Abstract
Diabetes alters cerebral metabolism, structure, and function. Both hyperglycemia and therapy-associated hypoglycemia are believed to have an impact on the brain, and this impact may depend on the age of the individual, their stage of neurological development, and whether they have Type 1 or Type 2 diabetes. Hypoglycemia in children with Type 1 has consistently been associated with a reduction in neurocognitive function, but such a finding has not been seen in adults with Type 1 diabetes. Both hypoglycemia and hyperglycemia have been linked with dementia in adults with Type 2 diabetes. In both Type 1 and Type 2 diabetes, recurrent episodes of treatment-associated hypoglycemia impair how well the brain can sense and respond to subsequent episodes of hypoglycemia. In this brief review, we will review how diabetes affects the brain with a focus on investigations done in our own laboratory. We have focused on using high magnetic field imaging and spectroscopy to identify subtle changes in brain structure and metabolism that may contribute to the long-term cerebral complications of diabetes. We have found evidence of microstructural changes in white matter regions, reduced gray matter density, and reduced activation of the thalamus in response to recurrent hypoglycemia in patients with Type 1 diabetes.
Keywords: diabetes, cerebral metabolism, brain structure, cognition, magnetic resonance imaging and spectroscopy
INTRODUCTION
Diabetes mellitus is a metabolic disease that afflicts more than 29 million Americans (1) and many more worldwide. The disease is increasing in prevalence, and in 2050, 1 in 3 Americans are expected to have sufficient hyperglycemia to meet the diagnostic criteria (2). Most of the morbidity and mortality associated with diabetes is due to the development of the complications of the disease. Macrovascular complications like coronary artery disease and stroke are a common cause of death in people with diabetes. The microvascular complications of retinopathy, nephropathy, and neuropathy are the number 1 causes of blindness, renal failure, and limb amputations in the United States (1). The risk of developing these microvascular complications is greatly reduced when treatment strategies are used to achieve near normal levels of glycemia. For patients with Type 1 diabetes, this strategy always requires the use of insulin since the autoimmune destruction of the pancreatic β cell that caused their disease renders than profoundly insulinopenic. Patients with Type 2 diabetes are usually managed with oral therapies early in the course of their disease but often go on to require insulin to control their blood sugars as the disease advances.
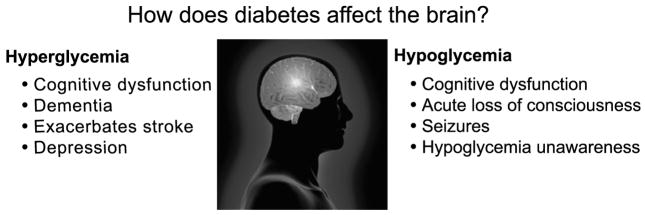
In recent years, it has become recognized that other organ systems not typically associated with diabetes complications are themselves altered by long-term exposure to the disease. In this report, we will review how diabetes affects the brain with a focus on investigations done in our own laboratory. In considering how diabetes impacts the cerebral structure and function, it is essential to consider how the extremes in glycemia experienced by patients with diabetes might affect the brain (Fig. 1). Although diabetes can be associated with changes in many physiological parameters including insulin sensitivity, hyperlipidemia, and hypertension, and both hyperglycemia and hypoglycemia are common in patients with the disease who are treated with insulin or drugs that increase insulin secretion like sulfonylureas. The primary adverse effect of both insulin and sulfonylureas is hypoglycemia. Thus, patients with both Type 1 and Type 2 diabetes who are treated with insulin or sulfonylureas may experience hyperglycemia because of their underlying disease and hypoglycemia as an adverse effect of their treatment.
FIGURE 1.
How does diabetes affect the brain? Effects of hyper- and hypoglycemia.
In the acute setting, hyperglycemia and hypoglycemia have been shown to impair cognitive function both in children and in adults. Several years ago, Gonder-Frederick et al. (3) asked school children with diabetes to perform simple tasks on a handheld computer at the time they checked their blood sugar. They found that task performance was worse when blood sugar was greater than 400 mg/dl (22.2 mM) and less than 54 mg/dl (3.0 mM), demonstrating that glycemia above and below normal has acute effects on cognitive function. The transient effect of acute hypoglycemia on cognition and mood in patients with Type 1 diabetes has been recently reviewed (4), and most studies have found a negative impact on mood and motivation. A linear relationship between poor performance on the California Learning Test and elevated glycosylated hemoglobin has also been demonstrated in older patients with Type 2 diabetes by Reaven et al. (5). Sommerfield and colleagues (6) also demonstrated impairments in mood state and cognitive performance in patients with Type 2 diabetes during experimental hyperglycemia.
