Abstract
The diagnosis of autoimmune gastrointestinal dysmotility requires a high level of clinical suspicion when standard work-up is unrevealing. We report the case of a 56-year-old male patient with history of tobacco use and a subacute presentation of weight loss, vomiting and cerebellar ataxia. The discovery of paraneoplastic type 1 antineuronal nuclear antibodies and neuronal acetylcholine receptor antibodies led to further directed imaging and diagnostic studies in spite of prior negative chest imaging. Bronchoscopy with endobronchial ultrasound was used to confirm a diagnosis of small cell lung cancer and paraneoplastic syndrome as the cause of the presenting upper gastrointestinal symptoms.
Keywords: Neurogastroenterology, Lung Cancer (oncology)
Background
Autoimmune autonomic disorders that affect the gastrointestinal tract may reflect an underlying malignancy. These patients may present with a variety of symptoms suggestive of gastrointestinal dysmotility, ranging from gastroparesis to constipation with accompanying neurological signs. Multiple cancers have been found to be associated with paraneoplastic syndromes, with small cell lung cancer (SCLC) being the most frequent. Treatment of malignancy-related autoimmune gastrointestinal dysmotility focuses on both tumour therapy and immune strategies targeting abnormal antibody production.
Case presentation
A 56-year-old man presented with a 3-month history of vomiting and weight loss. He reported non-bloody bilious vomiting accompanied by early satiety, epigastric abdominal discomfort, constipation and loss of 55 pounds. His history was significant for coronary artery disease, hypertension and a 30 pack-year smoking history. No significant family history was noted.
Physical examination revealed diffuse abdominal tenderness with no distention. Neurological examination was suggestive of cerebellar ataxia with slurred speech, gait instability and dysmetria on finger-to-nose testing. Evaluation of vital signs in the supine and standing position confirmed the presence of orthostatic hypotension.
Investigations
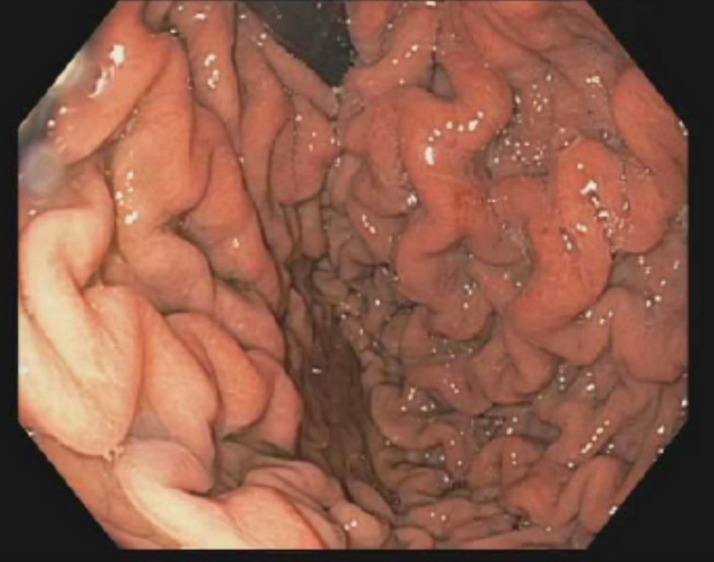
Initial gastrointestinal work-up included normal abdominal vascular studies to include mesenteric Doppler ultrasound and CT angiography. Upper gastrointestinal X-ray series confirmed a normal duodenal sweep. Upper endoscopy with endoscopic ultrasound demonstrated thickened gastric folds (figure 1). Mucosal biopsies were negative for Menetrier’s syndrome and congo red staining was negative for amyloidosis. Neurological work-up included normal brain MRI and CT angiography of the head and neck vessels. Laboratory studies were negative for adrenal insufficiency, negative Scl-70 antibody and negative serum protein electrophoresis. In concern for a paraneoplastic syndrome, initial CT scanning of the chest was negative for suspicious lung nodules.
Figure 1.
Thickened gastric folds on upper endoscopy.
