Abstract
Although direct muscle invasion by carcinoma is well recognised, skeletal muscle metastases are rare. Breast cancer very rarely metastasises to skeletal muscles. We present a case of breast cancer that metastasised to the biceps muscle. The woman developed breast cancer in 1990 and then developed axillary subcutaneous metastasis in 2001. In 2015, she presented with pain in the left forearm extending to the hand. Initial imaging showed no abnormalities, but the positron emission tomography-CT scanning revealed a hot spot in the left biceps muscle. Additionally, the nerve conduction study showed feature of carpal tunnel syndrome. The hot spot was deemed inconclusive in the view of normal CT and MRI scans, and the patient was treated with carpal tunnel decompression. A few months later, the patient developed a lump in the left biceps muscle, which appeared to be a metastatic lesion from her primary breast cancer. The patient was treated with radiotherapy and responded satisfactorily.
Keywords: breast cancer, pain (palliative care)
Background
Although direct muscle invasion by carcinoma is well recognised, skeletal muscle metastases are rare. They in fact account for only a small percentage of all cancer types in an autopsy series.1 The most common cancer to metastasise to the skeletal muscles is lung cancer, followed by gastrointestinal cancer and renal cell carcinoma.2 Distant metastases of breast cancer to the skeletal muscles are very rare. Few cases have been reported to the best of our knowledge.2–15 Muscle metastases often remain asymptomatic and with no physical signs, and they are difficult to detect through imaging.16 Being uncommon, skeletal muscle metastasis must always be kept in mind as its detection requires specific evaluation.
Case presentation
Our case is a 60-year-old woman who had left breast cancer in 1990. Her cancer was T2N1M0, grade 2 ductal carcinoma, oestrogen-receptor-positive (ER-positive) with axillary lymph nodes involvement (6 out of 10). The patient was treated with wide local excision, axillary clearance, chemotherapy (5-fluorouracil, epirubicin and cyclophosphamide) and radiotherapy without hormonal treatment at that time. In March 2001, she developed a lump (2 cm × 2 cm) in her left axilla. Fine needle aspiration revealed a breast cancer metastasis to the subcutaneous tissue, and histopathology and immunohistochemistry assay matched her previous primary breast cancer. The patient was treated with metastasectomy, chemotherapy (5-fluorouracil, epirubicin and cyclophosphamide) and tamoxifen for 5 years. In January 2015, she presented with a 6-month history of numbness, pain, coldness and needles sensation along the medial aspect of the left forearm extending to the thumb, index and middle finger. The symptoms were getting worse with time. On examination of the left upper limb, there were no obvious abnormalities on inspection or palpation. Neurological sensory examination revealed reduced sensation to pain and light touch over the thumb and the index finger. Motor examination was normal.
Investigations
We requested CT scan for the chest, abdomen and pelvis to exclude any recurrence. The scan did not reveal any abnormality apart from the surgical scarring in her left axilla. MRI of the axilla and the brachial plexus revealed no abnormalities in the nerves apart from the surgical scarring. Positron emission tomography-CT (PET-CT) scan showed a 22 mm zone of fluorodeoxyglucose (FDG)-avid uptake in the left biceps muscle 10 cm distal to the left axilla (figure 1). The radiologist questioned the significance of this hot spot in the view of normal CT and MRI scans. With the absence of any clue of recurrence, we decided to request nerve conduction study (NCS) to rule out carpal tunnel syndrome. The study of the left upper limb showed conductive abnormalities restricted to the median nerve at the level of carpal tunnel. Based on these results, the patient was referred to orthopaedics for carpal tunnel decompression. Four months later the symptoms persist and the patient developed a lump over the medial side of the left upper arm. The lump was 5–7 cm in diameter, soft and mobile. MRI scan of the left upper arm revealed a soft tissue mass involving the biceps musculature and the neurovascular bundle in the medial aspect of the left upper arm (figure 2). PET-CT scan showed a 33 mm zone of FDG uptake in the left biceps muscle 10 cm distal to the left axilla, and this was present in the last PET scan and it is bigger on this scan (figure 3). Core needle biopsy was performed, and histopathology showed metastatic breast cancer infiltrating the striated muscle tissue, and immunohistochemistry assay was ER-positive, progesterone-receptor-positive (PR-positive) and human epidermal growth factor-2 (HER2) 2+ (figure 4).
