Abstract
Objective
Oral anticoagulation (OAC) is state-of-the-art therapy for atrial fibrillation (AF), the most common arrhythmia worldwide. However, little is known about the perception of patients with AF and how it correlates with risk scores used by their physicians. Therefore, we correlated patients’ estimates of their own stroke and bleeding risk with the objectively predicted individual risk using CHA2DS2-VASc and HAS-BLED scores.
Design
Cross-sectional prevalence study using convenience sampling and telephone follow-up.
Settings
Eight hospital departments and one general practitioner in Austria. Patients’ perception of stroke and bleeding risk was opposed to commonly used risk scoring.
Participants
Patients with newly diagnosed AF and indication for anticoagulation.
Main outcome measures
Comparison of subjective risk perception with CHA2DS2-VASc and HAS-BLED scores showing possible discrepancies between subjective and objective risk estimation. Patients’ judgement of their own knowledge on AF and education were also correlated with accuracy of subjective risk appraisal.
Results
Ninety-one patients (age 73±11 years, 45% female) were included in this study. Subjective stroke and bleeding risk estimation did not correlate with risk scores (ρ=0.08 and ρ=0.17). The majority of patients (57%) underestimated the individual stroke risk. Patients feared stroke more than bleeding (67% vs 10%). There was no relationship between accurate perception of stroke and bleeding risks and education level. However, we found a correlation between the patients’ judgement of their own knowledge of AF and correct assessment of individual stroke risk (ρ=0.24, p=0.02). During follow-up, patients experienced the following events: death (n=5), stroke (n=2), bleeding (n=1). OAC discontinuation rate despite indication was 3%.
Conclusions
In this cross-sectional analysis of OAC-naive patients with AF, we found major differences between patients’ perceptions and physicians’ assessments of risks and benefits of OAC. To ensure shared decision-making and informed consent, more attention should be given to evidence-based and useful communication strategies.
Trial registration number
Keywords: anticoagulation
Strengths and limitations of this study.
The design of this cross-sectional study allowed the objective assessment of the patients’ risk perception immediately after initiation of anticoagulation for atrial fibrillation.
For generalisability, primary, secondary and tertiary healthcare centres were included in this study.
To evaluate long-time outcome, follow-up was obtained via telephone.
The study is statistically powered for the cross-sectional comparison, but the number of patients included does not allow association between baseline characteristics and events during follow-up.
Introduction
Atrial fibrillation (AF) is the most common significant arrhythmia worldwide, associated with a fivefold increase in risk for stroke1 and almost doubles the risk of mortality.2 In an ageing population, the number of individuals affected is projected to increase exponentially over the next decades.3 Since the early 1990s, oral anticoagulation (OAC) is the state-of-the-art therapy for reducing stroke and embolic events.2 OAC is considered a long-term, often lifelong medical intervention. Therefore, clinicians and particularly patients need to have a clear understanding of the related benefits and imminent harms.4 It serves as a reasonable background for shared decision-making of patients and their doctors, one of the most important principles for patients’ reliance, compliance and adherence to recommended medical strategies.5 6
Adequate information of patients7 and increased health literacy8 are of major importance for compliance and adherence to therapy. Patients’ knowledge also affects the perception of risk for stroke, embolic events and bleeding. It has been shown that the extent of information perceived influenced patients’ preferences towards or against OAC treatment the most.9
Clinicians use algorithms like CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke, vascular disease, age 65–74 years, sex category) and HAS-BLED (hypertension, abnormal renal and liver function, stroke, bleeding, labile INR, elderly, drugs or alcohol) scores10–12 to predict the balance of future risk for stroke and embolic events versus bleeding in an individual patient. A recent survey of the European Heart Rhythm Association proved that a considerable amount of time and resources are needed in daily clinical practice to communicate risk/benefit ratios to patients suffering from AF: several centres have established special OAC clinics and initial visits mostly lasted 21–30 min.13 However, decades after the introduction of OAC therapy, standardised and validated risk communication tools14–16 are still missing and adherence follow-up programmes are rare.13 Those programmes have an important impact on effectiveness of OAC: adherence to OAC is considered a key factor for preventing events,17 but it is still as low as 43%.18
Little is known about the perception of patients with AF and how it correlates with risk scores used by their physicians.19 A potential gap between subjective and objective assessments may increase the likelihood of non-compliance to OAC in patients with AF.20 Therefore, the study was designed to correlate the subjective stroke and bleeding risk with the objectively predicted individual risks calculated by CHA2DS2-VASc and HAS-BLED scores.
Methods
This work is a cross-sectional prevalence study, using convenience sampling by trained doctors at nine centres (representing primary, secondary and tertiary healthcare) in the province of Styria, Austria. Responsible institutional review boards approved the study (1376/2015 (BHB Graz, Austria), 28-004 ex 15/16 (Medical University of Graz, Austria)). Furthermore, the study was registered under the ClinicalTrials.gov number NCT03061123. Patients with first diagnosed and ECG-documented non-valvular AF and indication for OAC were included in the study. Exclusion criteria were pre-existing OAC therapy, valvular heart disease, history of valve surgery, denial or inability of informed consent.
