Abstract
Mycobacterium tuberculosis has spread worldwide and its mortality rate had been very high. The prevention technology and antituberculosis (TB) chemotherapy has improved its prognosis. However, immunocompromised patients, such as those who had HIV infection, older age and on haemodialysis, are still at high risk of TB infection. TB pericarditis is a common cause of constrictive pericarditis and its mortality remains high. Early diagnosis and initiation of appropriate therapy are critical to improve mortality. Additionally, detection of an elevation in the adenosine deaminase level in pericardial effusion is reported to be useful. We report the case of an immunocompromised patient with TB pericarditis, and steroid therapy could prevent him from progressing to constrictive pericarditis. The adenosine deaminase value of pericardial effusion was so helpful that we could promptly make clinical and therapeutic decisions.
Keywords: tb and other respiratory infections, heart failure
Background
Tuberculosis (TB) caused by Mycobacterium tuberculosis has spread worldwide, and its prognosis has improved owing to the introduction of prevention and anti-TB chemotherapy.1 Immunocompromised patients, such as those with HIV infection and those on haemodialysis, have a high risk of TB infection, and the risk has been reported to be 6 to 25-fold higher in these patients than in the general population.2 Additionally, the incidence of TB in patients on haemodialysis has been increasing,3 and there is a possibility of a TB outbreak in these patients.
Tuberculous pericarditis is a significant clinical problem, and it is the most frequent cause of constrictive pericarditis. The mortality and morbidity of tuberculous pericarditis remain high despite anti-TB chemotherapy and we often have difficulty in the diagnosis of tuberculous pericarditis. Early diagnosis and initiation of appropriate therapy are critical to improve mortality.4
We report a case of an immunocompromised patient with tuberculous pericarditis. Early administration of steroid could prevent him from progressing to constrictive pericarditis.
Case presentation
A 49-year-old man with chronic renal failure, who was on dialysis due to diabetic nephropathy, presented with dyspnoea on exertion. He experienced dyspnoea for 8 months before visiting our hospital. His dyspnoea gradually worsened despite intensive body fluid control with haemodialysis. Pericardial effusion was observed on echocardiography, and it gradually increased. He had sinus tachycardia, dyspnoea on effort and dialysis difficulty because of chronic pericardial effusion. He had a history of pulmonary TB, and he was successfully treated with the anti-TB drugs ethambutol, isoniazid, rifampicin and pyrazinamide at 37 years of age.
Chest radiography showed cardiomegaly, and ECG showed complete right bundle branch block (figure 1A). He was therefore admitted to our hospital. At admission, his blood pressure was 155/90 mm Hg, pulse rate was 101 bpm and body temperature was 37.2°C. Additionally, his respiratory rate was 25 bpm breathing room air with oxygen saturation of 96%. Physical examination found jugular venous distention and no peripheral oedema was present in the legs. The results of blood examination were as follows: white blood cell (WBC) count, 5630/μL; haemoglobin, 9.9 g/dL; C reactive protein (CRP), 1.61 mg/mL; brain natriuretic peptide, 292 pg/mL; troponin-I, 0.12 mg/dL; thyroid-stimulating hormone, 3.11 µIU/mL; free T3, 2.0 pg/mL and free T4, 1.06 ng/dL. His serum was negative for antinuclear antibody; however, the QuantiFERON TB-3G (Cellestis, Australia) test result was positive. Transthoracic echocardiography demonstrated preserved left ventricular ejection fraction and a large amount of pericardial effusion (figure 1B). His pericardial effusion was drained via the apical approach with ultrasound guidance, and the drainage tube was inserted for 3 days. Yellow transparent pericardial effusion was collected, and the total amount was 1250 mL. Bacterial culture of the pericardial effusion was negative. Additionally, PCR for M. tuberculosis and acid-fast bacilli culture of the pericardial effusion were negative. Cytology showed that lymphocytes were the dominant component, and no malignancy was noted. The levels of tumour markers (sIL-6, CEA and CA19-9) in the pericardial effusion were within the normal ranges; however, the level of adenosine deaminase (ADA) in the pericardial effusion was elevated (76.2 IU/L; normal range, 6.8–18.2 IU/L). Pericardial effusion did not occur again after drainage. Chest CT showed swelling of mediastinal lymph nodes and residual pericardial effusion (figure 1C). On transthoracic echocardiography performed 35 days after drainage, the pericardium showed partial calcification and an increase in brightness, especially at the posterior and inferior sides of the left ventricle. Additionally, the surface of the heart had a rough appearance with diminished movements (figure 1D). His inflammatory parameters (body temperature, WBC count and CRP level) worsened.
Figure 1.
