payments for surgery highlight savings potential from bundled payment programs. Health Aff Proj Hope. 2011;30:2107–15. doi: 10.1377/hlthaff.2011.0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shih Y-CT, Ward JF, Pettaway CA, et al. Comparative effectiveness, cost, and utilization of radical prostatectomy among young men within managed care insurance plans. Value Health. 2012;15:367–75. doi: 10.1016/j.jval.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Epstein AJ, Groeneveld PW, Harhay MO, Yang F, Polsky D. Impact of minimally invasive surgery on medical spending and employee absenteeism. JAMA Surg. 2013;148:641–7. doi: 10.1001/jamasurg.2013.131. [DOI] [PubMed] [Google Scholar]
- 33.Kim SP, Gross CP, Smaldone MC, et al. Perioperative outcomes and hospital reimbursement by type of radical prostatectomy: results from a privately insured patient population. Prostate Cancer Prostatic Dis. 2015;18:13–7. doi: 10.1038/pcan.2014.38. [DOI] [PubMed] [Google Scholar]
- 34.Bai G, Anderson GF. Extreme markup: the fifty US hospitals with the highest charge-to-cost ratios. Health Aff (Millwood) 2015;34:922–8. doi: 10.1377/hlthaff.2014.1414. [DOI] [PubMed] [Google Scholar]
- 35.Cooperberg MR, Ramakrishna NR, Duff SB, et al. Primary treatments for clinically localised prostate cancer: a comprehensive lifetime cost-utility analysis. BJU Int. 2013;111:437–50. doi: 10.1111/j.1464-410X.2012.11597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eldefrawy A, Katkoori D, Abramowitz M, Soloway MS, Manoharan M. Active surveillance vs. treatment for low-risk prostate cancer: a cost comparison. Urol Oncol. 2013;31:576–80. doi: 10.1016/j.urolonc.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 37.O’Malley SP, Jordan E. Review of a decision by the Medical Services Advisory Committee based on health technology assessment of an emerging technology: The case for remotely assisted radical prostatectomy. Int J Technol Assess Health Care. 2007;23:286–91. doi: 10.1017/S0266462307070390. [DOI] [PubMed] [Google Scholar]
- 38.Hohwü L, Borre M, Ehlers L, Venborg Pedersen K. A short-term cost-effectiveness study comparing robot-assisted laparoscopic and open retropubic radical prostatectomy. J Med Econ. 2011;14:403–9. doi: 10.3111/13696998.2011.586621. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen PL, Gu X, Lipsitz SR, et al. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol. 2011;29:1517–24. doi: 10.1200/JCO.2010.31.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yong JHE, Beca J, McGowan T, Bremner KE, Warde P, Hoch JS. Cost-effectiveness of intensity-modulated radiotherapy in prostate cancer. Clin Oncol. 2012;24:521–31. doi: 10.1016/j.clon.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Konski A. Cost-effectiveness of intensity-modulated radiation therapy. Expert Rev Pharmacoecon Outcomes Res. 2005;5:137–40. doi: 10.1586/14737167.5.2.137. [DOI] [PubMed] [Google Scholar]
- 42.Yong JHE, McGowan T, Redmond-Misner R, et al. Estimating the costs of intensity-modulated and 3-dimensional conformal radiotherapy in Ontario. Curr Oncol. 2016;23:e228–38. doi: 10.3747/co.23.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zemplényi At, Kaló Z, Kovács G, et al. Cost-effectiveness analysis of intensity-modulated radiation therapy with normal and hypofractionated schemes for the treatment of localised prostate cancer. Eur J Cancer Care (Engl) doi: 10.1111/ecc.12430. In press. http://dx.doi.org/10.1111/ecc.12430. [DOI] [PubMed]
- 44.Carter HE, Martin A, Schofield D, et al. A decision model to estimate the cost-effectiveness of intensity modulated radiation therapy (IMRT) compared to three dimensional conformal radiation therapy (3DCRT) in patients receiving radiotherapy to the prostate bed. Radiother Oncol. 2014;112:187–93. doi: 10.1016/j.radonc.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 45.Konski A, Speier W, Hanlon A, Beck JR, Pollack A. Is proton beam therapy cost effective in the treatment of adenocarcinoma of the prostate? J Clin Oncol. 2007;25:3603–8. doi: 10.1200/JCO.2006.09.0811. [DOI] [PubMed] [Google Scholar]
- 46.Lundkvist J, Ekman M, Ericsson SR, Jönsson B, Glimelius B. Proton therapy of cancer: potential clinical advantages and cost-effectiveness. Acta Oncol. 2005;44:850–61. doi: 10.1080/02841860500341157. [DOI] [PubMed] [Google Scholar]
- 47.Yaxley JW, Coughlin GD, Chambers SK, et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomised controlled phase 3 study. Lancet. 2016;388:1057–66. doi: 10.1016/S0140-6736(16)30592-X. [DOI] [PubMed] [Google Scholar]
- 48.Novara G, Ficarra V, Mocellin S, et al. Systematic review and meta-analysis of studies reporting oncologic outcome after robot-assisted radical prostatectomy. Eur Urol. 2012;62:382–404. doi: 10.1016/j.eururo.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 49.Ficarra V, Novara G, Rosen RC, et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol. 2012;62:405–17. doi: 10.1016/j.eururo.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 50.Ficarra V, Novara G, Ahlering TE, et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur Urol. 2012;62:418–30. doi: 10.1016/j.eururo.2012.05.046. [DOI] [PubMed] [Google Scholar]
- 51.Yu T, Zhang Q, Zheng T, et al. The effectiveness of intensity modulated radiation therapy versus three-dimensional radiation therapy in prostate cancer: a meta-analysis of the literatures. PLoS One. 2016;11:e0154499. doi: 10.1371/journal.pone.0154499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hall EJ, Wuu C-S. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol. 2003;56:83–8. doi: 10.1016/s0360-3016(03)00073-7. [DOI] [PubMed] [Google Scholar]
- 53.Proton therapy vs. IMRT for low or intermediate risk prostate cancer PARTIQoL. 2016 https://clinicaltrials.gov/ct2/show/NCT01617161.
