Abstract
Mycoplasmapneumoniae is a common cause of respiratory infections. Although most cases are mild, some patients have extrapulmonary complications including mucocutaneous eruptions including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN) and erythemamultiforme (EM). Recently, a new entity, called M. pneumoniae-induced rash and mucositis (MIRM) was described. The authors present a clinical case difficult to classify attending to the classical classification of epidermolytic syndromes that meets the criteria proposed for the diagnosis of MIRM. The mucocutaneous disease associated with M. pneumoniae presents predominant mucositis, with scarce or absent cutaneous involvement. Because of the distinct morphology, pathophysiology and benign clinical course, MIRM should be considered as a new entity, distinct from SJS/TEN and EM.
Keywords: Dermatology, Pneumonia (infectious Disease), Medical Education
Background
Mycoplasma pneumoniae is a well-known cause of respiratory infections, but has also been associated with extrapulmonary complications.1 In fact, studies show that M. pneumoniae can cause mucocutaneous eruptions in up to one-quarter of all cases.2 The spectrum of dermatological manifestations is wide and includes Raynaud’s disease, erythema nodosum, Kawasaki disease, erythema multiforme (EM), Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN).1 3 4 Recently, Canavan et al have proposed a new entity, distinct from SJS/TEN or EM called M. pneumoniae-induced rash and mucositis (MIRM).5
The authors present a case difficult to allocate into the classical classification of epidermolytic syndromes that meets the criteria proposed by Canavan et al for the diagnosis of MIRM.
Case presentation
The authors present an otherwise healthy 8-year-old boy that recurred to the emergency service for a 5-day history of dry cough and fever (maximum axillary temperature of 39.3°C). A chest radiograph showed an ill-defined opacity in the left lower lobe (figure 1). The patient was diagnosed with atypical pneumonia and treated in ambulatory with clarithromycin (15 mg/kg/day). Over the following 2 days, in addition to persistent fever and respiratory complains, the patient developed mucocutaneous lesions that led to his admission. He took no other medications and there was no evidence of previous herpetic infection.
Figure 1.
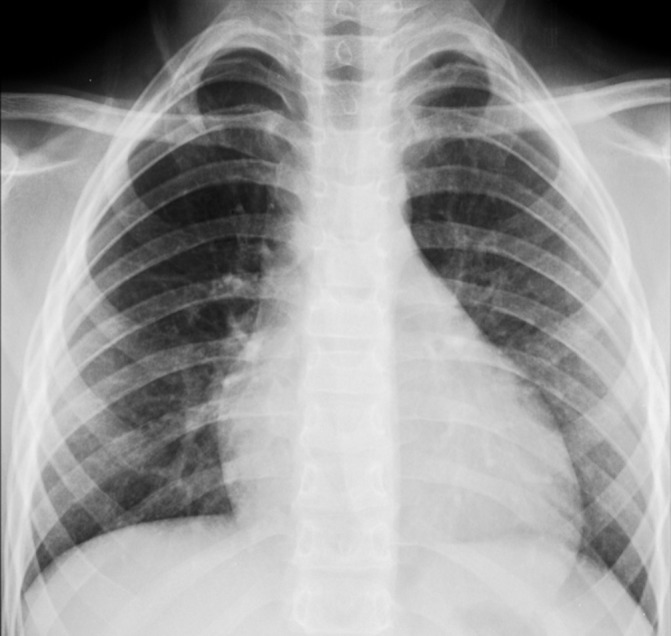
Chest radiograph: note the ill-defined opacity in the left lower lobe.