Figure 1.
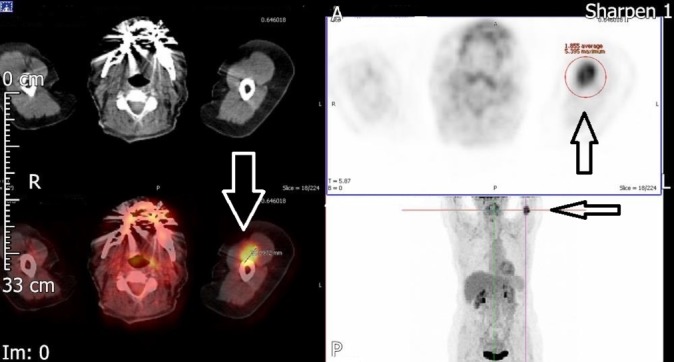
Solitary biceps muscle metastasis from breast cancer. Positron emission tomography-CT image. Axial and coronal images showing fluorodeoxyglucose-avid lesion (22 mm) in the medial aspect of the left upper arm (arrows).
Figure 2.
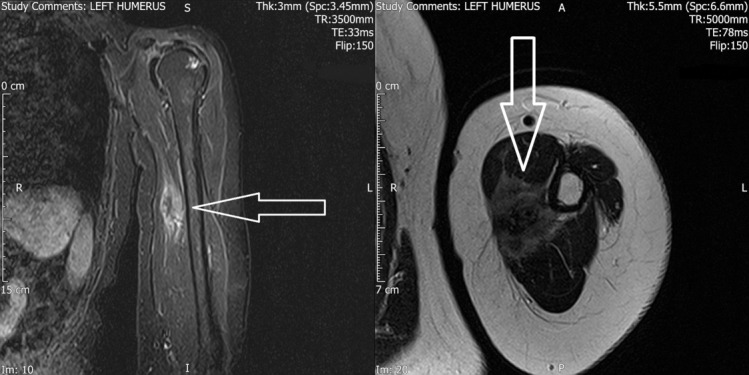
Solitary biceps muscle metastasis from breast cancer. (left) MRI scan, T1-weighted, sagittal image showing a mass near the medial aspect of the left humerus (arrow). (right) MRI scan, T2-weighted, axial image showing a mass near the medial aspect of the left humerus (arrow).
Figure 3.
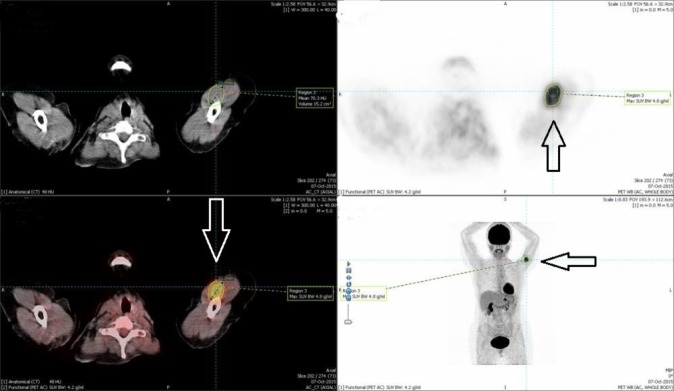
Solitary biceps muscle metastasis from breast cancer. Positron emission tomography (PET)-CT months after the first PET-CT. Axial and coronal images showing fluorodeoxyglucose-avid lesion (33 mm) in the medial aspect of the left upper arm (arrows).
Figure 4.