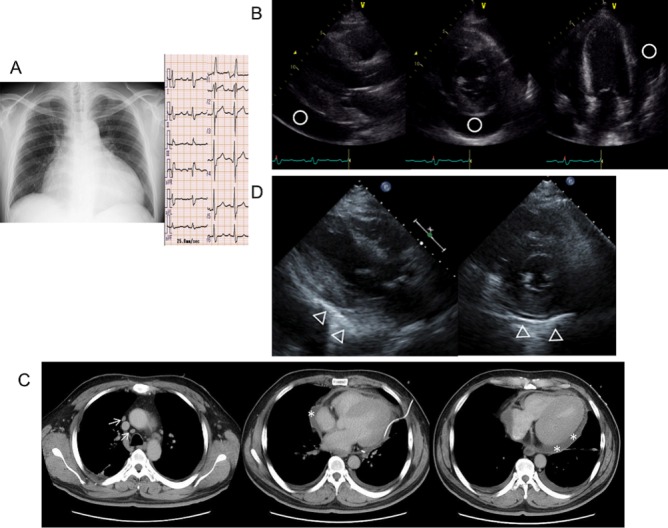
(A) Chest radiography and ECG at admission chest radiography shows cardiomegaly (cardiothoracic ratio, 69.6), and ECG shows sinus tachycardia and complete right bundle branch block. (B) Transthoracic echocardiography at admission. Left ventricular ejection fraction is preserved (53.4%), and there is a large amount of pericardial effusion (○). (C) Chest CT scan after pericardial effusion drainage. There is swelling of the mediastinal lymph nodes (↑) and residual pericardial effusion (*). (D) Transthoracic echocardiography performed 35 days after pericardial effusion drainage. Pericardial effusion is not noted; however, the pericardium shows partial calcification (Δ), especially at the posterior and inferior sides of the left ventricle. Additionally, the surface of the heart has a rough appearance with diminished movements.
We could not clinically diagnose but suspected the patient with tuberculous pericarditis based on the following: (1) his history of pulmonary TB; (2) swelling of multiple mediastinal lymph nodes; (3) partial calcification of the pericardium and (4) elevation of ADA in pericardial effusion. We tried to administer anti-TB drugs (ethambutol 250 mg, isoniazid 200 mg, rifampicin 600 mg and pyrazinamide 0.5 g). His inflammatory parameters improved after treatment and pericardial effusion did not occur. We were able to reduce his dry weight on haemodialysis. His dyspnoea disappeared, and he was discharged from the hospital. However, 24 days after starting anti-TB drug therapy, he showed sinus tachycardia, dyspnoea on effort and dialysis difficulty again without the elevation of his inflammatory parameters and was admitted to the hospital. Transthoracic echocardiography showed pericardial adhesions to the bilateral atria and ventricles. The pericardium of the anterior right ventricle was thickened approximately 4 mm, and that of the lateral part of the left ventricle was found to have calcification (figure 2A). The transmitral flow velocity variation with respiration was 32%, and the transtricuspid flow velocity variation with respiration was 84% (figure 2A), suggesting the development of partial constrictive pericarditis. Cardiac catheterisation showed elevation in the end-diastolic pressures of the bilateral ventricles and dip-and-plateau pattern in the right ventricular pressure (figure 3A). On echocardiography, there was a non-calcified area in the pericardium that adhered to the myocardium and was suspected transient constrictive pericarditis; therefore, we orally administered aspirin (2250 mg) to suppress the inflammation. However, the 7 days administration of aspirin did not have an effect on the inflammation of the pericardium. So we changed from aspirin to prednisolone (60 mg) and tapered off the steroid to half doze by week.
Figure 2.
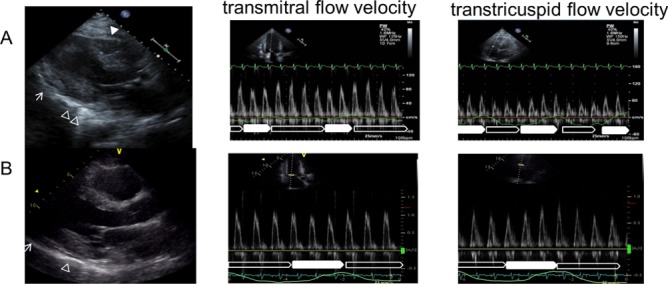
(A) Transthoracic echocardiography performed 14 days after anti-TB drug therapy was started. There is thickening of approximately 4 mm at the pericardium of the front part of the right ventricle (▲) and calcification of the lateral part of the left ventricle (Δ). On pulsed wave Doppler examination, the transmitral flow velocity variation with respiration is 32% and the transtricuspid flow velocity variation with respiration is 84%. (□: expiration, ■: inspiration). (B) Transthoracic echocardiography performed 30 days after steroid therapy was started. The rough appearance of the pericardium (↑) has disappeared and its movement has improved. On pulsed wave Doppler examination, the transmitral flow velocity variation with respiration improved from 32% to 12% and the transtricuspid flow velocity variation with respiration improved from 84% to 43%. (□: expiration, ■: inspiration). TB, tuberculosis.
