Abstract
Tracheobronchopathia osteochondroplastica is a rare airway disease of unknown aetiology. Due to overlapping symptomology and lack of awareness, the condition is often missed resulting in unnecessary medical or surgical treatment. A male patient presented with a long-standing history of hoarseness and had earlier received treatment for bronchial asthma and tuberculosis. On evaluation, he had typical submucosal calcified nodules distributed throughout the trachea sparing the posterior membranous part. Although the biopsy confirmed the diagnosis of tracheobronchopathia osteochondroplastica in our case, histopathological examination is not always needed to make this diagnosis. Our patient has been kept under conservative management and is having non-progression of disease at 1-year follow-up. After having reviewed the literature related to pathophysiology and management of tracheobronchopathia osteochondroplastica, we emphasise on the fact that the treating physicians’ awareness about this condition is the key to its diagnosis and management.
Keywords: ear, nose And throat/otolaryngology; respiratory medicine; otolaryngology/ent
Background
Tracheobronchopathia osteochondroplastica (TO) is a rare idiopathic disease of the tracheobronchial framework. Most often it is diagnosed incidentally and managed conservatively because of its self-limiting course. However, when symptomatic, it may mimic airway diseases like asthma, chronic obstructive pulmonary disease, sarcoidosis and so on.1 The non-specific symptomology and lack of awareness might lead to misdiagnosis of TO as some other condition resulting in unnecessary treatment.2 Through this case report of TO we are readdressing the classical clinicoradiological features of the condition, which are often diagnostic. Extensive but relevant literature has been presented in a systematic manner to create awareness about the condition.
Case presentation
A 56-year-old male patient came to us with recent worsening of hoarseness which was there with last 10 years. He had no history of aspiration or swallowing difficulty. The patient was on multidose inhaler therapy for bronchial asthma and had taken antitubercular treatment 27 years back for pulmonary tuberculosis. On examination, the supraglottis and the glottis were normal, but there was some nodular thickening in subglottis, as shown in figure 1A. The tracheoscopy also showed similar irregular nodular lesions distributed along the entire length of the trachea, except the posterior wall, as in figure 1B. Crosssection imaging revealed numerous irregular deposits of calcified nodules along the mucosal surface of the trachea, with no significant lumen narrowing, as shown in figure 2. A biopsy was taken from these lesions to rule out any neoplastic or chronic granulomatous aetiology. However, the histopathological examination was suggestive of TO, as shown in figure 3.
Figure 1.
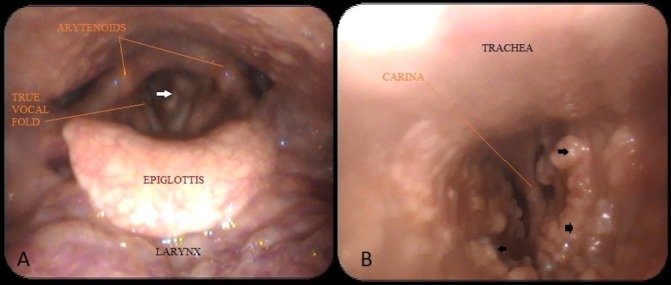
(A) Laryngoscopy showing subglottic narrowing (white arrow). (B) Fibre-optic bronchoscopic image with irregular nodular thickening (black arrows) along the entire length, sparing the posterior wall.
Figure 2.
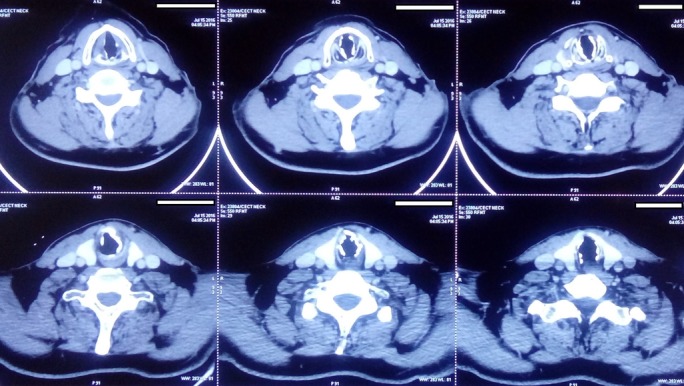
CT images showing irregular submucosal nodular calcification.