Chronic exposure to recurrent hyperglycemia and hypoglycemia has also been linked to changes in brain structure and function (7), but it has been difficult to determine what the relative contributions of hyperglycemia and hypoglycemia to these changes might be because insulin- and sulfonylurea-treated patients generally experience both. In addition, the impact of extremes in glycemia on the brain may depend on the age of the patient. In children with Type 1 diabetes, those who have experienced episodes of severe hypoglycemia at an age less than 5 years seem to have reduced performance on measures of spatial intelligence and delayed recall when compared with children who did not experience severe hypoglycemia at a young age, but both sets of children with Type 1 diabetes had reduced measures of verbal intelligence compared with non diabetic sibling controls (8). Structural changes in children with Type 1 diabetes have also been identified, but hyperglycemia and hypoglycemia seem to affect different regions differently (9).
Adults with diabetes have also been found to have structural changes, including atrophy (10) and leukoariosis (11), but the relative impact of hyperglycemia versus hypoglycemia on these changes remains uncertain. In the Diabetes Control and Complications Trial/Epidemiology of Diabetes Complications cohort of adults with Type 1 diabetes, no differences were found in cognitive function measured at year 18 between those who did and did not experience episodes of severe hypoglycemia or between those randomized to intensive versus standard glucose control during the active treatment period.(12) Interestingly, moderate reductions in performance on tests of motor speed and psychomotor efficiency were significantly related to higher glycated hemoglobin in this population (12). In large epidemiological studies, dementia seems to be more common in adults with diabetes (primarily Type 2 diabetes) than in adults without the disease (13), perhaps as a result of chronic hyperglycemia. However, severe hypoglycemia that required a visit to the emergency department was significantly associated with the development of dementia in the subsequent 1 to 22 years in a study that examined health records from more than 16,000 adults enrolled in an integrated care system California (14). Although it remains unclear if the episodes of severe hypoglycemia caused the dementia or were merely early signs of cognitive impairment, diabetes and the extremes in glycemia experienced by patients with Type 1 and advanced Type 2 diabetes seem to have an impact of brain structure and function.
IMPACT OF LONGSTANDING TYPE 1 DIABETES
Adults with Type 1 diabetes have repeatedly been found to have reduced performance on tests of information processing, psychomotor efficiency, attention, visuconstructive ability, and mental flexibility (15). These cognitive domains are believed to reside primarily in white matter, and Kodl et al. (16) tested the hypothesis that changes in neurocognitive performance in adults with Type 1 diabetes can be linked to changes in white matter microstructure. In this study, 25 adults with long-standing Type 1 diabetes (mean duration = 30.3 [10.8] years) were recruited to participate in a comprehensive battery of neurocognitive testing and to undergo magnetic resonance imaging. They were compared with 25 age-, sex-, and educational level–matched controls without diabetes. No significant changes were found in performance on the neurocognitive battery, but the participants with diabetes tended to perform more poorly on the Rey-Osterreith Complex Figure Drawing test of visuospatial orientation and planning (scores for group with diabetes versus scores for controls = 31 [0.6] versus 33 ± 0.6, p = .063). White matter microstructure was measured using diffusion tensor imaging, and significant differences were identified between the participants with and without diabetes in the corona radiata and optic radiations, suggesting that the participants with diabetes had microstructural abnormalities in these regions. The magnitude of these abnormalities, measured as fractional anisotropy, correlated significantly with age, diabetes duration, and A1c at the time of the magnetic resonance imaging. Across the entire study population, the fractional anisotropy in the posterior corona radiata correlated significantly with the score on the Rey-Osterreith Complex Figure Drawing test of visuospatial orientation and planning (p = .0018), with the participants with diabetes having the lowest level of performance and lowest measure of white matter microstructural integrity.