Figure 3.
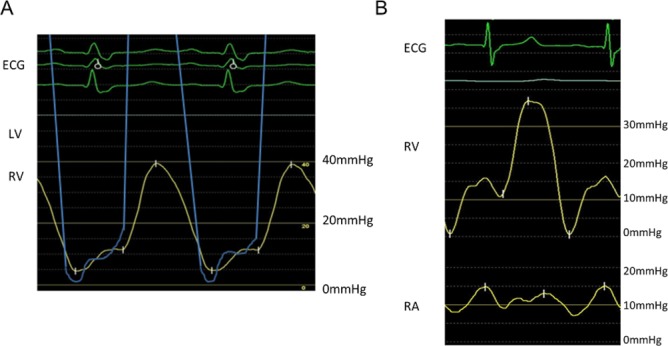
(A) Cardiac catheterisation performed 14 days after anti-TB drug therapy was started. There is elevation of the end-diastolic pressures of the bilateral ventricles and dip-and-plateau pattern in the right ventricular pressure. (B) Cardiac catheterisation performed after completion of steroid therapy showed the reduction of the end-diastolic pressures in the right ventricle. LV, left ventricle, RA, right atrium; RV, right ventricle.
On echocardiography performed 30 days after the steroid therapy was started, the adhesion of the non-calcified parts of the pericardium to the heart and the ventricular inflow variation with respiration showed improvement (figure 2B). Additionally, cardiac catheterisation showed the reduction of the end-diastolic pressures in the right ventricle (figure 3B). He did not experience dyspnoea on effort, and there were no dialysis difficulties. Therefore, surgery was not required.
Discussion
Tuberculous pericarditis shows various clinical manifestations, and it should be considered in all cases of pericarditis without a rapidly self-limited course. TB-associated pericardial effusion usually develops insidiously, presenting with non-specific systemic symptoms, such as fever, night sweats, fatigue and weight loss.5
Tuberculous pericarditis onset can be acute or chronic. Chronic tuberculous pericarditis is often accompanied with ‘dry’ pericarditis and is identified for the first time with constrictive pericarditis. On the other hand, acute tuberculous pericarditis is often accompanied with pericardial effusion. In the present case, acute tuberculous pericarditis was initially suspected and the pericarditis advanced,resulting in the development of constrictive pericarditis.6
TB infection usually spreads lymphatically from the peritracheal, peribronchial or mediastinal lymph nodes or spreads haematogenously from the primary TB infection site.7 8 Chest CT shows typical changes in the mediastinal lymph nodes in almost 100% of cases of tuberculous pericarditis. In the present case, he had a history of pulmonary TB and showed swelling of the mediastinal lymph nodes on chest CT. Furthermore, there was no evidence of TB in blood culture. If his pericarditis was due to TB infection, TB might have spread lymphatically.
The diagnosis of tuberculous pericarditis can sometimes be difficult. The definite diagnosis of tuberculous pericarditis is based on the demonstration of tubercle bacilli in pericardial fluid or in a histological section of the pericardium, while the probable diagnosis is based on evidence of TB elsewhere in a patient with unexplained pericarditis, lymphocytic pericardial effusion with elevated ADA levels and/or appropriate response to a trial of anti-TB chemotherapy. In patients with tuberculous pericarditis, the diagnostic sensitivity of the direct detection of tubercle bacilli in pericardial effusion is approximately 50%,8 while the diagnostic sensitivity of the direct detection of tubercle bacilli in a pericardial biopsy specimen is 10%–64%.9 Therefore, negative findings on culture of pericardial effusion and pericardial biopsy do not exclude the possibility of tuberculous pericarditis. PCR has been suggested for detecting M. tuberculosis DNA in pericardial fluid, and although PCR is a fast process, the sensitivity of PCR with pericardial fluid is only approximately 50%.8 10 Several studies have demonstrated that an elevated pericardial ADA level is suggestive of tuberculous pericarditis,10–12 and it has been shown that an ADA cut-off level of 35 U/L has a sensitivity and specificity of 90% and 74%, respectively, for the diagnosis of tuberculous pericarditis.12 In the present case, the ADA level was elevated, and therefore, we decided to start anti-TB therapy.