Figure 3.
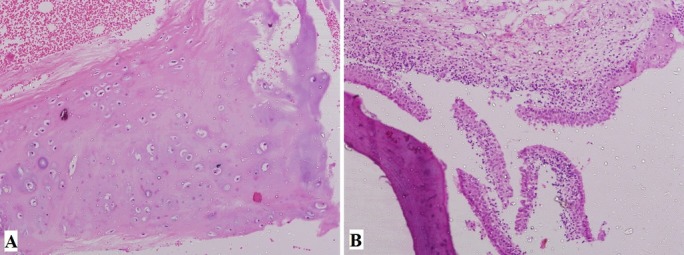
Histopathological pictures. (A) Typical submucosal cartilaginous and osseous deposits underneath the respiratory epithelium (40×). (B) Squamous metaplasia of the ciliated columnar epithelium of respiratory mucosa representing chronic inflammatory reaction (40×).
Outcome and follow-up
The patient was kept under conservative management with the close observation and has a non-progressive course at 1-year follow-up.
Discussion
The reported incidence of TO is 0.1%.3 Although the aetiopathology of this condition is elusive, it is characterised by inflammatory osseocartilaginous deposits underneath the mucosa of the tracheobronchial tree. The infection has been proposed as probable aetiology responsible for TO, as the affected individuals usually have a history of recurrent respiratory infections.4 Reported cases of TO associated with the IgA deficiency also support this theory.5 Certain bacteria has also been isolated from bronchoalveolar lavage of the affected individual.6 TO mostly affects elderly in the sixth decade and has a male predilection.3 7 8 However, there are few reports of TO affecting children.4 6 In the clear majority of the affected individuals, this condition is diagnosed incidentally.9 10 However, symptomatic patients may present with a long-standing history of an unresolved non-productive cough, change in voice and/or breathing difficulty.11–13 Cough is the most common complaint, seen in 66% of the symptomatic patients.14 Due to non-specific and inexplicit symptoms, TO may mimic laryngeal amyloidosis or sarcoidosis clinically. There are reports of TO being misdiagnosed as asthma or chronic obstructive pulmonary disease.1 6 15 Our patient has also received treatment for bronchial asthma and tuberculosis in the past, which could have been avoided by appropriate diagnosis.
The key in identifying the patient with TO is ‘the awareness’ about the condition. All these disease entities have similar and overlapping symptomatology, but the signs of TO are quite peculiar and diagnostic. The fibre-optic bronchoscopy (FOB) reveals characteristic whitish submucosal hard nodules along the walls of the trachea, with pathognomonic sparing of the membranous part. These lesions are ‘rock-garden’ like thickenings representing osseous or metaplastic cartilaginous nodules of 1–3 mm underneath normal respiratory mucosal layer.6 Amyloidosis, sarcoidosis and papillomatosis could also have a similar endoscopic appearance, but none of these would selectively spare posterior membranous wall.
CT shows irregular calcification along the inner lining of the tracheobronchial tree, typically, sparing the posterior membranous trachea.14 16 This nodular lesions on the inner surface of tracheal cartilage are usually sessile and diffuse, but could be focal and may not show calcification at times.14 17 The other conditions with possible similar radiological picture include relapsing polychondritis and old age. However, in contrast to TO, the cartilage gets thickened and deformed without any intraluminal nodules in relapsing polychondritis. The calcification due to ageing is usually uniform and not extensive like that of TO.16
Symptomology of TO may overlap with that of laryngeal amyloidosis, sarcoidosis, respiratory papillomatosis, dystrophic calcification of trachea, relapsing polychondritis and other connective tissue disorders. As the prevalence of TO is rare, these differential diagnoses should be considered while making the diagnosis of TO. However, most of these lesions have clinical appearances and radiological features that are distinct from characteristic clinicoradiological features of TO. Nevertheless, the presence of concomitant systemic features should not be overlooked and, whenever in doubt, further investigations should be done accordingly, which may include the biopsy from the lesion(s).