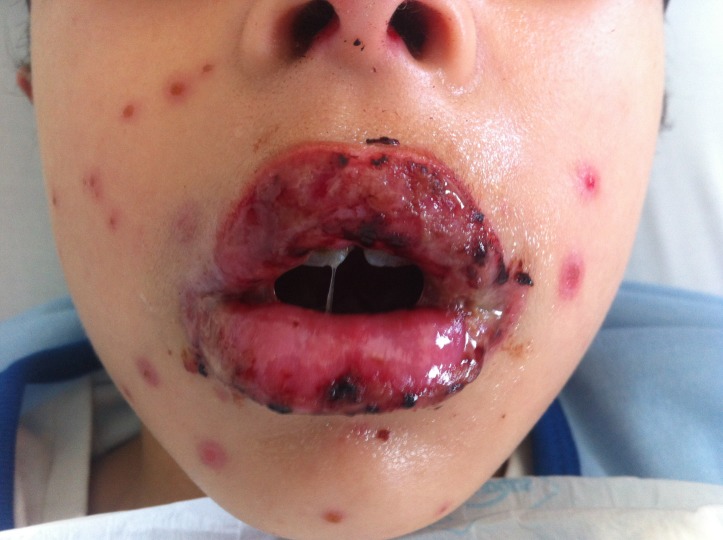
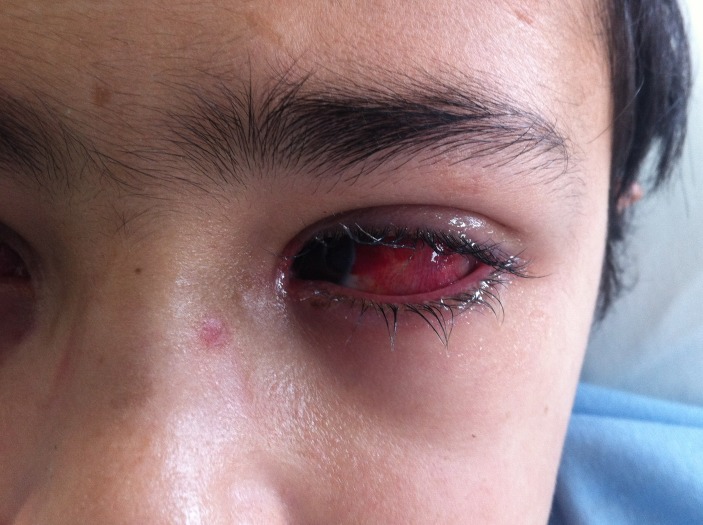
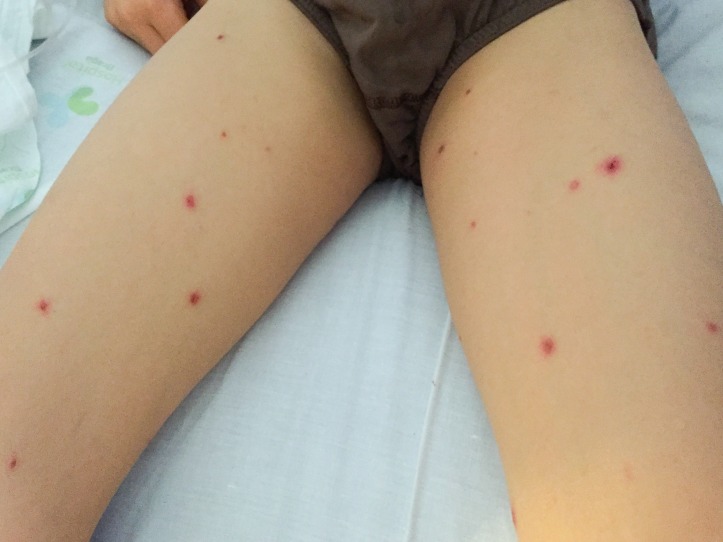
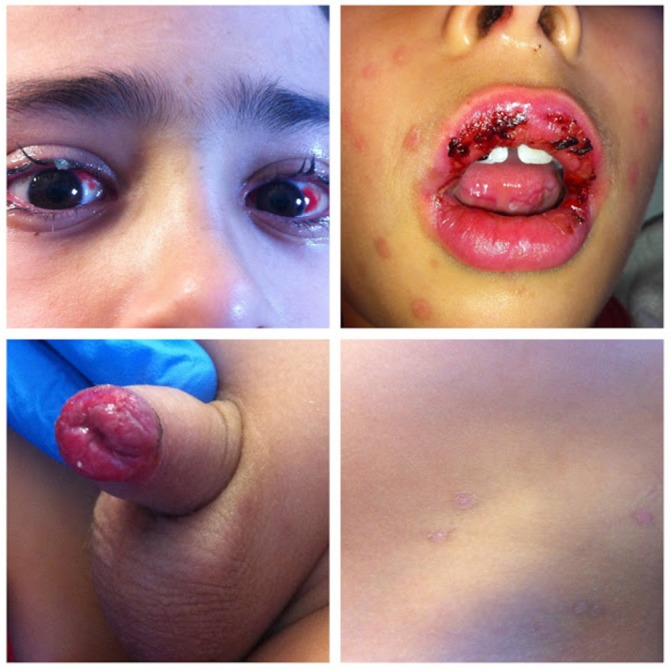
The clinical observation revealed painful erosions on the lips and oral mucosa partially covered by haemorrhagic crusts (figure 2) as well as bilateral conjunctival hyperaemia (figure 3). Erythema and oedema of the glans penis and prepuce that was painful to retract was also found (figure 4). On the remaining skin, scattered asymptomatic erythematous papules and atypical target lesions were noticed symmetrically on the lower limbs and face (figures 2 and 5). The trunk, palms and plants were unaffected.
Figure 2.