The data collected during this study were further analyzed using diffusion tensor imaging tractography to identify the cortical regions that had high functional connectivity to the posterior white matter tracts with reduced fractional anisotropy in the participants with diabetes (17). The cortical thickness of the gray matter with functional connections to the areas with reduced white matter fractional anisotropy was significantly reduced. This observation suggests that diabetes leads to changes both in white matter microstructure and in gray matter density in regions with high connectivity. Prospective study of a younger population will be necessary to determine which type of tissue shows the earliest structural changes and to define the impact these changes on overall intellectual performance as the patient ages.
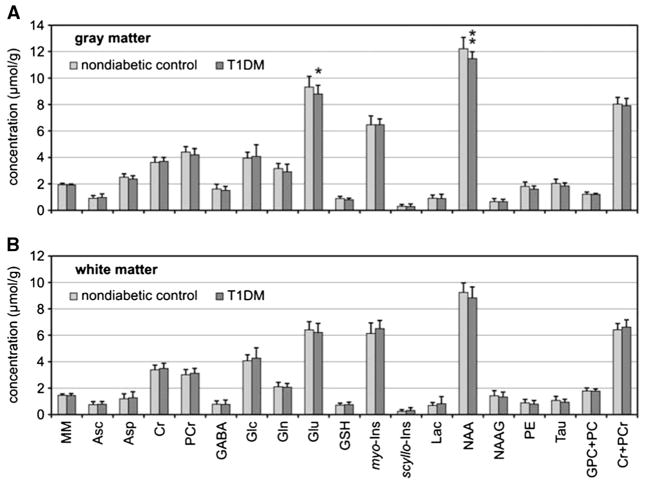
Magnetic resonance spectroscopy can be used to define the chemical composition of tissue, and our group has applied this method to determine if long-standing Type 1 diabetes alters the neurochemical profiles of gray and white matter regions in the occipital lobe. In this investigation, spectroscopic data collected for a study of brain glucose metabolism were reanalyzed using LCModeling to quantify 19 different chemical species (18), as is shown in Figure 2. Thirteen participants with long-standing Type 1 diabetes were compared with 32 healthy volunteers. The groups were similar with respect to age (41 [11] versus 36 [10] years, diabetic group versus controls; p = .16) and body mass index (26 [3] versus 27 [6] kg/m2; diabetic group versus controls; p = .5). The participants with Type 1 diabetes were found to have significant reductions in the gray matter content of N-acetylaspartate and glutamate relative to controls (Fig. 1). Because these compounds are found in neurons themselves, the observations suggest that patients with long-standing Type 1 diabetes may have partial neuronal loss or dysfunction as a result of their disease.
FIGURE 2.
Comparison of neurochemical profiles of patients with T1DM (n = 13) relative to nondiabetic controls (n = 32). Metabolite concentrations were measured from the gray matter–rich occipital lobe (A) and white matter–rich parieto-occipital region (B). Error bars indicate significance levels: *p < .05 and **p < .01. MM is quantified in arbitrary units. T1DM = Type 1 diabetes; MM = macromolecules; Asp = aspartate; Asc = ascorbate; Cr = creatine; GABA = g-aminobutyric acid; Glc = glucose; Gln = glutamine; Glu = glutamate; GSH = glutathione; myo-Ins = myo-inositol; scyllo-Ins = scyllo-inositol; Lac = lactate; NAA = N-acetylaspartate; NAAG = N-acetylaspartylglutamate; PE = phosphoethanolamine; Tau = taurine; GPC+PC = the sum of glycerophosphocholine and phosphocholine; Cr = creatine; PCr = phosphocreatine. Reprinted by permission from Macmillan Publishers Ltd: Journal of Cerebral Blood Flow and Metabolism (18).
IMPACT OF RECURRENT HYPOGLYCEMIA ON THE DEVELOPMENT OF HYPOGLYCEMIA UNAWARENESS IN PATIENTS WITH TYPE 1 DIABETES
Hypoglycemia is the factor that limits patients from achieving the level of glycemic control necessary to reduce the microvascular complications of diabetes. Hypoglycemia is uncomfortable and requires a person to interrupt what they are doing to ingest carbohydrate. Hypoglycemia can also lead to confusion, loss of consciousness, and death. To prevent hypoglycemia, humans have developed an elaborate set of redundant mechanisms that ensure that blood glucose remains in the normal range, even during prolonged fasting. The first defense against hypoglycemia in a healthy person is a reduction in endogenous insulin secretion. The second line of defense should the sugar continue to fall is an increase in glucagon and epinephrine secretion and activation of the sympathetic nervous system. Together, this response increases hepatic glucose production and limits glucose uptake into the muscle to allow blood glucose to rise. The release of epinephrine and activation of the sympathetic nervous system also causes symptoms of palpitations, sweating, and anxiety that drive a person to eat.