The histopathological feature of distinct cartilaginous, as well as mineralised osseous elements underneath intact mucosa, confirms the diagnosis of TO, but a biopsy is not necessary in all cases.1 2 18 TO has been found to be associated with certain malignancies like skin cancer and multiple myeloma.3 9 However, this association is probably coincidental as there is no pathophysiological correlation between TO and malignancy.8 Various other diseases like polyarteritis nodosa and IgA deficiency have also been found concomitantly with TO.5 11 In the index case, there were no signs and symptoms of malignancy, bronchiectasis, polyarteritis nodosa or any other previously described associations.
TO is a begin disease with the stable course over years. It doesn't progress in half of the cases on repeated bronchoscopic examinations and most of these patients are managed conservatively without any active treatment.1 4 8 Affected individuals have had a non-progressive course as long as 20 years after diagnosis.19 However, it is important to explain the patients about the condition along with implications and consequences. These patients may have exertional distress and should have adequate treatment available to relieve distress at that time. They should be warned about the risk of respiratory failure during an episode of superadded airway infection, which is due to further narrowing of the lumen.20 Affected individuals are also at a risk of difficult intubation during any elective surgery.10 If such a need arises, patients should be able to discuss the diagnosis with concerned anaesthesiologists to avoid unanticipated complicated airway. Topical steroids and chest physiotherapy could be beneficial in controlling cough and dyspnoea.2 15 Surgical intervention is indicated for lesions causing symptomatic narrowing.11 12 18 Endobronchial Nd:YAG laser, coring with a rigid bronchoscope and endoluminal stents are the various surgical modalities used to provide adequate airway in these cases.21
Patient's perspective.
The patient is very happy to get the right diagnosis and management after many years of onset of symptoms. The patient himself along with family members has voluntarily insisted on this publication so that it generates interests among medical professionals and helps further patients of the condition.
Learning points.
The possibility of tracheobronchopathia osteochondroplastica must be exercised while dealing with tracheobronchial disease, especially in adults with a long-standing history.
The typical clinicoradiological features are often diagnostic, but the treating doctor should be aware of the condition.
This may help in avoiding the morbidity of unnecessary treatment.
Footnotes
Contributors: All the authors: involved in the manuscript preparation. KD, PS and AS: involvedin concepts and design; planning; definition of intellectual content;literature search; clinical work; data acquisition; data analysis; manuscriptpreparation; manuscript editing; and manuscript review.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Luo S, Wu L, Zhou J, et al. Tracheobronchopathia osteochondroplastica: two cases and a review of the literature. Int J Clin Exp Pathol 2015;8:9681–6. [PMC free article] [PubMed] [Google Scholar]
- 2. Wang N, Long F, Jiang S. Tracheobronchopathia Osteochondroplastica: two cases reports and review of Literature. Medicine 2016;95:e3396 10.1097/MD.0000000000003396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agarwal A, Klair J, Joiner A, et al. Nodular trachea: tracheobronchopathia osteochondroplastica. BMJ Case Rep 2015;2015:bcr2015209860 10.1136/bcr-2015-209860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sant'Anna CC, Pires-de-Mello P, Morgado MF, et al. Tracheobronchopathia osteochondroplastica in a 5 year-old girl. Indian Pediatr 2012;49:985–6. 10.1007/s13312-012-0223-1 [DOI] [PubMed] [Google Scholar]