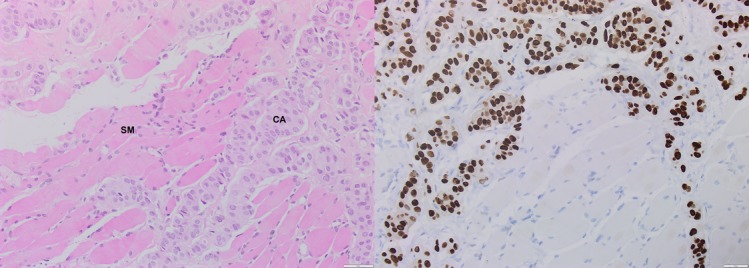
Solitary biceps muscle metastasis from breast cancer. Microscopic appearance of metastatic breast cancer in the biceps muscle (×200). (left) H&E staining shows ductal-type metastatic carcinoma of breast origin (CA) invading the skeletal muscle tissue (SM). (right) Immunohistochemistry stain showing strong nuclear Oestrogen receptor expression in the carcinoma.
Differential diagnosis
Sarcoma is one of the important differential diagnoses when there is a skeletal muscle mass.
Intramuscular abscess.
Carpal tunnel syndrome in view of the pain in the upper limb and the positive NCS result.
Treatment
The case was discussed in the local breast multidisciplinary meeting, and the decision of the clinical oncologist was to start the patient on letrozole (2.5 mg daily) and to offer palliative radiotherapy (15 fractions of 40.05 Gy). Surgical intervention was discussed in the sarcoma multidisciplinary meeting, and they advised that the surgical removal of the mass would carry a high risk of neurovascular bundle injury as the lesion is involving the neurovascular bundle. The clinical oncologist discussed the options of radiotherapy and surgery with the patient. The patient’s preference was to start radiotherapy and not to proceed with surgery.
Outcome and follow-up
After the course of radiotherapy, the patient’s symptoms improved considerably. An MRI scan of the left upper limb 6 months after the radiotherapy revealed significant decrease in the size of the tumour mass (figure 5).
Figure 5.
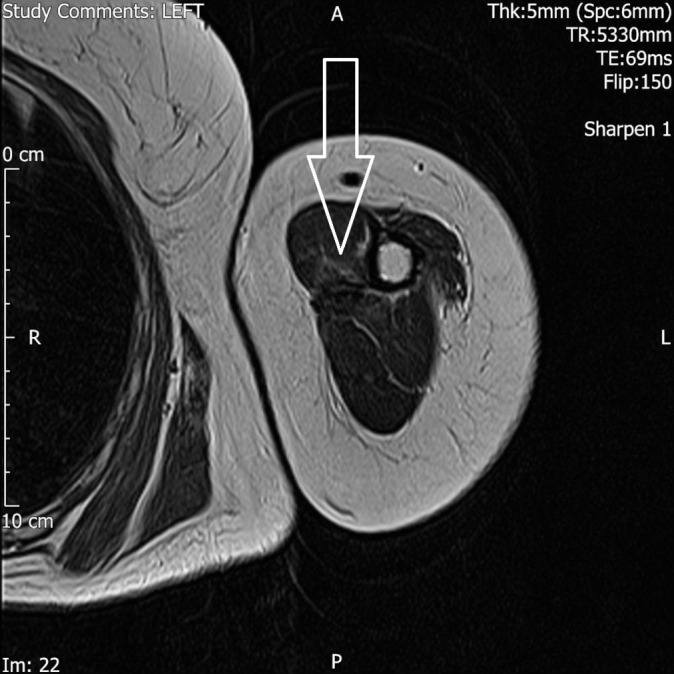
Solitary biceps muscle metastasis from breast cancer. MRI scan, T2-weighted, axial image showing significant decrease in the size of the tumour mass after radiotherapy.