Patients with Type 1 and advanced Type 2 diabetes are at particular risk for the development of hypoglycemia because this counterregulatory response becomes impaired (19). Such patients cannot reduce endogenous insulin secretion in response to falling blood sugars because they are unable to make insulin. They also cannot respond to hypoglycemia by increasing glucagon secretion, probably because the αcell must see a reduction of insulin from the β cell in the setting of hypoglycemia for glucagon secretion to be stimulated. The only line of defense in these patients is an increase in epinephrine secretion and activation of the sympathetic nervous system, but recurrent episodes of hypoglycemia drive down the glucose level that elicits this response. As a result, patients with recurrent hypoglycemia may not develop symptoms of hypoglycemia before they become unconscious from neuroglycopenia. Impaired awareness of hypoglycemia occurs in up to 20 % of patients with Type 1 diabetes (20) and is a serious consequence of efforts to achieve near-normal levels of glycemia.
The underlying mechanisms that lead to the development of impaired awareness of hypoglycemia in patients with diabetes have been the topic of much research (see Ref. (19) and references therein), and one hypothesis states that recurrent hypoglycemia alters glucose sensing in the brain. To test this hypothesis, our group used the method of arterial spin labeling to determine which areas of the brain are activated during hypoglycemia (21). With this approach, regional changes in cerebral blood flow that occur when a region is activated by a stimulus can be detected and used to identify the brain regions involved.
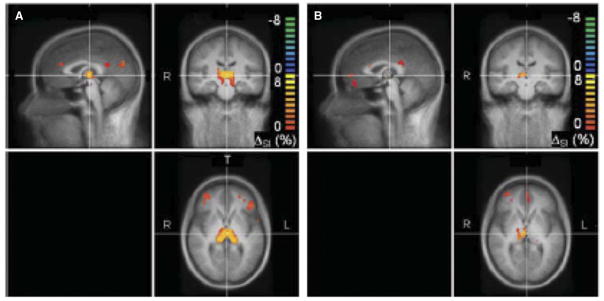
In this study, 12 people with long-standing Type 1 diabetes (mean duration = 26 [13] years) and impaired awareness of hypoglycemia underwent magnetic resonance imaging at 3 T under first euglycemic and then hypoglycemic conditions, all in a single session. These individuals were all categorized as hypoglycemia unaware by the standard Cox questionnaire, which requires them to have a history of severe hypoglycemia that required the assistance of another person to both recognize and treat the episode. The data collected from the patients with diabetes were compared with those collected from healthy volunteers without diabetes. As is shown in Figure 3, control participants showed activation in the region of the thalamus during hypoglycemia, whereas participants with Type 1 diabetes and hypoglycemia unawareness did not. Recurrent hypoglycemia sufficiently severe to render a patient with Type 1 diabetes unaware of hypoglycemia significantly reduces the thalamic response to hypoglycemia. This suggests that the thalamus plays a role in sensing hypoglycemia and/or in the coordination of the counterregulatory response to hypoglycemia.
FIGURE 3.
Cerebral blood flow responses to hypoglycemia in 12 controls (C) and 11 participants with Type 1 diabetes and hypoglycemia unawareness (D). t Scores were evaluated where the change in %SI differed from zero. Red to yellow (green to blue) indicates larger (smaller) changes in cerebral blood flow in hypoglycemia versus euglycemia. %SI = signal intensity. Reprinted with permission from Macmillan Publishers Ltd: Journal of Cerebral Blood Flow and Metabolism (21).
How recurrent hypoglycemia might alter glucose sensing in the thalamus or any other brain region remains uncertain. One possibility is that the brain compensates for recurrent hypoglycemia by increasing brain glucose transport so that more glucose passes from the blood into the brain during subsequent episodes of hypoglycemia. In animal models, glucose transporter proteins (22) and glucose transport (23) are shown to be up-regulated in response to hypoglycemia. In humans, we have shown that individuals with Type 1 diabetes and hypoglycemia unawareness have higher steady-state brain glucose concentrations than do controls studied at the same level of glycemia (24), suggesting that glucose transport may be increased in this group.