This study was designed to comply with standard operating procedures of individual centres for initiation of OAC therapy. Responsible physicians were asked to include all eligible patients. Immediately after the pretreatment interviews, which included the discussion of benefits, harms and side effects of OAC, patients were asked to participate in the study. After informed consent was signed, a standardised questionnaire was handed out to all patients (see table S1 in the online supplemental file 1)
bmjopen-2017-018242supp001.pdf (361.8KB, pdf)
Questionnaire
The survey was conducted using a standardised questionnaire with two parts (see table S1 in the online supplementary file 1). The patient-oriented part consisted of seven questions covering subjective perception of patients with regard to general individual risk/benefit ratios of OAC in AF, the willingness of therapy continuation even in the possible case of minor adverse effects (haematoma, minor bleeding) and the individually discerned level of information. We used three-point and four-point verbal rating scales to comply with the patients’ categorical perception of checks and balances.21
Physicians in charge of patients filled the second part, which included patient demographics, CHA2DS2-VASc and HAS-BLED scores, as well as the intended OAC therapy.
CHA2DS2-VASc and HAS-BLED scores were stratified into four risk categories each corresponding to the four different risk levels for stroke/embolic events and bleeding interrogated by the patient questionnaire. Risk estimations were based on published data from large population studies. Regarding CHA2DS2-VASc score, patients with zero points (stroke rate 0%–1%/year) were considered low risk, one point (stroke rate 1%–2%/year) intermediate risk, 2–4 points (stroke rate 2%–7%/year) high risk and≥5 points (stroke rate >7 %/year) very high risk cohort.10 22 23 The corresponding categories concerning HAS-BLED score were as follows: no or one risk factor (low risk group, bleeding rate 0%–4%/year), two risk factors (intermediate risk group, bleeding rate 4%–6%/year), three or four risk factors (high risk group, bleeding rate 6%–10%/year) and five or more risk factors (very high risk group, bleeding rate >10 %/year).11 22
For assessing the awareness of general benefit of OAC, we asked patients to estimate their appraisal of relative risk reduction (RRR) for stroke and embolic events. We defined high (RRR 50%–74%) as an accurate answer,24 others were low (RRR 0%–24%), intermediate (RRR 25%–49%) and very high (RRR 75%–100%). We extrapolated predicted HRs of bleeding due to OAC from meta-analyses24–27 and defined the general risk of OAC as intermediate (HR 1.25–1.49). Other options were low (HR 1.00–1.24), high (HR 1.50–2.00) and very high (HR >2.00). Subjective scales were interpreted as ‘correct’ if they corresponded correctly to individual objective risk groups.
Follow-up
Follow-up was obtained by phone calls. Patients were asked about their current status of OAC therapy and the occurrence of cardiovascular or bleeding events.
Statistical analysis
Sample size calculation was performed using the freeware tool G*Power by Heinrich Heine University Düsseldorf (http://www.gpower.hhu.de). We sought to oppose the self-reported benefits and risks of OAC with an actual assessment using validated data (including CHA2DS2-VASc score and HAS-BLED score). To prove correlation (|ρ|<0.3) with type I error (α) of 0.05 and power (1−β) of 80%, at least 84 patients had to be included into the study.
Two-sided significance level was 0.05. Data are presented as mean±SD deviation, median (IQR) or count (proportion), where appropriate. Pearson’s test and Spearman’s rank correlation coefficient were used to correlate ordinal variables (eg, subjective perceptions and risk scores). Correlation coefficients (ie, |r|, |ρ|) were interpreted as follows: negligible correlation (0.0–0.3), low correlation (0.3–0.5), moderate correlation (0.5–0.8) and strong correlation (0.8–1.0).28
Data were analysed with IBM SPSS Statistics V.23 (IBM Corporation, Armonk, New York, USA). All raw data can be found in the online supplementary file 1.
Results
Patient population
From September 2015 to March 2016, 91 patients (age 73±11 years, 45% female) from nine centres were included in this study (see table S2 in the online supplementary file 1). As highest educational attainment, lower secondary education (International Standard Classification of Education (ISCED) level 2, n=32, 35%) and higher secondary vocational education (ISCED level 3B, n=25, 28%) were most prevalent. New oral anticoagulants were used most frequently (n=75, 82%). Vitamin K antagonists (n=14, 15%) and low-molecular weight heparin (n=2, 2%) were given to remaining patients.