Discussion
It is poorly understood why skeletal muscles metastases are rare, even though skeletal muscles form large part of the body mass and cardiac output. Several factors have been suggested to contribute to the resistance of the skeletal muscle to metastatic cancer, such as intramuscular blood pressure, blood flow per unit of weight and local changes in pH; local temperature distribution may also be involved.17 Intramuscular blood flow seems to play an important role in tumour implantation. Organs like lung, liver and bones have much higher incidence of tumour metastasis than skeletal muscles because these organs have constant blood flow, whereas skeletal muscles have variable blood flow.18 Although some have suggested that protease inhibitors in the extracellular matrix of the muscle may resist tumour cell invasion,19 it may be the production of lactic acid and other metabolites by skeletal muscle that inhibits tumour cell growth.20 Even though skeletal muscles are resistant to metastasis, properties of the primary tumour may also have some role in the metastasis process. Two-thirds of all cancers metastatic to skeletal muscles are solid carcinomas, and only about one-third are from haematological malignancies, and few cases originated from melanomas.21
Several pathophysiological mechanisms have been suggested for the development of muscle metastases, but the haematogenous spread is the most probable pathway.1 Although the most common clinical presentation is a painful mass,22 in our case the mass was painless and the referred pain was due to involvement of the adjacent nerves. Therefore, if a patient presents with mild pain without clear evidence of a mass on physical examination, as occurred with our case when she presented for the first time, the metastatic tumour may be easily missed.
Although fine needle aspiration biopsy is the less traumatic and the less likely to contaminate the tissue plans, core needle biopsy is the main diagnostic technique.23 MRI is considered as the best imaging tool for diagnosis of muscle metastasis.11 Although MRI has been considered to be superior to CT,11 CT-guided fine needle aspiration provides a rapid, minimally invasive means of diagnosis.24 PET-CT is a sensitive tool in the diagnosis of muscle metastasis,4 and it has been reported that it may detect unsuspected metastasis.25 Intramuscular hot spots on PET-CT scans should be considered as a sign of metastatic lesions even in the absence of any abnormality on CT scans.15
Differential diagnosis of skeletal muscle metastasis mainly includes sarcoma, myopathy and intramuscular abscess.25 Differentiating a muscle metastasis from sarcoma is of paramount importance because the treatment approach is quite different.26 Due to the variety of clinical and radiological features, misinterpretation of muscle metastasis is not infrequent,27 and this is what happened in our case when the radiologist was not convinced that the hot spot in the first PET-CT was a metastatic lesion because the previous scans did not show any structural abnormalities. Therefore, in patients with history of cancer, FDG-avid zones in skeletal muscles on PET-CT scan should always raise suspicion of metastatic disease even in the absence of structural abnormalities on other imaging modalities.
Treatment options for muscle metastasis should be individualised depending on the patient’s clinical condition regarding the underlying disease and its prognosis.28 Treatment options may include radiotherapy, chemotherapy or surgical excision.29 Surgical excision may be considered in carefully selected cases such as patients with painful masses and in patients with a long disease-free interval.22 The surgical option was not considered in our case as the metastatic lesion was invading the adjacent nerves and blood vessels. Radiotherapy has been reported as effective in controlling pain and metastatic lesion but has been associated with local complications such as skin burn and muscle contracture.29
The prognosis of patients with muscle metastasis is poor, and most of the patients die within few months despite the treatment.30 In Mathis et al’s31 series, less than 2.5% of 174 patients with skeletal muscle metastasissurvived beyond 72 months.
In conclusion, distant metastasis to skeletal muscle rarely occurs in patients with breast cancer. MRI and PET-CT are the preferable diagnostic modalities in the detection of skeletal muscle metastases. Core needle biopsy is necessary to confirm the diagnosis. The treatment of muscle metastasis is always palliative, involving radiotherapy, chemotherapy or surgery.
Learning points.
Painful mass in the skeletal muscles of patients with cancer should be addressed carefully.
In patients with a history of cancer, fluorodeoxyglucose-avid zone in the skeletal muscles on positron emission tomography scan should always raise suspicion of metastasis even in the absence of structural abnormalities on other imaging modalities.
Palliative radiotherapy is very effective in controlling the pain and the growth of the metastatic tumours in skeletal muscles.