Erosions on the lips and oral mucosa partially covered by haemorrhagic crusts. Also note some atypical targets with central bulla on the face.
Figure 3.
Conjunctival hyperaemia.
Figure 4.
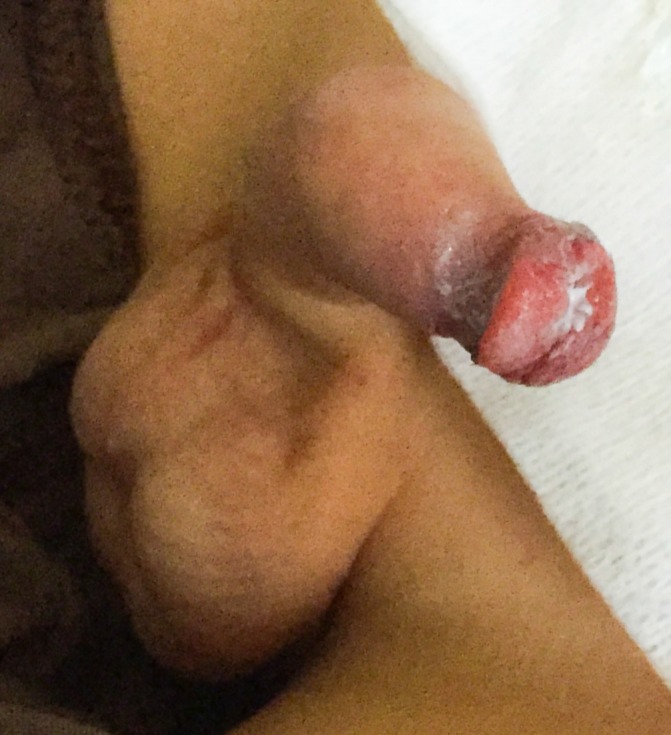
Erythema and oedema of the glans penis and prepuce.
Figure 5.
Scattered erythematous papules and atypical targets symmetrically distributed on the lower limbs.
Investigations
Blood examinations showed leucocytosis (16.1×103 µL) with neutrophilia (78.4%) and elevated C reactive protein levels (12.00 mg/L). The serum enzyme immunoassay revealed a positive M. pneumoniae IgM. Serologic tests for herpes simplex virus, cytomegalovirus and Epstein-Barr virus yielded negative results.
