Abstract
Retinoids, natural and synthetic derivatives of vitamin A, induce cellular changes by activating nuclear retinoic acid receptors (RAR) and retinoid X receptors (RXR). Although the ability of retinoids to govern gene expression is exploited clinically for cancer therapeutics, the full benefit of retinoid-based strategies is unrealized due to detrimental side effects. Delineating the receptors that prompt cellular outcomes is critical to advancing retinoid-based approaches. Here we identify the receptors that evoke multiple responses in cutaneous T cell lymphoma (CTCL). The data demonstrate that RARα drives integrin β7-dependent adhesion and CCR9-mediated chemotaxis in CTCL cells. Of note, concomitant activation of RARα and RXR nuclear receptors yielded synergistic increases in adhesion and migration at concentrations where single agents were ineffective. As the established paradigm of retinoid action in CTCL is apoptosis and growth arrest, the role of RARα/RXR in these events was studied. As with adhesion and migration, RARα/RXR synergism prompted apoptosis and dampened CTCL cell proliferation. Strikingly, RARα/RXR synergism induced responses from CTCL cell lines previously reported to be unresponsive to retinoids. These data provide a novel framework that may further refine a proven CTCL therapy.
Keywords: vitamin A, retinoid, integrin, chemotaxis, lymphoma
Introduction
Retinoids are compounds structurally related to parental vitamin A (retinol) including natural metabolites and synthetic analogs [1]. Classically, retinoic acid (RA)1 isomers induce genetic responses by binding and subsequently activating two distinct families of nuclear steroid receptors known as the retinoic acid receptor (RAR) and retinoid X receptor (RXR) family [2, 3]. Each family exhibits three main isotypes, designated α, β, γ. Ligand-dependent conformational changes culminate in the dissociation of transcriptional corepressors and localization of coactivators to retinoic acid response elements within target genes [4]. The RAR receptor family forms heterodimers with RXR members. In contrast, RXRs are capable of forming heterodimers with additional nuclear receptors such as thyroid hormone receptor, vitamin D receptor, and peroxisome proliferator-activated receptors (PPAR). The promiscuous pairing nature of RXRs accounts for more pleiotropic cellular effects as compared to RAR activity.
The particular cellular response evoked by retinoids is contingent upon the identity and pharmacological properties of the retinoid administered, nuclear receptor repertoire expressed by a cell, and the physical pairings of the receptors expressed. RAR/RXR heterodimers are nonpermissive as RXR agonism alone is insufficient for inducing transcription, RAR ligands can solely increase transcription, and simultaneous RAR/RXR agonism results in robust, synergistic changes in gene expression [5, 6]. In contrast, permissive receptors are activated with RXR ligand alone and arise when RXR members dimerize with non-RAR nuclear receptors such as PPARs. As retinoids embody agents capable of prompting changes in gene expression, the exploitation of RA and other structural analogs for therapeutic purposes is extensive [7]. In particular, natural and synthetic vitamin A derivatives are noted for their clinical efficacy in multiple human cancers including leukemia, breast, hormone-refractory prostate, and lung [8]. In particular, the use of ATRA in the treatment of acute promyelocytic leukemia is commonly referred to as a milestone in molecular based cancer therapy [9]. However, the full clinical potential of retinoid-based strategies has yet to be realized as toxicity and detrimental side effects hinder use. These limitations occur largely due to the supraphysiological doses required to achieve a desired therapeutic outcome [10].