Objective risk estimation
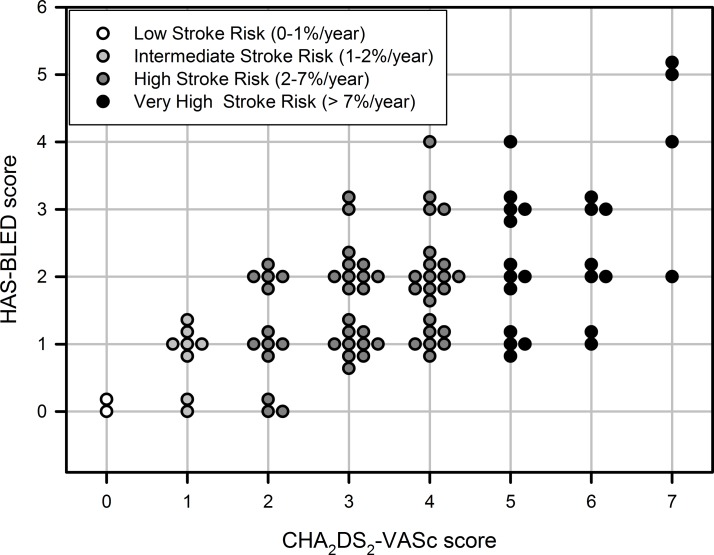
Median CHA2DS2-VASc score was 4 (IQR 2–5). Therefore, we summarised most patients on high risk for stroke or embolic events (CHA2DS2-VASc score 2–4, stroke risk 2%–7%/year, figure 1). Most common risk factors were arterial hypertension and age>75 years (table 1). In terms of HAS-BLED score, most of the patients were in low (0–1 points, bleeding risk 0%–4%) and intermediate risk groups (two points, bleeding risk 4%–6%; figure 1).
Figure 1.
CHA2DS2-VASc and HAS-BLED scores of individual patients, including our classification into low, intermediate, high and very high stroke risk groups (stratified by CHA2DS2-VASc score).
Table 1.
CHA2DS2-VASc and HAS-BLED scores and individual risk factors
| CHADS2 score | 2 (1–3) |
| CHA2DS2-VASc score | 4 (2–5) |
| CHA2DS2-VASc score≥2 | 81 (89%) |
| Congestive heart failure | 14 (15%) |
| Hypertension (diagnosis of arterial hypertension) | 75 (82%) |
| Age>75 years | 48 (53%) |
| Diabetes mellitus | 18 (20%) |
| Stroke or TIA | 15 (17%) |
| Vascular disease | 27 (30%) |
| Age 65–75 years | 25 (28%) |
| Female sex | 41 (45%) |
| HAS-BLED score | 2 (1–2) |
| HAS-BLED score≥3 | 17 (19%) |
| Hypertension (systolic blood pressure>160 mm Hg) | 42 (46%) |
| Abnormal kidney/liver function | 8 (9%) |
| Stroke | 14 (15%) |
| Bleeding | 1 (1%) |
| Labile INR values | 1 (1%) |
| Elderly (age>65 years) | 72 (79%) |
| Drugs or alcohol (one point) | 16 (18%) |
| Drugs and alcohol (two points) | 2 (2%) |
INR, international normalised ratio; TIA, transient ischaemic attack.
Perception of individual risk
Many patients (n=41, 45%) interpreted risk for stroke and embolic events in atrial fibrillation as high (corresponding stroke risk 2%–7% per year). Bleeding risk was estimated mainly as intermediate (corresponding bleeding risk 4%–6% per year, n=40, 44%). Patients feared stroke more than bleeding (67% vs 10%) and only 9% would discontinue OAC therapy if minor bleeding complications (eg, epistaxis) would occur. Patients estimated their personal level of information as good or adequate in 41% and 34%, respectively.