Outcome and follow-up
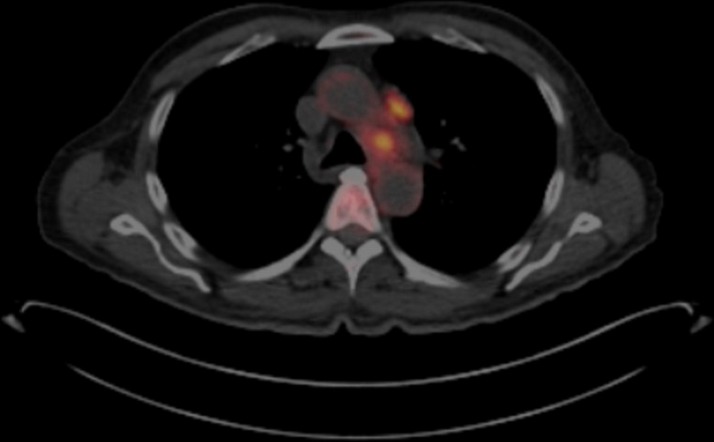
Given persistent suspicion for an occult malignancy, a paraneoplastic panel ultimately identified positive titres for type 1 antineuronal nuclear antibody (ANNA-1) at 1:61 440 and neuronal acetylcholine receptor antibody at 0.05 (ref range <0.02 nmol/L). A positron emission tomography (PET) scan was performed revealing hypermetabolic activity in the mediastinum, with standardised uptake values maximum of 7.1 (figure 2). This scan was completed 1 month after the negative CT scan of the chest. Subsequent bronchoscopy with endobronchial ultrasound was performed, with transbronchial needle aspiration of the lesion in the left paratracheal area. Cytology confirmed the diagnosis of limited-stage small cell carcinoma of the lung; no additional immunohistochemical stains were performed (figure 3).
Figure 2.
Positron emission tomography scan with hypermetabolic mediastinal lymph nodes.
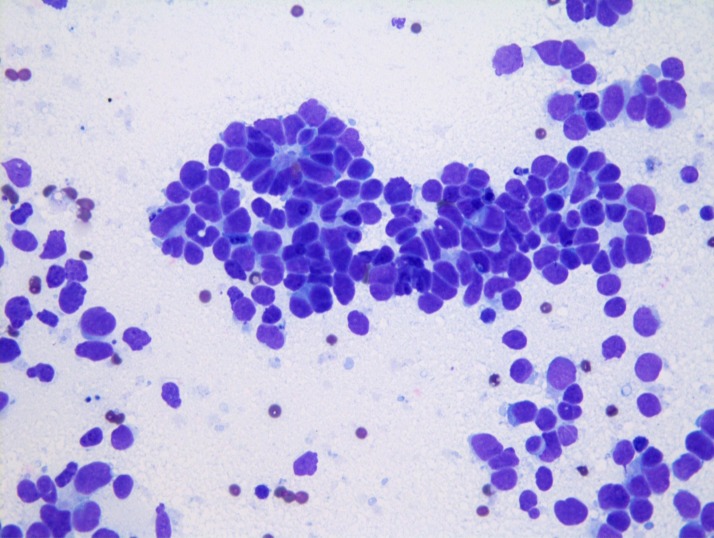
Figure 3.
Cytological examination with Wright Giemsa stain demonstrates large round cells with scant cytoplasm, fine chromatin and absent nucleoli confirming small cell lung cancer. Permission was obtained from the patient to include radiographical and diagnostic images used in the development of this case.
He was referred to oncology and started on a chemotherapy regimen with carboplatin and etoposide, a previously reported therapy for limited-stage SCLC chosen by his oncologist. Chemotherapy was accompanied by thoracic radiation therapy. Therapy was associated with improvement in his speech and strength. Repeat PET/CT imaging 4 months after initial assessment demonstrated no recurrent or metastatic lung cancer.
Discussion
Autoimmune gastrointestinal dysmotility is a form of autoimmune dysautonomia that can occur idiopathically or as a result of an anatomically remote cancer.1 When the motility disorder occurs as a result of an established cancer, it is commonly referred to as paraneoplastic dysmotility.2 While the exact mechanism for the formation of antibodies to the cancer is unclear, they are known to target onconeural antigens shared by enteric neurons and the tumour cells.2 The most common antibody isolated in paraneoplastic dysmotility is ANNA-1, which recognises the nuclear protein Hu and is expressed in the neurons of the central, peripheral and enteric nervous system.2 Tumours that are associated with the expression of ANNA-1 include SCLC, breast, prostate and ovarian cancer.2 The exact mechanism by which ANNA-1 antibodies result in dysfunction of the enteric nervous system and subsequent dysmotility is unclear. In an in vitro model, anti-Hu antibodies from patients with paraneoplastic dysmotility as well as commercial anti-Hu antibodies resulted in activation of the apoptotic cascade in cultured myenteric neurons and a neuroblastoma cell line.3 In a case report of paraneoplastic dysmotility in a patient with SCLC and antibodies to ANNA-1 as well as the P/Q-type calcium channel, full thickness small bowel sections demonstrated decreased and disorganised staining of the interstitial cells of Cajal at the level of the myenteric plexus.4
In this study, we present the case of a 56-year-old male patient with clinical gastrointestinal dysmotility and cerebellar ataxia who was found to have paraneoplastic antibodies to both ANNA-1 and neuronal acetylcholine receptor antibodies. The investigation by the paraneoplastic panel was pursued after the PET scan revealed hypermetabolic lymph nodes suggestive of malignancy. While the PET scan has high sensitivity for excluding SCLC, a case has been reported of benign-appearing imaging and only mild fluoro-deoxy-D-glucose uptake in a patient with limited-stage SCLC who presented with ANNA-1-positive paraneoplastic autonomic neuropathy.5 In a study examining 162 patients from the Mayo Clinic identified as ANNA-1-positive, 88% were found to have an underlying cancer and 81% had SCLC. In 97% of the patients with SCLC, the diagnosis was made after the onset of paraneoplastic symptoms, highlighting the immunological role of the antibodies directed against the malignancy.6