This principle is exemplified by the use of Bexarotene (Targretin®), an RXR selective agonist, in the treatment of cutaneous T cell lymphoma (CTCL). CTCL is a heterogeneous group of skin-tropic T cell malignancies that account for nearly 4 percent of all non-Hodgkin lymphoma [11, 12]. Retinoids have been employed for over three decades to treat CTCL. Currently, Bexarotene is the only FDA approved retinoid to treat CTCL at all stages. Zhang and colleagues identified Bexarotene exposure culminates in CTCL cell apoptosis [13], and this original mechanism has since been verified and expanded to include cell cycle growth arrest [14, 15]. However, the maximal therapeutic benefit of Bexarotene is offset by interrelated, adverse metabolic side effects namely, hyperlipidemia, hypercholesterolemia, and hypothyroidism [16, 17]. Metabolic complications likely arise since RXRs play a vital role in metabolic integration among tissues as RXRs pair with liver X receptor and thyroid hormone receptor [18]. Considering the average therapeutic serum concentration among patients is 10 μM [19], activation of multiple RXR-containing nuclear receptors by Bexarotene is unavoidable. The severity of these unwanted side effects warrants administration of additional therapies or cessation of retinoid therapy [20]. In light of the strong clinical promise of vitamin A derivatives, mechanistic studies merit investigation. As retinoid receptor activation likely accounts for both desired clinical outcomes as well as confounding side-effects, identifying the receptor isotypes that transduce retinoid exposure into cellular responses is paramount for advancing retinoid based therapies in CTCL.
Methods
Cell culture
The human T leukemia/lymphoma cell lines Jurkat and CCRFCEM and human cutaneous T cell lymphoma lines HuT78, MJ and HuT102 were purchased from ATCC (Manassas, VA). SeAx and MyLa lines were the generous gift of Dr. Robert Gniadecki (University of Copenhagen). All cell lines were maintained in 5% CO2 incubator with RPMI 1640 media supplemented with 1 mM sodium pyruvate, 10% (v/v) fetal bovine serum, 10 mM HEPES, 1% L-glutamine and 1% penicillin-streptomycin. SeAx and HuT102 media also contained 10 U/ml of recombinant IL-2.
Reagents and chemicals
Recombinant human MAdCAM-1-Fc and CCL25 were purchased from R&D Systems. (Minneapolis, MN). The colorimetric phosphatase substrate (p-nitrophenyl phosphate), ATRA, SR11237, and Bexarotene were obtained from Sigma (St. Louis, MO). RARα agonist AM580, RARβ agonist CD2314, and RARγ agonist BMS961 were obtained from TOCRIS bioscience through Sigma, as were the selective receptor antagonists for RARα (ER5089), RARβ/γ (CD2665), and RARβ (LE135). Retinoids were dissolved at desired concentrations in DMSO and handled accordingly to minimize light exposure. Receptor agonists were administered at 1× or 2×EC50 to ensure activation of the targeted receptor isotype and minimize activation of other receptor subtypes [21–24]. All values equated to nM levels of agonist (1×EC50 for α, β, γ agonist used were 0.3, 145 and 30 nM, respectively).
Monoclonal antibodies (mAb) against human RARβ and CCR9 were purchased from R&D Systems, Inc. (Minneapolis, MN). FIB27 (anti-β7) mAb was obtained from BD Biosciences (San Jose, CA). Antibodies used for immuno-detection of survivin, bcl-2, RARα and RARγ were purchased from Santa Cruz Biotechnology. Caspase-3 antibody was from Cell Signaling Technology (Danvers, MA). Secondary goat anti-mouse IgG FITC-conjugated antibody and goat anti-rat IgG (H+L) fluorescein-conjugated antibody were obtained from Millipore (Temecula, CA). The anti β-tubulin mAb was generously provided by Dr. Bill Angus (East Carolina University, Greenville, NC). Millicell 24-well Hanging Cell Culture Insert (PET 8.0 μm) and Brdu Cell Proliferation Kit were purchased from EMD Millipore Corporation (Billerica, MA). Calcein AM was obtained from Thermo Scientific (Waltham, MA).