Correlations
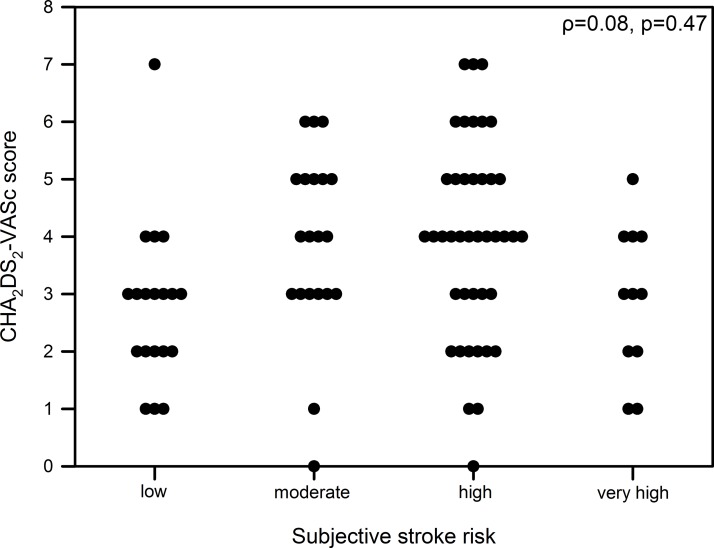
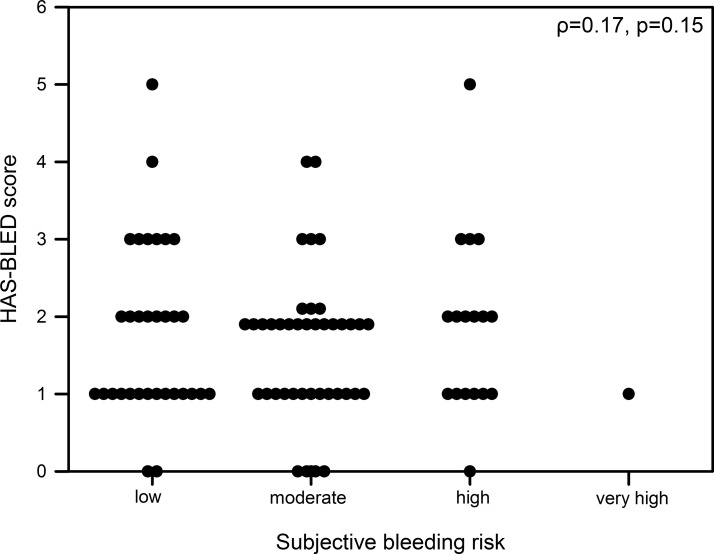
Patients estimated their risk for stroke or embolic events in concordance to the individual CHA2DS2-VASc score in 28% (n=25) of cases, but by the majority (n=52, 57%) risk was under-rated. Bleeding risk was assumed accurately in 41% (n=37), but overestimated in 31 cases (34%). There were no significant correlations neither between objectively assessed and subjectively expected risk for stroke nor for bleeding (ρ=0.08, p=0.47, figure 2 and ρ<0.01, p=0.98, figure 3).
Figure 2.
Correlation of CHA2DS2-VASc score and subjective assessed stroke risk.
Figure 3.
Correlation of HAS-BLED score and subjective assessed bleeding risk.
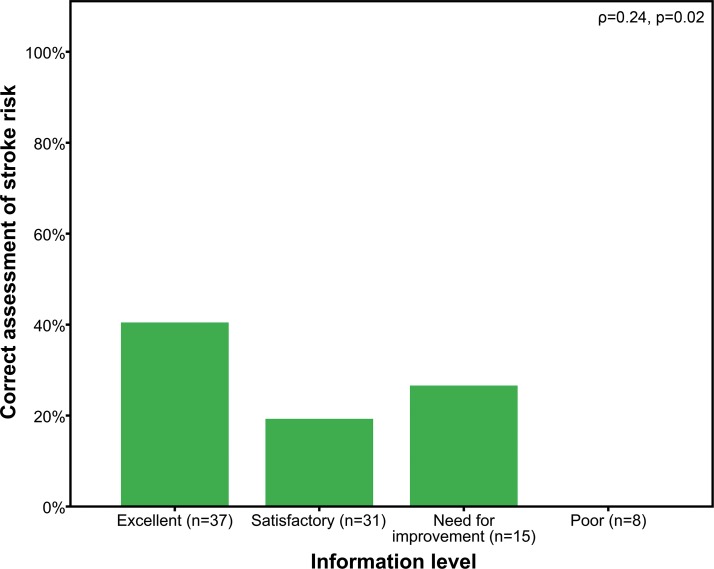
Analogies in patients’ answers and CHA2DS2-VASc and HAS-BLED scores did not correlate to the levels of highest education (ρ=−0.06, p=0.64 and ρ=0.17, p=0.15). However, we observed a significant correlation between patients’ judgement of their knowledge of AF with regard to concordant assumptions of stroke risk and CHA2DS2-VASc score (ρ=0.24, p=0.02, figure 4). No correlation was observed between patients’ judgement of AF knowledge and concordance with subjectively assumed and objectively predicted risk for bleeding events (ρ=0.08, p=0.45).
Figure 4.
Amount of correct answered assessment of stroke risk in patients with different self-assessed levels of information.
Perception of general risk
Most patients (n=51, 56%) assumed score-predicted effectiveness of OAC in AF as high (corresponding stroke risk reduction 50%–74%). Other answers were very high (RRR 75%–100%; n=23, 25%), intermediate (RRR 25%–49%; n=15, 17%) or low (RRR 0%–24%; n=1, 1%). The estimated general risk of bleeding caused by OAC was considered by patients as intermediate (HR for bleeding 1.25–1.49; n=37, 41%) and low (HR 1.00–1.24; n=30, 33%). Only three patients (3%) estimated the bleeding risk associated with OAC as very high (HR>2.00).