Differential diagnosis
Although the mucosal involvement suggested SJS, there was a sparse cutaneous involvement. That observation allied to the evidence, both clinical and radiological, of atypical pneumonia and the increase in M. pneumoniae IgM antibodies prompted to the diagnosis of a recently described entity of the epidermolytic dermopathies spectrum called MIRM.5
There are some key features that may help to distinguish MIRM from EM or SJS/TEN: first of all, the young age characteristic of most patients with MIRM; second, the nosology (evidence of atypical pneumonia in MIRM as opposed to the herpes simplex virus association of EM and the medication aetiology in SJS/TEN); third and probably most important, the scarce or absent cutaneous involvement in MIRM; and fourth, the better prognosis of MIRM even with just support treatment.5 6
Treatment
Ophthalmological consultation revealed bilateral conjunctival hyperaemia and a corneal ulceration of the right eye. These ophtalmological findings were treated with eye occlusion and topic oxytetracycline ointment. Supportive care including pain management, intravenous hydration and mucosal care (oral suspension of sucralfate for oral mucosa and an ointment containing prednisolone acetate, neomycin sulfate and sodium sulfacetamide for the genital lesions) was also administered.
The patient was treated with intravenous immunoglobulins at a dosage of 1 g/kg/day for 3 days with a rapid improvement in the ocular, oral and urinary symptoms as well as the skin lesions.
Outcome and follow-up
The patient was discharged after 8 days with noticeable clinical improvement (figure 6).
Figure 6.
Clinical improvement of the cutaneous and mucosal lesions at discharge.
Discussion
The classical classification of epidermolytic dermopathies that encompassed EM (minor and major), SJS and TEN as part of a spectrum of disease has been updated, when, in 1993 Bastuji-Garin et al proposed a novel classification: EM as a different entity from SJS/TEN, with its own aetiology, pathophysiology and clinical course.7 Since then, another update was achieved, when, in 2013 the drug reaction with eosinophilia and systemic symptoms syndrome was recognised as a separate entity, outside the SJS spectrum.8
Recently, another breakthrough in this area was made by Canavan et al, in 2015, by describing a new syndrome called MIRM.5 This new entity helps to classify several cases with mucosal findings consistent with SJS, but without (or scarce) cutaneous involvement, preceded by a recognised M. pneumoniae infection. These cases have been classified as ‘atypical’ or ‘incomplete SJS’ but also as ‘Fuchs syndrome’.5
M. pneumoniae is the most common infectious agent associated with acute epidermolytic dermopathies, particularly in children.6 Mucocutaneous eruptions associated with M. pneumoniae infection are more often characterised by moderate to severe involvement of two or more mucosal sites and sparse or even absent skin involvement. However, it is uncertain whether M. pneumoniae-associated mucositis with minimal or no skin involvement represents an atypical SJS variant or is a distinct entity as proposed by Canavan et al.5 6 9 10
In the study performed by Canavan et al, 202 cases compatible with MIRM were found with a male preponderance and a markedly young age (mean 11.9 years old).5 All patients had prodromal symptoms (cough, fever, malaise) the week prior to the eruption.5 MIRM has a pleomorphic presentation, but the most common morphologies are vesiculobullous (77%), targetoid lesions (48%), papules (14%) and macules (12%).5 The cutaneous involvement is sparse (a few scattered lesions) in about half the cases or absent (MIRM sine rash) in one-third of cases.5 When present, the cutaneous lesions have a predominantly acral distribution, but the trunk and face can also be affected.5 The mucosal involvement is critical to the diagnosis, with a mean of 2.5 mucosal sites affected. The oral mucosa is affected in the vast majority of cases (94%, varying from isolated erosions to significant denudation of the entire buccal mucosa), followed by the ocular