Patients with autoimmune dysmotility can present with a wide array of gastrointestinal symptoms to include achalasia, gastroparesis, chronic intestinal pseudo-obstruction and constipation.2 Among patients with ANNA-1 antibodies, gastrointestinal symptoms occur in 23% of individuals, with gastroparesis being the most common symptom.6 These patients can also present with neurological symptoms including cerebellar ataxia and orthostasis, such as in the case of our patient. Patients with SCLC and anti-Hu antibodies can develop paraneoplastic cerebellar degeneration, which manifests with ataxia, dysarthria and vertigo. Other areas of the nervous system may be affected as well, including the development of autonomic neuropathy, symptoms of which include orthostasis and gastroparesis.7
Management of autoimmune gastrointestinal dysmotility depends on the underlying aetiology. Management of paraneoplastic dysmotility is compounded by the difficulty in maintaining nutritional support in the setting of active cancer and weight loss. The mainstay of treatment is management of the active cancer, while management strategies previously investigated include immune suppression with steroids, cyclophosphamide, plasmapheresis and intravenous immunoglobulin, without convincing success.2 Management of non-paraneoplastic gastrointestinal dysmotility is directed at the underlying immune-mediated process with therapies to include immune suppression, plasmapheresis and acetylcholinesterase inhibitors.2
Our case highlights the important symptomatic presentation of SCLC as a result of symptoms secondary to paraneoplastic syndrome. The constellation of early satiety and weight loss suggested an autonomic dysmotility, which presented concurrently with the development of orthostasis and cerebellar ataxia. The isolation of the positive paraneoplastic antibodies led to further imaging studies demonstrating hypermetabolic lymph nodes and subsequent pathological confirmation of SCLC.
Learning points.
Paraneoplastic dysmotility can present with a wide range of gastrointestinal symptoms to include achalasia, gastroparesis, chronic intestinal pseudo-obstruction and constipation.
Tumours that are associated with the expression of type 1 antineuronal nuclear antibodies include small cell lung cancer, breast, prostate, ovarian cancer and lymphoma.
Treatment of malignancy-related autoimmune gastrointestinal dysmotility focuses on both tumour therapy and immune strategies targeting abnormal antibody production.
When symptoms are suspicious for an occult malignancy resulting in a paraneoplastic syndrome, a paraneoplastic panel may reveal positive antibodies even in the setting of negative imaging studies.
Footnotes
Contributors: AML and DM drafted the manuscript. AC assisted with drafting of the manuscript. EMD assisted with critically revising the manuscript and is the article guarantor.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Dhamija R, Tan KM, Pittock SJ, et al. Serologic profiles aiding the diagnosis of autoimmune gastrointestinal dysmotility. Clin Gastroenterol Hepatol 2008;6:988–92. 10.1016/j.cgh.2008.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kashyap P, Farrugia G. Enteric autoantibodies and gut motility disorders. Gastroenterol Clin North Am 2008;37:397–410. 10.1016/j.gtc.2008.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Giorgio R, Bovara M, Barbara G, et al. Anti-HuD-induced neuronal apoptosis underlying paraneoplastic gut dysmotility. Gastroenterology 2003;125:70–9. 10.1016/S0016-5085(03)00664-4 [DOI] [PubMed] [Google Scholar]
- 4. Pardi DS, Miller SM, Miller DL, et al. Paraneoplastic dysmotility: loss of interstitial cells of Cajal. Am J Gastroenterol 2002;97:1828–33. 10.1111/j.1572-0241.2002.05851.x [DOI] [PubMed] [Google Scholar]
- 5. Block MS, Vassallo R. Lack of FDG uptake in small cell carcinoma associated with ANNA-1 positive paraneoplastic autonomic neuropathy. J Thorac Oncol 2008;3:542–4. 10.1097/JTO.0b013e31816e23ba [DOI] [PubMed] [Google Scholar]
- 6. Lucchinetti CF, Kimmel DW, Lennon VA. Paraneoplastic and oncologic profiles of patients seropositive for type 1 antineuronal nuclear autoantibodies. Neurology 1998;50:652–7. 10.1212/WNL.50.3.652 [DOI] [PubMed] [Google Scholar]
- 7. Newton HB, Malkin MG. Neurological complications of systemic cancer and antineoplastic therapy. New York: CRC Press, 2016. [Google Scholar]