Follow-up
Follow-up via telephone was obtained 18±2 months after enrolment from 84 patients (92%). The remaining seven patients were lost to follow-up because of missing contact details (n=6, 7%) or denial to participate (n=1, 1%). The following events were reported during follow-up: death of unknown cause (n=5, 5%), ischaemic stroke (n=2, 2%) and epistaxis requiring hospitalisation (n=1, 1%). All patients with ischaemic or bleeding events were under OAC therapy and had continued it until follow-up.
At time of follow-up, four patients had discontinued OAC therapy intermittently (n=1, 1%) or permanently (n=3, 3%). One female patient with CHA2DS2-VASc score of 2 reported that OAC therapy was terminated due to successful pulmonary vein isolation without any recurrence of AF during 9 months of event recorder monitoring. Three patients (CHA2DS2-VASc score between 3 and 7) discontinued OAC therapy on their own; although one patient reinitiated OAC therapy after discussion with his general practitioner.
Patients, who stopped OAC therapy on their own, believed that their current condition ‘had no indication’ for OAC therapy. Two of them had underestimated their individual stroke risk at baseline interrogation, while one had overestimated it. Two stoppers feared the risk of bleeding more than the risk for ischaemic events.
Discussion
This cross-sectional questionnaire study in 91 OAC-naive patients with non-valvular AF shows that (1) patients generally underestimated their risk of stroke, (2) they perceived their individual stroke risk to higher extent than bleeding risk and (3) there was a significant correlation between accuracy in answers and patients’ judgement of their knowledge of AF. During follow-up, we observed OAC discontinuation despite clear indication in 3% of patients.
Due to the high prevalence of AF in the western world, non-adherence to OAC in patients with AF has a tremendous impact on our society. Despite the availability of adequate therapy, AF-related strokes are still estimated to cost US$ 8 billion annually in the USA29 30 or over 9000 GBP per stroke in the UK.31 The increased severity of AF-related strokes compared with other aetiologies32 may even increase the negative effect of general embolic events on quality of life.33 As a consequence, it is urgently necessary to ameliorate adherence to OAC therapy for AF. We proved underjudgement of stroke risk and, therefore, postulate better patient education as a possibility to overcome this problem.
No correlation between subjective assessment and objective risk
To our knowledge, this is the first study that compares the subjective risk perception of patients with AF with evidence-based risk scores used in daily clinical practice. We found no significant correlation between subjective and objective assessment of stroke or bleeding risk. Therefore, our study provides evidence that a perception gap remains after informed consent discussion before OAC initiation. Although not powered for it, we provide preliminary data on the OAC discontinuation rate 1 year after OAC initiation. Two of three patients, who stopped OAC on their own, had underestimated their stroke risk at baseline.
If this finding remains constant in larger trials, it has a direct impact on clinical practice. A perception gap between subjective and objective assessment of stroke or bleeding risk is considered a major obstacle at the start of a lifelong medical intervention. It hinders not only shared decision-making, but may also worsen treatment compliance and adherence.34
Previous studies already evaluated the levels of information in patients after initiation of OAC treatment.19 35–39 In a survey of 711 patients with AF that were on OAC for at least 1 year, only 7% knew the purpose of anticoagulation in AF.38 Lane et al 35 observed that 51% of patients with AF with OAC therapy for ≥3 months could not name their cardiac condition. Furthermore, the knowledge could not be increased by a brief educational intervention. McCabe et al 40 showed considerable knowledge deficits already 2 weeks after initial diagnosis of AF. A recent qualitative systematic review postulated the lack of patient information as one of the most important reasons for vitamin K antagonists underuse.41
Although Dantas et al 37 demonstrated that only minimal knowledge of patients is needed to allow acceptance of OAC, doctors should seek shared decisions. This is even more important, when evidence for drug treatment is marginal,42 which is definitely not the case in patients with high risk scores for AF.2 However, the physician’s perspective of shared decision-making may not be congruent to the patient’s perceptions.43 LaHaye et al 44 demonstrated high interpatient variability regarding individual treatment thresholds. Consequently, we propose that health literacy of patients should be enhanced before OAC initiation, especially regarding the individual risk/benefit ratio. Thus, patients may be able to participate in decision-making of therapy initiation. Patients also seem to have difficulties regarding verbal descriptions of risk.45 Therefore, graphical information might help overcome this problem.7 14 One promising example is an electronic prototype for the translation of Grading of Recommendations Assessment, Development and Evaluation summaries46 into decision aids using interactive formats to present evidence summaries at varying levels of detail.16 Another possibility is the establishment of a Fact Box, which describes evidence of benefits and harms without making recommendations.15 Further theory-driven educational interventions have been shown to increase OAC control47 or knowledge of international normalised ratio targets.35
Stroke risk is topping bleeding risk
In our study, most of the patients assumed their personal stroke risk to be the most frequent and serious complication of untreated atrial fibrillation in their setting. However, the majority (57%) underestimated their stroke risk while 41% interpreted their bleeding risk accurately. In other studies, patients were keen on avoiding stroke more than bleeding48 and placed even more importance on stroke prevention than doctors49 with higher tolerance of adverse bleeding events.50 Nevertheless, with increased duration of OAC therapy, knowledge about OAC in the indication of AF seems to deteriorate.38
Factors influencing correct risk estimation
We found out that the highest level of educational attainment did not correlate with analogies in risk estimation in our analysis. Our results therefore indicate that understanding of individuals’ risk is not correlated with formal education levels. However, the preservation of knowledge might be correlated with better education.40 Lip et al 39 showed differences of AF perceptions in different ethnical groups. We could not add evidence to this factor as we included only Caucasian patients.