Acknowledgments
Authors would like to thank Dr Angus Molyneux (consultant pathologist) for his contribution in reporting the slides and preparing the images.
Footnotes
Contributors: HE has contributed in facilitating the acquisition of the patient details. MA prepared the draft of the work and collected the patient’s details with important intellectual input from HE, who also revised the draft critically. Both authors approved the final draft.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Surov A, Hainz M, Holzhausen HJ, et al. Skeletal muscle metastases: primary tumours, prevalence, and radiological features. Eur Radiol 2010;20:649–58. 10.1007/s00330-009-1577-1 [DOI] [PubMed] [Google Scholar]
- 2.Salemis NS. Skeletal muscle metastasis from breast cancer: management and literature review. Breast Dis 2015;35:37–40. 10.3233/BD-140384 [DOI] [PubMed] [Google Scholar]
- 3.Liu CH, Chang C, Sy E, et al. Metaplastic breast carcinoma with multiple muscle metastasis: a case report. Medicine 2015;94:e662 10.1097/MD.0000000000000662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuroi K, Tominaga T. Metastasis of breast carcinoma to rectus abdominis muscle. Breast J 2003;9:58–9. 10.1046/j.1524-4741.2003.09112.x [DOI] [PubMed] [Google Scholar]
- 5.Plaza JA, Perez-Montiel D, Mayerson J, et al. Metastases to soft tissue: a review of 118 cases over a 30-year period. Cancer 2008;112:193–203. 10.1002/cncr.23151 [DOI] [PubMed] [Google Scholar]
- 6.Kouvaris JR, Gkongkou PV, Papadimitriou CA, et al. Bilateral metastases to extraocular muscles from lobular breast carcinoma. Onkologie 2008;31:387–9. 10.1159/000129689 [DOI] [PubMed] [Google Scholar]
- 7.Bello-Roufai D, Soares DG, Kerrou K, et al. Long-term complete response in a breast cancer patient with skeletal muscle metastases diagnosed using 18F-FDG-PET. Oxf Med Case Reports 2017;2017:omx002 10.1093/omcr/omx002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Surov A, Hainz M, Holzhausen HJ, et al. Skeletal muscle metastases: primary tumours, prevalence, and radiological features. Eur Radiol 2010;20:649–58. 10.1007/s00330-009-1577-1 [DOI] [PubMed] [Google Scholar]
- 9.Surov A, Köhler J, Wienke A, et al. Muscle metastases: comparison of features in different primary tumours. Cancer Imaging 2014;14:21 10.1186/1470-7330-14-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schultz SR, Bree RL, Schwab RE, et al. CT detection of skeletal muscle metastases. J Comput Assist Tomogr 1986;10:81–3. 10.1097/00004728-198601000-00018 [DOI] [PubMed] [Google Scholar]
- 11.Glazer LC, Harris GJ, Simons KB. Orbital metastasis as the presenting sign of adenocarcinoma of the breast. Ophthal Plast Reconstr Surg 1991;7:252–5. 10.1097/00002341-199112000-00003 [DOI] [PubMed] [Google Scholar]
- 12.Sahai S, Leigh D. Metastasis of adenocarcinoma of breast to gluteus medius. Iowa Med 1995;85:369–70. [PubMed] [Google Scholar]
- 13.Peckham EL, Giblen G, Kim AK, et al. Bilateral extraocular muscle metastasis from primary breast cancer. Neurology 2005;65:74 10.1212/01.WNL.0000156359.93206.99 [DOI] [PubMed] [Google Scholar]