mucosa (82%, with purulent bilateral conjunctivitis and photophobia) and the genital mucosa (63%, with vesiculobullous and ulcerations).5 There are no guidelines for the treatment of MIRM.5 11 12 The majority of cases are treated with antibiotics, systemic corticosteroids, intravenous immunoglobulin, supportive care or a combination of the above.5 MIRM seems to have an overall good prognosis as the majority of patients recover without sequels and the recurrences are rare (8%).5 Among the possible complications the most common are synechiae (ocular, oral and genital).13 Rare complications do sometimes occur as persistent cutaneous lesions or B-cell lymphopaenia.13 A 3% of fatalities due to pulmonary complications has been published, but these cases (a total of four) refer to a preantibiotic era and therefore does not affect the actual good prognosis of the disease.5
The aetiopathophysiology of MIRM is still unclear, but may be distinct from EM or SJS/TEN. EM is strongly associated with herpes simplex virus and SJS/TEN is traditionally linked to medication exposure (despite other possible causes, including M. pneumoniae infection). MIRM is associated with M. pneumoniae infection. It was hypothesised that antibody production, immunocomplex deposition and complement activation would result in mucocutaneous damage.2 Molecular mimicry between Mycoplasma adhesion molecules and a keratinocyte antigen is also a hypothesis.14 15 This proposed pathophysiology is clearly different from EM and SJS/TEN that likely involves a type IV delayed-type hypersensitivity reaction and cytotoxicity due to a Fas-ligand mechanism.16–19
Canavan et al propose a set of diagnostic criteria for cases of MIRM.5 This encompasses skin detachment of less than 10% of the body surface area, at least two mucosal sites involved, few skin lesions including vesiculobullous or atypical targets and evidence of atypical pneumonia (clinical and laboratorial).5 It should be emphasised the importance of definitive M. pneumoniae identification to the correct diagnosis of MIRM and even more to differentiate from herpes simplex virus-associated EM and drug-associated SJS/TEN.5 20 Our patient met most of this criteria as he was diagnosed with atypical pneumonia, had positive IgM antibodies for M. pneumoniae, had three affected mucosal sites (ocular, oral and genital) and a few scattered atypical targets on the skin. Also the favourable clinical evolution and good outcome is in accordance with the diagnosis.
Attending to the methodic study of Canavan et al, demonstrating MIRM as a separate entity due to the young age of patients, predominance (or only) mucosal involvement and excellent prognosis we highlight the usefulness of this diagnosis to the classification of this case. Previously, a patient like ours, with predominant mucosal involvement but scarce or none cutaneous findings was difficult to classify and if so, was probably termed as ‘incomplete’ SJS. However, SJS, even ‘incomplete’, has a far worse prognosis than MIRM and so an accurate diagnosis is of critical importance for the correct management and prognosis.
Learning points.
Mycoplasma pneumoniae can cause mucocutaneous eruptions, including a new entity from the epidermolytic dermopathies spectrum, called M. pneumoniae-induced rash and mucositis (MIRM).
The mucocutaneous disease associated with M. pneumoniae presents predominant mucositis, with scarce or absent cutaneous involvement.
The aetiopathophysiology of MIRM is still unclear, but may be distinct from erythema multiforme or Stevens-Johnson syndrome/toxic epidermal necrolysis.
The majority of cases are treated with antibiotics, systemic corticosteroids, intravenous immunoglobulins and supportive care.
MIRM has an overall good prognosis as the majority of patients recover without sequels and the recurrences are rare.
Footnotes
Contributors: RPS and MS contributed to the planning, conducting and reporting of the work. RPS, APV and CB contributed to the conception and design of the work. All the authors are responsible for the overall content.
Competing interests: None declared.