Patients that felt better informed had an improved understanding of their individual risks in this study. Consequently, we encourage to evaluate patients’ information level repeatedly by asking how informed they felt and to take appropriate measures to enhance the patient’s level of information if required.
Limitations
Our study has several limitations. First, due to the absence of a screening log, consecutive patient enrolment cannot be guaranteed. Second, the study was powered for cross-sectional analysis, but not for associations between baseline parameters and OAC adherence or events at follow-up. Therefore, we can only speculate that higher levels of information might be associated with better adherence and outcomes as results of previous studies suggested. Third, we did not evaluate other bleeding risk scores, such as AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA)51 or Outcomes Registry for Better Informed Treatment (ORBIT),52 into the analysis. Lastly, we intended to concentrate on the risk perception of individual patients and did not evaluate the general knowledge of AF and stroke prevention per se in a standardised questionnaire.53 Due to this fact, we kept the questionnaire short and tried to minimise bias due to selection of motivated patients that may not be representative of the general AF population.19
Conclusion
In this cross-sectional analysis of OAC-naive patients with AF, we found major differences between patients’ perceptions and physicians’ assessments of risks and benefits of OAC. To ensure shared decision-making and informed consent, more attention should be given to evidence-based and useful communication strategies.
Supplementary Material
Acknowledgments
We appreciate the helpful assistance of Stefan Zweiker in the preparation of the manuscript. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Footnotes
Contributors: RZ, MS and NW designed the study. RZ, EW, KR, MS, VS, PK, NB, MH, GR, GZ, MS and NW were involved in conduction of the study and data collection. DZ and NW performed the statistical analysis. DZ, RZ, MS and NW wrote the manuscript. All authors have read and approved the last version of the manuscript.
Competing interests: NB reports personal fees from Bayer, Medtronic, Daiichi-Sankyo, Servier, AstraZeneca, other from Boehringer-Ingelheim, Bayer, Lilly outside the submitted work. HM, PK, GR, SM, MS, VS, EW, DZ and GZ have nothing to disclose. NW reports personal fees from Lectures and Consulting outside the submitted work. RZ reports grants from Lilly, personal fees from Boehringer Ingelheim, Bayer and Daiichi-Sankyo outside the submitted work.
Ethics approval: IRB of the Medical University of Graz, IRB of the Hospital Barmherzige Brueder Graz.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All raw data are available in the supplementary appendix.
References
- 1. Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369–429. 10.1093/eurheartj/ehq278 [DOI] [PubMed] [Google Scholar]
- 2. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESCEndorsed by the European Stroke Organisation (ESO). Eur Heart J 2016;37:2893–962. [DOI] [PubMed] [Google Scholar]
- 3. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370–5. [DOI] [PubMed] [Google Scholar]
- 4. Coulter A, Ellins J. Effectiveness of strategies for informing, educating, and involving patients. BMJ 2007;335:24–7. 10.1136/bmj.39246.581169.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spatz ES, Krumholz HM, Moulton BW. The new era of informed consent: getting to a reasonable-patient standard through shared decision making. JAMA 2016;315:2063–4. 10.1001/jama.2016.3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deyo RA. A key medical decision maker: the patient. BMJ 2001;323:466–7. 10.1136/bmj.323.7311.466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lane DA, Barker RV, Lip GY. Best practice for atrial fibrillation patient education. Curr Pharm Des 2015;21:533–43. 10.2174/1381612820666140825125715 [DOI] [PubMed] [Google Scholar]
- 8. Health literacy: report of the Council on Scientific Affairs. Ad Hoc Committee on Health Literacy for the Council on Scientific Affairs, American Medical Association. JAMA 1999;281:552–7. [PubMed] [Google Scholar]
- 9. Wilke T, Bauer S, Mueller S, et al. Patient preferences for oral anticoagulation therapy in atrial fibrillation: a systematic literature review. Patient 2017;10:17- 37. 10.1007/s40271-016-0185-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263–72. 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 11. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093–100. 10.1378/chest.10-0134 [DOI] [PubMed] [Google Scholar]
- 12. January CT, Wann LS, Alpert JS, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1–76. 10.1016/j.jacc.2014.03.022 [DOI] [PubMed] [Google Scholar]