Flow cytometry
Cells (2×105/condition) were incubated with primary monoclonal antibodies recognizing β7 or CCR9 for 30 min. Cells were subsequently fixed in 0.37% formalin/1×phosphate-buffered saline (PBS), pH 7.4 for 15 min. Cells were washed twice in FACS buffer [1×PBS, 2.5% (v/v) fetal calf serum, and 0.02% (v/v) NaN3, pH 7.4] and stained with fluorescent secondary antibody for 15 min in the dark. Cells resuspended in 300 μl of FACS buffer were analyzed using a FACS Calibur flow cytometer w CellQuest-Pro software.
Static cell adhesion
Assays were adapted from established protocols [25]. Ligands were immobilized at the desired concentrations on Immulon-2 HB microtiter wells (Thermo Scientific, Waltham, MA) in 0.1 M NaHCO3. Nonspecific adhesion was minimized by blocking wells with 2% (w/v) bovine serum albumin (BSA) in HEPES-Tyrodes buffer at room temperature for 30 min. Cells were washed twice and enumerated. Cells (2×105/well) were incubated with ligands for 1 to 2 hrs at 37°C in 5% CO2. After three consecutive washes, adhered cells were determined by addition of acid phosphatase activity assay buffer (1% v/v Triton X-100, 50 mM sodium acetate at pH 5.0 and 6 mg/ml p-nitrophenyl phosphate) for 30 min at 37°C. Absorbance values were obtained at 405 nm after addition of 50 μl/well of 1 N NaOH. Cell adhesion to wells coated solely with BSA was considered as background values for all experimental conditions. Background BSA values were subtracted before reporting final values. Average A405=Average A405(ligand) − Average A405(BSA).
Chemotaxis
Directed migration was quantitated using 24-well hanging cell culture insert Millicell® system (PET 8.0 μm). Cells (1×105) were added to the upper chamber of each well in a total volume of 100 μl serum-free culture medium. Cells were cultured in the presence of retinoids or DMSO control in complete medium for indicated time before addition to upper chambers. Chemotaxis to the lower chamber containing 100 ng/ml CCL25 was allowed to occur over 1–3 hr depending upon cell line at 37°C, after which time migrated cells were collected and stained with Calcein AM for 1 hr in the dark. Fluorescence values were obtained at 485/535 nm.
Apoptosis
Apoptosis assay was carried out with the Annexin V-FITC Detection Kit II (BD PharMingen, San Diego, CA) according to the manufacturer’s instructions. Cells were washed twice with cold PBS, centrifuged at 1000 rpm for 5 min, and resuspended in 1×binding buffer at a concentration of 1×106 cells/ml. Then 100 μl of the solution was assessed for each condition by addition of annexin V-FITC and PI. Cells were incubated for 15 min at room temperature in the dark. An additional, 400 μl of binding buffer was added to each tube, and samples were analyzed by FACScan flow cytometry (Becton Dickinson). For each sample, 10,000 ungated events were acquired.
Western Blot
Cell lysates were prepared with RIPA buffer containing protease inhibitors from Roche. Cell suspensions were sonicated for 15 minutes, and cell debris was pelleted. After BCA quantitation of supernatant, protein amounts indicated in the corresponding figure legends were loaded and separated under reducing conditions.
BrdU cell proliferation
Cell proliferation was quantitated by BrdU cell proliferation assay kit from Millipore. Briefly, cells were seeded at 2×105 cell/ml in 100 μL/well in 96-well tissue culture plate. Retinoids were supplemented at 2× the final desired concentration in 100 μL/well of media. BrdU was added 24 hrs prior to harvesting. After cell fixation, diluted anti-BrdU monoclonal antibody was added 100 μL/well for 1 hr. Cells were washed again and incubated with 100 μL filtered peroxidase conjugated goat anti-mouse IgG, for 30 min at room temperature. After washing, 100 μL/well TMB substrate was added and incubated for 30 min at room temperature in the dark. Plates were read at 450/550 nm.