- 5. Dincer HE, Dunitz JM. Tracheobronchopathia osteochondroplastica and selective IgA deficiency. J Bronchology Interv Pulmonol 2012;19:54–6. 10.1097/LBR.0b013e3182446949 [DOI] [PubMed] [Google Scholar]
- 6. Fois AG, Arcadu A, Santoru L, et al. Tracheobronchopathia Osteochondroplastica: a rare case report of a non-smoker and non-atopic patient, with a long history of wheezing since childhood. Multidiscip Respir Med 2016;11:16 10.1186/s40248-016-0050-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Willms H, Wiechmann V, Sack U, et al. Tracheobronchopathia osteochondroplastica: a rare cause of chronic cough with haemoptysis. Cough 2008;4:4 10.1186/1745-9974-4-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leske V, Lazor R, Coetmeur D, et al. Tracheobronchopathia osteochondroplastica: a study of 41 patients. Medicine 2001;80:378–90. [DOI] [PubMed] [Google Scholar]
- 9. Laine M, Elfihri S, Kettani F, et al. Tracheobronchopathia osteochondroplastica associated with skin Cancer: a case report and review of the literature. BMC Res Notes 2014;7:637 10.1186/1756-0500-7-637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Warner MA, Chestnut DH, Thompson G, et al. Tracheobronchopathia osteochondroplastica and difficult intubation: case report and perioperative recommendations for anesthesiologists. J Clin Anesth 2013;25:659–61. 10.1016/j.jclinane.2013.05.010 [DOI] [PubMed] [Google Scholar]
- 11. Uchimura K, Yamasaki K, Yatera K, et al. Multiple Tracheobronchial Polyposis caused by Tracheobronchopathia Osteochondroplastica. Intern Med 2016;55:3165–7. 10.2169/internalmedicine.55.6774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nielsen SW, Stevens JR, Dion GR, et al. Dyspnea, Dysphonia, and Cough: varied presentations of Tracheobronchopathia Osteochondroplastica. Ann Otol Rhinol Laryngol 2015;124:829–33. 10.1177/0003489415586845 [DOI] [PubMed] [Google Scholar]
- 13. Simmons C, Vinh D, Donovan DT, et al. Tracheobronchopathia osteochondroplastica. Laryngoscope 2016;126:2006–9. 10.1002/lary.25813 [DOI] [PubMed] [Google Scholar]
- 14. Ribeiro GM, Natal MR, Silva EF, et al. Tracheobronchopathia osteochondroplastica: computed tomography, bronchoscopy and histopathological findings. Radiol Bras 2016;49:56–7. 10.1590/0100-3984.2014.0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hayes D. Tracheopathia osteoplastica misdiagnosed as asthma. J Asthma 2007;44:253–5. 10.1080/02770900701246782 [DOI] [PubMed] [Google Scholar]
- 16. Jindal S, Nath A, Neyaz Z, et al. Tracheobronchopathia osteochondroplastica--a rare or an overlooked entity? J Radiol Case Rep 2013;7:16–25. 10.3941/jrcr.v7i3.1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Raess PW, Cowan SW, Haas AR, et al. Tracheobronchopathia osteochondroplastica presenting as a single dominant tracheal mass. Ann Diagn Pathol 2011;15:431–5. 10.1016/j.anndiagpath.2011.04.009 [DOI] [PubMed] [Google Scholar]
- 18. Chroneou A, Zias N, Gonzalez AV, et al. Tracheobronchopathia osteochondroplastica. An underrecognized entity? Monaldi Arch Chest Dis 2008;69:65–9. 10.4081/monaldi.2008.398 [DOI] [PubMed] [Google Scholar]
- 19. Brandén E. A 20-year follow-up of a case with tracheobronchopathia osteochondroplastica. J Bronchology Interv Pulmonol 2013;20:84–6. 10.1097/LBR.0b013e31827d13bc [DOI] [PubMed] [Google Scholar]
- 20. Danckers M, Raad RA, Zamuco R, et al. A complication of tracheobronchopathia osteochondroplastica presenting as acute hypercapnic respiratory failure. Am J Case Rep 2015;16:45–9. 10.12659/AJCR.892427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jabbardarjani HR, Radpey B, Kharabian S, et al. Tracheobronchopathia osteochondroplastica: presentation of ten cases and review of the literature. Lung 2008;186:293–7. 10.1007/s00408-008-9088-4 [DOI] [PubMed] [Google Scholar]