- 54.Moon DH, Efstathiou JA, Chen RC. What is the best way to radiate the prostate in 2016? Urol Oncol. 2017;35:59–68. doi: 10.1016/j.urolonc.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 55.Fang P, Mick R, Deville C, et al. A case-matched study of toxicity outcomes after proton therapy and intensity-modulated radiation therapy for prostate cancer. Cancer. 2015;121:1118–27. doi: 10.1002/cncr.29148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gray PJ, Paly JJ, Yeap BY, et al. Patient-reported outcomes after 3-dimensional conformal, intensity-modulated, or proton beam radiotherapy for localized prostate cancer. Cancer. 2013;119:1729–35. doi: 10.1002/cncr.27956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoppe BS, Michalski JM, Mendenhall NP, et al. Comparative effectiveness study of patient-reported outcomes after proton therapy or intensity-modulated radiotherapy for prostate cancer. Cancer. 2014;120:1076–82. doi: 10.1002/cncr.28536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sheets NC, Goldin GH, Meyer A-M, et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA. 2012;307:1611–20. doi: 10.1001/jama.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen C, Ackerly DC. Beyond ACOs and bundled payments: Medicare’s shift toward accountability in fee-for-service. JAMA. 2014;311:673. doi: 10.1001/jama.2014.11. [DOI] [PubMed] [Google Scholar]
- 60.Porter ME. What Is value in health care? N Engl J Med. 2010;363:2477–81. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 61.Laviana AA, Ilg AM, Veruttipong D, et al. Utilizing time-driven activity-based costing to understand the short and long-term costs of treating localized, low-risk prostate cancer: TDABC and low-risk prostate cancer. Cancer. 2016;122:447–55. doi: 10.1002/cncr.29743. [DOI] [PubMed] [Google Scholar]
- 62.Gandaglia G, Bray F, Cooperberg MR, et al. Prostate cancer registries: current status and future directions. Eur Urol. 2016;69:998–1012. doi: 10.1016/j.eururo.2015.05.046. [DOI] [PubMed] [Google Scholar]
- 63.Lotan Y, Bolenz C, Gupta A, et al. The effect of the approach to radical prostatectomy on the profitability of hospitals and surgeons. BJU Int. 2010;105:1531–5. doi: 10.1111/j.1464-410X.2009.08996.x. [DOI] [PubMed] [Google Scholar]
- 64.Bolenz C, Gupta A, Hotze T, et al. The influence of body mass index on the cost of radical prostatectomy for prostate cancer. BJU Int. 2010;106:1188–93. doi: 10.1111/j.1464-410X.2010.09242.x. [DOI] [PubMed] [Google Scholar]
- 65.Kim SP, Shah ND, Karnes RJ, et al. Hospitalization costs for radical prostatectomy attributable to robotic surgery. Eur Urol. 2013;64:11–6. doi: 10.1016/j.eururo.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 66.Yu H, Hevelone ND, Lipsitz SR, Kowalczyk KJ, Hu JC. Use, costs and comparative effectiveness of robotic assisted, laparoscopic and open urological surgery. J Urol. 2012;187:1392–8. doi: 10.1016/j.juro.2011.11.089. [DOI] [PubMed] [Google Scholar]
- 67.Hall RM, Linklater N, Coughlin G. Robotic and open radical prostatectomy in the public health sector: cost comparison. ANZ J Surg. 2014;84:477–80. doi: 10.1111/ans.12097. [DOI] [PubMed] [Google Scholar]
- 68.Tomaszewski JJ, Matchett JC, Davies BJ, Jackman SV, Hrebinko RL, Nelson JB. Comparative hospital cost-analysis of open and robotic-assisted radical prostatectomy. Urology. 2012;80:126–9. doi: 10.1016/j.urology.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 69.Chang SL, Kibel AS, Brooks JD, Chung BI. The impact of robotic surgery on the surgical management of prostate cancer in the USA. BJU Int. 2015;115:929–36. doi: 10.1111/bju.12850. [DOI] [PubMed] [Google Scholar]