- 13. Potpara TS, Pison L, Larsen TB, et al. How are patients with atrial fibrillation approached and informed about their risk profile and available therapies in Europe? Results of the European heart rhythm association survey. Europace 2015;17:468–72. 10.1093/europace/euv025 [DOI] [PubMed] [Google Scholar]
- 14. Man-Son-Hing M, Laupacis A, O’Connor AM, et al. A patient decision aid regarding antithrombotic therapy for stroke prevention in atrial fibrillation: a randomized controlled trial. JAMA 1999;282:737–43. 10.1001/jama.282.8.737 [DOI] [PubMed] [Google Scholar]
- 15. McDowell M, Rebitschek FG, Gigerenzer G, et al. A simple tool for communicating the benefits and harms of health interventions: a guide for creating a fact box. MDM Policy & Practice 2016;1:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Agoritsas T, Heen AF, Brandt L, et al. Decision aids that really promote shared decision making: the pace quickens. BMJ 2015;350:g7624 10.1136/bmj.g7624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van der Pol M, Hennessy D, Manns B. The role of time and risk preferences in adherence to physician advice on health behavior change. Eur J Health Econ 2017;18 10.1007/s10198-016-0800-7 [DOI] [PubMed] [Google Scholar]
- 18. Yao X, Abraham NS, Alexander GC, et al. Effect of adherence to oral anticoagulants on risk of stroke and major bleeding among patients with atrial fibrillation. J Am Heart Assoc 2016;5:e003074 10.1161/JAHA.115.003074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aliot E, Breithardt G, Brugada J, et al. An international survey of physician and patient understanding, perception, and attitudes to atrial fibrillation and its contribution to cardiovascular disease morbidity and mortality. Europace 2010;12:626–33. 10.1093/europace/euq109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao S, Zhao H, Wang X, et al. Factors influencing medication knowledge and beliefs on warfarin adherence among patients with atrial fibrillation in China. Patient Prefer Adherence 2017;11:213–20. 10.2147/PPA.S120962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lloyd AJ. The extent of patients’ understanding of the risk of treatments. Qual Health Care 2001;10:14–18. 10.1136/qhc.0100014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. LaHaye SA, Gibbens SL, Ball DG, et al. A clinical decision aid for the selection of antithrombotic therapy for the prevention of stroke due to atrial fibrillation. Eur Heart J 2012;33:2163–71. 10.1093/eurheartj/ehs167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Olesen JB, Lip GY, Hansen PR, et al. Bleeding risk in ‘real world’ patients with atrial fibrillation: comparison of two established bleeding prediction schemes in a nationwide cohort. J Thromb Haemost 2011;9:1460–7. 10.1111/j.1538-7836.2011.04378.x [DOI] [PubMed] [Google Scholar]
- 24. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857–67. 10.7326/0003-4819-146-12-200706190-00007 [DOI] [PubMed] [Google Scholar]
- 25. Investigators AF. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994;154:1449–57. [PubMed] [Google Scholar]
- 26. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American college of chest physicians evidence-based clinical practice guidelines (8th edition). Chest 2008;133:257–98. [DOI] [PubMed] [Google Scholar]
- 27. You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012;141:531S–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mukaka MM. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med J 2012;24:69–71. [PMC free article] [PubMed] [Google Scholar]
- 29. Caro JJ. An economic model of stroke in atrial fibrillation: the cost of suboptimal oral anticoagulation. Am J Manag Care 2004;10:S451–8. [PubMed] [Google Scholar]
- 30. Casciano JP, Dotiwala ZJ, Martin BC, et al. The costs of warfarin underuse and nonadherence in patients with atrial fibrillation: a commercial insurer perspective. J Manag Care Pharm 2013;19:302–16. 10.18553/jmcp.2013.19.4.302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ali AN, Howe J, Abdel-Hafiz A. Cost of acute stroke care for patients with atrial fibrillation compared with those in sinus rhythm. Pharmacoeconomics 2015;33:511–20. 10.1007/s40273-015-0263-1 [DOI] [PubMed] [Google Scholar]
- 32. Lang C, Seyfang L, Ferrari J, et al. Do women with atrial fibrillation experience more severe strokes? Results from the austrian stroke unit registry. Stroke 2017;48 10.1161/STROKEAHA.116.015900 [DOI] [PubMed] [Google Scholar]
- 33. King RB. Quality of life after stroke. Stroke 1996;27:1467–72. 10.1161/01.STR.27.9.1467 [DOI] [PubMed] [Google Scholar]