Another possibility is that hypoglycemia-induced changes in brain glycogen metabolism might support the fuel deficit present during subsequent periods of hypoglycemia. Glycogen localized in the astrocyte is present in the brain at a concentration of approximately 3.5 μmol/gram (25). Glycogen content decreases during hypoglycemia in human and is restored to a superphysiological level after an episode of prolonged hypoglycemia (26). We have proposed that this hypoglycemia-induced increase in brain glycogen provides additional substrate to maintain brain energy metabolism during subsequent periods of hypoglycemia, and we are currently performing experiments to test this hypothesis.
CONCLUSIONS
Diabetes alters cerebral metabolism, structure, and function. Although a direct correlation between these subtle changes and life-altering disability has not yet been made, future study must address this issue. As patients with diabetes are living longer and better lives, and as the global epidemic in diabetes increases the number of people with diabetes everyday, we must understand how diabetes interacts with the aging process that so often leads to cognitive decline. With such knowledge, we will be able to identify those at particular risk and develop new approaches to prevent and treat this potentially devastating complication of diabetes.
Acknowledgments
Source of Funding: Funded by National Institute of Health Grants R01 NS035192 and DK62440 (both to ERS), 2 T32 DK7203, P41 RR008079, P41 EB015894, P30 NS057091, P30 NS5076408, and 5M01 RR0400.
Footnotes
Conflicts of Interest: No conflicts of interest to declare.
References
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Vol. 2014 Atlanta, GA: US Department of Health and Human Services; 2014. [Google Scholar]
- 2.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:12. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonder-Frederick LA, Zrebiec JF, Bauchowitz AU, Ritterband LM, Magee JC, Cox DJ, Clarke WL. Cognitive function is disrupted by both hypo- and hyperglycemia in school-aged children with Type 1 diabetes: a field study. Diabetes Care. 2009;32:1001–6. doi: 10.2337/dc08-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inkster B, Frier BM. The effects of acute hypoglycaemia on cognitive function in Type 1 diabetes. Br J Diabetes Vasc Dis. 2012;12:221–6. [Google Scholar]
- 5.Reaven GM, Thompson LW, Nahum D, Haskins E. Relationship between hyperglycemia and cognitive function in older NIDDM patients. Diabetes Care. 1990;13:16–21. doi: 10.2337/diacare.13.1.16. [DOI] [PubMed] [Google Scholar]
- 6.Sommerfield AJ, Deary IJ, Frier BM. Acute hyperglycemia alters mood state and impairs cognitive performance in people with Type 2 diabetes. Diabetes Care. 2004;27:2335–40. doi: 10.2337/diacare.27.10.2335. [DOI] [PubMed] [Google Scholar]
- 7.Stiles MC, Seaquist ER. Cerebral structural and functional changes in Type 1 diabetes. Minerva Med. 2010;101:105–14. [PubMed] [Google Scholar]
- 8.Perantie DC, Lim A, Wu J, Weaver P, Warren SL, Sadler M, White NH, Hershey T. Effects of prior hypoglycemia and hyperglycemia on cognition in children with Type 1 diabetes mellitus. Pediatr Diabetes. 2008;9:87–95. doi: 10.1111/j.1399-5448.2007.00274.x. [DOI] [PubMed] [Google Scholar]
- 9.Perantie DC, Wu J, Koller JM, Lim A, Warren SL, Black KJ, Sadler M, White NH, Hershey T. Regional brain volume differences associated with hyperglycemia and severe hypoglycemia in youth with Type 1 diabetes. Diabetes Care. 2007;30:2331–7. doi: 10.2337/dc07-0351. [DOI] [PubMed] [Google Scholar]