- 70.Chughtai B, Scherr D, Del Pizzo J, et al. National trends and cost of minimally invasive surgery in urology. Urol Pract. 2015;2:49–54. doi: 10.1016/j.urpr.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 71.Fabbro E, Crivellaro S, DalleFratte CF, et al. Robot-assisted laparoscopic prostatectomy: an economic analysis for decision-making in a university hospital of Northern Italy. Epidemiol Biostat Public Health. 2014;12:1–9. [Google Scholar]
- 72.Faiena I, Dombrovskiy VY, Modi PK, et al. Regional cost variations of robot-assisted radical prostatectomy compared with open radical prostatectomy. Clin Genitourin Cancer. 2015;13:447–52. doi: 10.1016/j.clgc.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yanamadala S, Chung BI, Hernandez-Boussard TM. Robot-assisted versus open radical prostatectomy utilization in hospitals offering robotics. Can J Urol. 2016;23:8279–84. [PubMed] [Google Scholar]
- 74.Gagnon L-O, Goldenberg SL, Lynch K, Hurtado A, Gleave ME. Comparison of open and robotic-assisted prostatectomy: the University of British Columbia experience. Can Urol Assoc J. 2014;8:92–7. doi: 10.5489/cuaj.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burgess SV, Atug F, Castle EP, Davis R, Thomas R. Cost analysis of radical retropubic, perineal, and robotic prostatectomy. J Endourol. 2006;20:827–30. doi: 10.1089/end.2006.20.827. [DOI] [PubMed] [Google Scholar]
- 76.Prasad SM, Gu X, Lavelle R, Lipsitz SR, Hu JC. Comparative effectiveness of perineal versus retropubic and minimally invasive radical prostatectomy. J Urol. 2011;185:111–5. doi: 10.1016/j.juro.2010.08.090. [DOI] [PubMed] [Google Scholar]
- 77.Lowrance WT, Eastham JA, Yee DS, et al. Costs of medical care after open or minimally invasive prostate cancer surgery: a population-based analysis. Cancer. 2012;118:3079–86. doi: 10.1002/cncr.26609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hofer MD, Meeks JJ, Cashy J, Kundu S, Zhao LC. Impact of increasing prevalence of minimally invasive prostatectomy on open prostatectomy observed in the national inpatient sample and national surgical quality improvement program. J Endourol. 2013;27:102–7. doi: 10.1089/end.2012.0315. [DOI] [PubMed] [Google Scholar]
- 79.Anderson JE, Chang DC, Parsons JK, Talamini MA. The first national examination of outcomes and trends in robotic surgery in the United States. J Am Coll Surg. 2012;215:107–14. doi: 10.1016/j.jamcollsurg.2012.02.005. discussion 114–6. [DOI] [PubMed] [Google Scholar]
- 80.Gandaglia G, Sammon JD, Chang SL, et al. Comparative effectiveness of robot-assisted and open radical prostatectomy in the postdissemination era. J Clin Oncol. 2014;32:1419–26. doi: 10.1200/JCO.2013.53.5096. [DOI] [PubMed] [Google Scholar]
- 81.Hyams ES, Mullins JK, Pierorazio PM, Partin AW, Allaf ME, Matlaga BR. Impact of robotic technique and surgical volume on the cost of radical prostatectomy. J Endourol. 2013;27:298–303. doi: 10.1089/end.2012.0147. [DOI] [PubMed] [Google Scholar]
- 82.Sugihara T, Yasunaga H, Horiguchi H, et al. Robot-assisted versus other types of radical prostatectomy: population-based safety and cost comparison in Japan, 2012–2013. Cancer Sci. 2014;105:1421–6. doi: 10.1111/cas.12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Konski A, Watkins-Bruner D, Feigenberg S, et al. Using decision analysis to determine the cost-effectiveness of intensity-modulated radiation therapy in the treatment of intermediate risk prostate cancer. Int J Radiat Oncol. 2006;66:408–15. [Google Scholar]
- 84.Hummel SR, Stevenson MD, Simpson EL, Staffurth J. A model of the cost-effectiveness of intensity-modulated radiotherapy in comparison with three-dimensional conformal radiotherapy for the treatment of localised prostate cancer. Clin Oncol. 2012;24:e159–67. doi: 10.1016/j.clon.2012.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