- 34. Berkman ND, Sheridan SL, Donahue KE, et al. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med 2011;155:97–107. 10.7326/0003-4819-155-2-201107190-00005 [DOI] [PubMed] [Google Scholar]
- 35. Lane DA, Ponsford J, Shelley A, et al. Patient knowledge and perceptions of atrial fibrillation and anticoagulant therapy: effects of an educational intervention programme. The West Birmingham Atrial Fibrillation project. Int J Cardiol 2006;110:354–8. 10.1016/j.ijcard.2005.07.031 [DOI] [PubMed] [Google Scholar]
- 36. Arnsten JH, Gelfand JM, Singer DE. Determinants of compliance with anticoagulation: a case–control study. Am J Med 1997;103:11–17. 10.1016/S0002-9343(97)90048-6 [DOI] [PubMed] [Google Scholar]
- 37. Dantas GC, Thompson BV, Manson JA, et al. Patients’ perspectives on taking warfarin: qualitative study in family practice. BMC Fam Pract 2004;5:15 10.1186/1471-2296-5-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lip GY, Agnelli G, Thach AA, et al. Oral anticoagulation in atrial fibrillation: a pan-European patient survey. Eur J Intern Med 2007;18:202–8. 10.1016/j.ejim.2006.11.005 [DOI] [PubMed] [Google Scholar]
- 39. Lip GY, Kamath S, Jafri M, et al. Ethnic differences in patient perceptions of atrial fibrillation and anticoagulation therapy: the West Birmingham atrial fibrillation project. Stroke 2002;33:238–42. [DOI] [PubMed] [Google Scholar]
- 40. McCabe PJ, Schad S, Hampton A, et al. Knowledge and self-management behaviors of patients with recently detected atrial fibrillation. Heart Lung 2008;37:79–90. 10.1016/j.hrtlng.2007.02.006 [DOI] [PubMed] [Google Scholar]
- 41. Mas Dalmau G, Sant Arderiu E, Enfedaque Montes MB, et al. Patients’ and physicians’ perceptions and attitudes about oral anticoagulation and atrial fibrillation: a qualitative systematic review. BMC Fam Pract 2017;18:3 10.1186/s12875-016-0574-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Charles C, Whelan T, Gafni A. What do we mean by partnership in making decisions about treatment? BMJ 1999;319:780–2. 10.1136/bmj.319.7212.780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Borg Xuereb C, Shaw RL, Lane DA. Patients’ and health professionals’ views and experiences of atrial fibrillation and oral-anticoagulant therapy: a qualitative meta-synthesis. Patient Educ Couns 2012;88:330–7. 10.1016/j.pec.2012.05.011 [DOI] [PubMed] [Google Scholar]
- 44. Lahaye S, Regpala S, Lacombe S, et al. Evaluation of patients’ attitudes towards stroke prevention and bleeding risk in atrial fibrillation. Thromb Haemost 2014;111:465–73. 10.1160/TH13-05-0424 [DOI] [PubMed] [Google Scholar]
- 45. Fuller R, Dudley N, Blacktop J. Risk communication and older people-understanding of probability and risk information by medical inpatients aged 75 years and older. Age Ageing 2001;30:473–6. 10.1093/ageing/30.6.473 [DOI] [PubMed] [Google Scholar]
- 46. Vandvik PO, Brandt L, Alonso-Coello P, et al. Creating clinical practice guidelines we can trust, use, and share: a new era is imminent. Chest 2013;144:381–9. 10.1378/chest.13-0746 [DOI] [PubMed] [Google Scholar]
- 47. Clarkesmith DE, Pattison HM, Lip GY, et al. Educational intervention improves anticoagulation control in atrial fibrillation patients: the TREAT randomised trial. PLoS One 2013;8:e74037 10.1371/journal.pone.0074037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. MacLean S, Mulla S, Akl EA, et al. Patient values and preferences in decision making for antithrombotic therapy: a systematic review: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-based Clinical Practice guidelines. Chest 2012;141:e1S–23. 10.1378/chest.11-2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Devereaux PJ, Anderson DR, Gardner MJ, et al. Differences between perspectives of physicians and patients on anticoagulation in patients with atrial fibrillation: observational study. BMJ 2001;323:1218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Alonso-Coello P, Montori VM, Díaz MG, et al. Values and preferences for oral antithrombotic therapy in patients with atrial fibrillation: physician and patient perspectives. Health Expect 2015;18:2318–27. 10.1111/hex.12201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) study. J Am Coll Cardiol 2011;58:395–401. 10.1016/j.jacc.2011.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. O’Brien EC, Simon DN, Thomas LE, et al. The ORBIT bleeding score: a simple bedside score to assess bleeding risk in atrial fibrillation. Eur Heart J 2015;36:ehv476–64. 10.1093/eurheartj/ehv476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hendriks JM, Crijns HJ, Tieleman RG, et al. The atrial fibrillation knowledge scale: development, validation and results. Int J Cardiol 2013;168:1422–8. 10.1016/j.ijcard.2012.12.047 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-018242supp001.pdf (361.8KB, pdf)