- 14.Lee JY, Kang HW, Jung SN, et al. Solitary gluteus maximus muscle metastasis in a breast cancer patient. Arch Plast Surg 2015;42:661–3. 10.5999/aps.2015.42.5.661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YW, Seo KJ, Lee SL, et al. Skeletal muscle metastases from breast cancer: two case reports. J Breast Cancer 2013;16:117–21. 10.4048/jbc.2013.16.1.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arpaci T, Ugurluer G, Akbas T, et al. Imaging of the skeletal muscle metastases. Eur Rev Med Pharmacol Sci 2012;16:2057–63. [PubMed] [Google Scholar]
- 17.Fidler IJ. Hart IR: Principles of cancer biology, biology of cancer metastasis : DeVita VT, Hellman S, Rosenberg SA, Cancer: principles and practice of oncology. Philadelphia: JB Lippincott Co, 1982:80–92. [Google Scholar]
- 18.Seely S. Possible reasons for the high resistance of muscle to cancer. Med Hypotheses 1980;6:133–7. 10.1016/0306-9877(80)90079-1 [DOI] [PubMed] [Google Scholar]
- 19.Pauli BU, Schwartz DE, Thonar EJ, et al. Tumor invasion and host extracellular matrix. Cancer Metastasis Rev 1983;2:129–52. 10.1007/BF00048966 [DOI] [PubMed] [Google Scholar]
- 20.Seely S. The evolution of human longevity. Med Hypotheses 1980;6:873–82. 10.1016/0306-9877(80)90011-0 [DOI] [PubMed] [Google Scholar]
- 21.Auerbach O, Garfinkle L. Parks VR: histologic types of lung cancer in relation to smoking habits, year of diagnosis and sites of metastases. Chest 1975;65:382–7. [DOI] [PubMed] [Google Scholar]
- 22.Molina-Garrido MJ, Guillén-Ponce C. Muscle metastasis of carcinoma. Clin Transl Oncol 2011;13:98–101. 10.1007/s12094-011-0625-x [DOI] [PubMed] [Google Scholar]
- 23.Ogiya A, Takahashi K, Sato M, et al. Metastatic breast carcinoma of the abdominal wall muscle: a case report. Breast Cancer 2015;22 10.1007/s12282-012-0352-3 [DOI] [PubMed] [Google Scholar]
- 24.Herring CL, Harrelson JM. Scully SP: Metastatic carcinoma to skeletal muscle. a report of 15 patients. Clin Orthop 1998;355:272–81. [DOI] [PubMed] [Google Scholar]
- 25.Khandelwal AR, Takalkar AM, Lilien DL, et al. Skeletal muscle metastases on FDG PET/CT imaging. Clin Nucl Med 2012;37:575–9. 10.1097/RLU.0b013e3182443e32 [DOI] [PubMed] [Google Scholar]
- 26.Plaza JA, Perez-Montiel D, Mayerson J, et al. Metastases to soft tissue: a review of 118 cases over a 30-year period. Cancer 2008;112:193–203. 10.1002/cncr.23151 [DOI] [PubMed] [Google Scholar]
- 27.Herring CL, Harrelson JM, Scully SP. Metastatic carcinoma to skeletal muscle. A report of 15 patients. Clin Orthop Relat Res 1998;355:272–81. [DOI] [PubMed] [Google Scholar]
- 28.Damron TA, Heiner J. Distant soft tissue metastases: a series of 30 new patients and 91 cases from the literature. Ann Surg Oncol 2000;7:526–34. 10.1007/s10434-000-0526-7 [DOI] [PubMed] [Google Scholar]
- 29.Tuoheti Y, Okada K, Osanai T, et al. Skeletal muscle metastases of carcinoma: a clinicopathological study of 12 cases. Jpn J Clin Oncol 2004;34:210–4. 10.1093/jjco/hyh036 [DOI] [PubMed] [Google Scholar]
- 30.Magee T, Rosenthal H. Skeletal muscle metastases at sites of documented trauma. AJR Am J Roentgenol 2002;178:985–8. 10.2214/ajr.178.4.1780985 [DOI] [PubMed] [Google Scholar]
- 31.Mathis S, Fromont-Hankard G, du Boisguéheneuc F, et al. Muscular metastasis. Rev Neurol 2010;166:295–304. 10.1016/j.neurol.2009.05.020 [DOI] [PubMed] [Google Scholar]