Constrictive pericarditis is one of the most serious tuberculous pericarditis conditions, even after prompt anti-TB chemotherapy. Although pericardiectomy is indicated for patients with calcific constrictive pericarditis, the operative mortality is high at 5.6%–19%.4 Steroids can be used to prevent the progression to constrictive pericarditis and the need for pericardiectomy.6 However, the timing of starting steroid administration is debatable. In the present case, although anti-TB drug therapy was started, inflammation remained and thickening of the pericardium progressed. Therefore, we started steroid therapy, and the patient's condition improved.
In conclusion, we experienced the case of an immunocompromised patient with tuberculous pericarditis. Early administration of steroid could prevent him from progressing to constrictive pericarditis. Detection of an elevation in the ADA level in pericardial effusion might be helpful, and the early administration of anti-TB drugs and steroids can improve cardiac function and help avoid pericardiectomy.
Learning points.
We should consider the recurrence of tuberculous (TB) as a differential diagnosis in an immunocompromised patient with the treatment history of pulmonary tuberculous pericarditis.
The elevated adenosine deaminase value of pericardial effusion might be useful as a supportive diagnosis of TB pericarditis.
The early administration of anti-TB drugs and steroids can improve cardiac function and could prevent him from progressing to constrictive pericarditis.
Footnotes
Contributors: RA did the data collection, interpretation and drafting the article. JI and YT did the critical revision of the article. KH did the final approval of the version to be published.
Disclaimer: I certify that neither this manuscript nor one with substantially similar content under my authorship has been published or is being considered for publication elsewhere (except as indicated in an attachment). I have access to any data upon which the manuscript is based and will provide such data upon request to the editors or their assignees. I agree to allow the corresponding author to correspond with the editorial office, to review the uncorrected proof copy of the manuscript and to make decisions regarding release of information in the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Zumla A, George A, Sharma V, et al. The WHO 2014 global tuberculosis report--further to go. Lancet Glob Health 2015;3:e10–12. 10.1016/S2214-109X(14)70361-4 [DOI] [PubMed] [Google Scholar]
- 2.Dobler CC, McDonald SP, Marks GB. Risk of tuberculosis in dialysis patients: a nationwide cohort study. PLoS One 2011;6:e29563 10.1371/journal.pone.0029563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas B, Wulf S, Bikbov B, et al. Maintenance dialysis throughout the world in years 1990 and 2010. J Am Soc Nephrol 2015;26:2621–33. 10.1681/ASN.2014101017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayosi BM, Burgess LJ, Doubell AF. Tuberculous pericarditis. Circulation 2005;112:3608–16. 10.1161/CIRCULATIONAHA.105.543066 [DOI] [PubMed] [Google Scholar]
- 5.Desai HN. Tuberculous pericarditis. A review of 100 cases. S Afr Med J 1979;55:877–80. [PubMed] [Google Scholar]
- 6.Strang JI, Kakaza HH, Gibson DG, et al. Controlled clinical trial of complete open surgical drainage and of prednisolone in treatment of tuberculous pericardial effusion in Transkei. Lancet 1988;2:759–64. 10.1016/S0140-6736(88)92415-4 [DOI] [PubMed] [Google Scholar]
- 7.Cherian G, Habashy AG, Uthaman B, et al. Detection and follow-up of mediastinal lymph node enlargement in tuberculous pericardial effusions using computed tomography. Am J Med 2003;114:319–22. 10.1016/S0002-9343(02)01521-8 [DOI] [PubMed] [Google Scholar]
- 8.Cegielski JP, Devlin BH, Morris AJ, et al. Comparison of PCR, culture, and histopathology for diagnosis of tuberculous pericarditis. J Clin Microbiol 1997;35:3254–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komsuoğlu B, Göldelï O, Kulan K, et al. The diagnostic and prognostic value of adenosine deaminase in tuberculous pericarditis. Eur Heart J 1995;16:1126–30. 10.1093/oxfordjournals.eurheartj.a061057 [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Lee CW, Lee SG, et al. Comparison of polymerase chain reaction with adenosine deaminase activity in pericardial fluid for the diagnosis of tuberculous pericarditis. Am J Med 2002;113:519–21. 10.1016/S0002-9343(02)01261-5 [DOI] [PubMed] [Google Scholar]
- 11.Aggeli C, Pitsavos C, Brili S, et al. Relevance of adenosine deaminase and lysozyme measurements in the diagnosis of tuberculous pericarditis. Cardiology 2000;94:81–5. 10.1159/000047296 [DOI] [PubMed] [Google Scholar]
- 12.Burgess LJ, Reuter H, Carstens ME, et al. The use of adenosine deaminase and interferon-gamma as diagnostic tools for tuberculous pericarditis. Chest 2002;122:900–5. 10.1378/chest.122.3.900 [DOI] [PubMed] [Google Scholar]