Patient consent: Obtained from guardian.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev 2004;17:697–728. 10.1128/CMR.17.4.697-728.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schalock PC, Dinulos JG. Mycoplasma pneumoniae-induced cutaneous disease. Int J Dermatol 2009;48:673–81. 10.1111/j.1365-4632.2009.04154.x [DOI] [PubMed] [Google Scholar]
- 3.Grosber M, Alexandre M, Poszepczynska-Guigné E, et al. Recurrent erythema multiforme in association with recurrent Mycoplasma pneumoniae infections. J Am Acad Dermatol 2007;56(5 Suppl):S118–S119. 10.1016/j.jaad.2006.05.047 [DOI] [PubMed] [Google Scholar]
- 4.Tay YK, Huff JC, Weston WL. Mycoplasma pneumoniae infection is associated with Stevens-Johnson syndrome, not erythema multiforme (von Hebra). J Am Acad Dermatol 1996;35(5 Pt 1):757–60. 10.1016/S0190-9622(96)90732-X [DOI] [PubMed] [Google Scholar]
- 5.Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol 2015;72:239–45. 10.1016/j.jaad.2014.06.026 [DOI] [PubMed] [Google Scholar]
- 6.Vujic I, Shroff A, Grzelka M, et al. Mycoplasma pneumoniae-associated mucositis--case report and systematic review of literature. J Eur Acad Dermatol Venereol 2015;29:595–8. 10.1111/jdv.12392 [DOI] [PubMed] [Google Scholar]
- 7.Bastuji-Garin S, Rzany B, Stern RS, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol 1993;129:92–6. 10.1001/archderm.1993.01680220104023 [DOI] [PubMed] [Google Scholar]
- 8.Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol 2013;169:1071–80. 10.1111/bjd.12501 [DOI] [PubMed] [Google Scholar]
- 9.Schalock PC, Dinulos JG. Mycoplasma pneumoniae-induced Stevens-Johnson syndrome without skin lesions: fact or fiction? J Am Acad Dermatol 2005;52:312–5. 10.1016/j.jaad.2004.07.044 [DOI] [PubMed] [Google Scholar]
- 10.Martínez-Pérez M, Imbernón-Moya A, Lobato-Berezo A, et al. Mycoplasma pneumoniae-Induced Mucocutaneous Rash: A New Syndrome Distinct from Erythema Multiforme? Report of a New Case and Review of the Literature. Actas Dermosifiliogr 2016;107:e47–e51. 10.1016/j.adengl.2016.06.005 [DOI] [PubMed] [Google Scholar]
- 11.Alcántara-Reifs CM, García-Nieto AV. Mycoplasma pneumoniae-associated mucositis. CMAJ 2016;188:753 10.1503/cmaj.151017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varghese C, Sharain K, Skalski J, et al. Mycoplasma pneumonia-associated mucositis. BMJ Case Rep 2014;2014:bcr2014203795 10.1136/bcr-2014-203795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martire B, Foti C, Cassano N, et al. Persistent B-cell lymphopenia, multiorgan disease, and erythema multiforme caused by Mycoplasma pneumoniae infection. Pediatr Dermatol 2005;22:558–60. 10.1111/j.1525-1470.2005.00140.x [DOI] [PubMed] [Google Scholar]
- 14.Baseman JB, Tully JG. Mycoplasmas: sophisticated, reemerging, and burdened by their notoriety. Emerg Infect Dis 1997;3:21–32. 10.3201/eid0301.970103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernald GW. Immunological interactions between host cells and mycoplasmas: an introduction. Rev Infect Dis 1982;4 Suppl:S201–S204. 10.1093/clinids/4.Supplement_1.S201 [DOI] [PubMed] [Google Scholar]
- 16.Chung WH, Hung SI, Yang JY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med 2008;14:1343–50. 10.1038/nm.1884 [DOI] [PubMed] [Google Scholar]
- 17.Kusunoki S, Shiina M, Kanazawa I. Anti-Gal-C antibodies in GBS subsequent to mycoplasma infection: evidence of molecular mimicry. Neurology 2001;57:736–8. 10.1212/WNL.57.4.736 [DOI] [PubMed] [Google Scholar]
- 18.Grimwood R, Huff JC, Weston WL. Complement deposition in the skin of patients with herpes-associated erythema multiforme. J Am Acad Dermatol 1983;9:199–203. 10.1016/S0190-9622(83)70128-3 [DOI] [PubMed] [Google Scholar]
- 19.Safai B, Good RA, Day NK. Erythema multiforme: report of two cases and speculation on immune mechanisms involved in the pathogenesis. Clin Immunol Immunopathol 1977;7:379–85. 10.1016/0090-1229(77)90072-1 [DOI] [PubMed] [Google Scholar]
- 20.Canavan TN, Mathes EF, Frieden IJ, et al. Reply to: "Diagnosing Mycoplasma pneumoniae-induced rash and mucositis (MIRM) in the emergency room". J Am Acad Dermatol 2015;73:e69 10.1016/j.jaad.2015.04.046 [DOI] [PubMed] [Google Scholar]