- 10.Moran C, Phan TG, Chen J, Blizzard L, Beare R, Venn A, Munch G, Wood AG, Forbes J, Greenaway TM, Pearson S, Srikanth V. Brain atrophy in Type 2 diabetes regional distribution and influence on cognition. Diabetes Care. 2013;36:4036–42. doi: 10.2337/dc13-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manschot SM, Brands AMA, van der Grond J, Kessels RPC, Algra A, Kappelle LJ, Biessels GJ Utrecht Diabetic Encephalopathy Study Group. Brain magnetic resonance imaging correlates of impaired cognition in patients with Type 2 diabetes. Diabetes. 2006;55:1106–13. doi: 10.2337/diabetes.55.04.06.db05-1323. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson AM, Musen G, Ryan CM, Silvers N, Cleary P, Waberski B, Burwood A, Weinger K, Bayless M, Dahms W, Harth J. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med. 2007;356:1842–52. doi: 10.1056/NEJMoa066397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: the Rotterdam study. Neurology. 1999;53:1937–42. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 14.Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP, Selby JV. Hypoglycemic Episodes and Risk of Dementia in Older Patients With Type 2 Diabetes Mellitus. JAMA. 2009;301:1565–72. doi: 10.1001/jama.2009.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev. 2008;29:494–511. doi: 10.1210/er.2007-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kodl CT, Franc DT, Rao JP, Anderson FS, Thomas W, Mueller BA, Lim KO, Seaquist ER. Diffusion tensor imaging identifies deficits in white matter microstructure in subjects with Type 1 diabetes that correlate with reduced neurocognitive function. Diabetes. 2008;57:3083–9. doi: 10.2337/db08-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franc DT, Kodl CT, Mueller BA, Muetzel RL, Lim KO, Seaquist ER. High connectivity between reduced cortical thickness and disrupted white matter tracts in long-standing Type 1 diabetes. Diabetes. 2011;60:315–9. doi: 10.2337/db10-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mangia S, Kumar AF, Moheet AA, Roberts RJ, Eberly LE, Seaquist ER, Tkac I. Neurochemical profile of patients with Type 1 diabetes measured by H-1-MRS at 4 T. J Cerebr Blood Flow Metab. 2013;33:754–9. doi: 10.1038/jcbfm.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tesfaye N, Seaquist ER. Neuroendocrine responses to hypoglycemia. In: Powers AC, Ahima RS, editors. Year in Diabetes and Obesity. Malden: Wiley-Blackwell; 2010. pp. 12–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geddes J, Schopman JE, Zammitt NN, Frier BM. Prevalence of impaired awareness of hypoglycaemia in adults with Type 1 diabetes. Diabet Med. 2008;25:501–4. doi: 10.1111/j.1464-5491.2008.02413.x. [DOI] [PubMed] [Google Scholar]
- 21.Mangia S, Tesfaye N, De Martino F, Kumar AF, Kollasch P, Moheet AA, Eberly LE, Seaquist ER. Hypoglycemia-induced increases in thalamic cerebral blood flow are blunted in subjects with Type 1 diabetes and hypoglycemia unawareness. J Cerebr Blood Flow Metab. 2012;32:2084–90. doi: 10.1038/jcbfm.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumagai AK, Kang YS, Boado RJ, Pardridge WM. Upregulation of blood-brain barrier GLUT1 glucose transporter protein and mRNA in experimental chronic hypoglycemia. Diabetes. 1995;44:1399–404. doi: 10.2337/diab.44.12.1399. [DOI] [PubMed] [Google Scholar]
- 23.Herzog RI, Jiang LH, Herman P, Zhao C, Sanganahalli BG, Mason GF, Hyder F, Rothman DL, Sherwin RS, Behar KL. Lactate preserves neuronal metabolism and function following antecedent recurrent hypoglycemia. J Clin Invest. 2013;123:1988–98. doi: 10.1172/JCI65105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Criego A, Kumar A, Tran N, Tkac I, Gruetter R, Seaquist ER. Brain glucose concentrations in patients with Type 1 diabetes and hypoglycemia unawareness. J Neurosci Res. 2005;79:42–7. doi: 10.1002/jnr.20296. [DOI] [PubMed] [Google Scholar]
- 25.Oz G, Seaquist ER, Kumar A, Criego AB, Benedict LE, Rao JP, Henry PG, Van De Moortele PF, Gruetter R. Human brain glycogen content and metabolism: implications on its role in brain energy metabolism. Am J Physiol Endocrinol Metab. 2007;292:E946–51. doi: 10.1152/ajpendo.00424.2006. [DOI] [PubMed] [Google Scholar]
- 26.Oz G, Kumar A, Rao JP, Kodl CT, Chow L, Eberly LE, Seaquist ER. Human brain glycogen metabolism during and after hypoglycemia. Diabetes. 2009;58:1978–85. doi: 10.2337/db09-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]