Quantitative RT-PCR
RNA was isolated from cells treated with or without 0.3 nM RARα/5nM Bexarotene for 72 hrs using RNeasy Plus Mini Kit (Qiagen). cDNAs were reverse-transcribed from 1 μg of RNA by Verso cDNA Synthesis Kit (ThermoFisher) and subjected to qPCR using iQ SYBER Green Supermix (Bio-Rad, Hercules, CA). Amplification and detection was performed using an iCycler IQ real-time PCR detection system. Primer optimization identified 60°C as ideal and all amplifications were followed by melt-curve analysis. α4 upstream primer: 5′-AAC ACG CTG TTC GGC TAC TC-3′; downstream primer: 5′-CCT TCC GAT CCT GCA TCT GT-3′. β7 upstream primer: 5′-TGC AGG AAG TCA CCC ATT CT-3′; downstream primer: 5′-AAA GCT GAA TGG TGA CTG GC-3′. Relative mRNA levels were calculated using the ddCt method: mRNA fold-change level = (2−ddCt), where ddCt = [dCt gene of interest in treated group − [dCt of gene of interest in control group], where dCt = the [Ct gene of interest] − [Ct of reference gene]. Ct values were normalized to those of GAPDH.
Statistical analyses
A student’s t-test was utilized to establish significance. When appropriate for multiple comparisons, a one-way ANOVA analysis with a Bonferonni post hoc test was employed. Asterisks denote significance (*p<0.05, **p<0.01). Error bars represent standard deviation in all figures. Unless otherwise indicated, results are representative of at least three independent experiments each done in triplicate. GraphPad Prism 6.0 software (La Jolla, CA) was used to generate plots and statistically analyze all data.
Results
RARα activity predominately drives integrin β7 expression and function in CTCL cells
An early cellular marker of CTCL response to retinoids entails the functional induction of the β7-integrin [26]. We sought to identify the receptor isotypes that transduce retinoid exposure into this initial CTCL response. The extensive conservation between all three RXR isotypes has confounded the generation of isotype selective agents [27]. Due to the paucity of RXR isotype selective reagents, our initial approach was to investigate which RAR isotype(s) contribute to the heightened β7 expression and function.
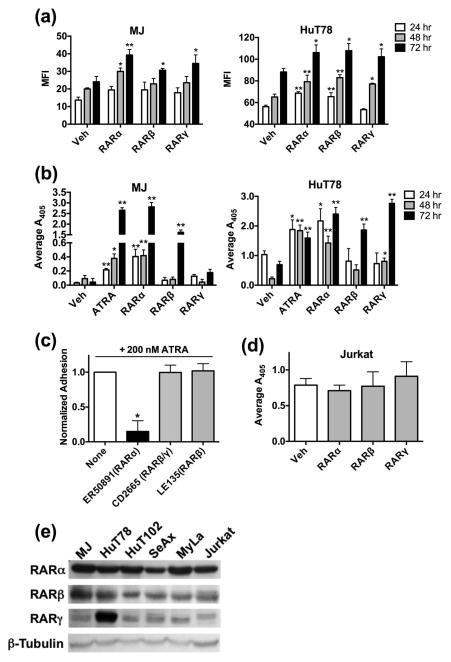
We first undertook studies with the MJ and HuT78 cell lines as these lines represent the two major variants of CTCL, Mycosis Fungoides and Sézary Syndrome, that account for over 70% of total CTCLs [28, 29]. Exposure of MJ and HuT78 cells to RAR isotype selective agonists demonstrated that all three RAR isotypes could induce cell surface β7 expression (Fig. 1a). Integrin β7 expression was induced in the MJ cell line at 48 and 72 hrs post exposure as compared to the HuT78 line in which β7 expression emerged as early as 24 hrs.
Figure 1.
RARα activation prompts β7 expression and function in CTCL. (a) MJ or HuT78 cells were treated with DMSO or 2×EC50 of well-established RAR isotype agonists for 24, 48 or 72 hrs. Surface β7 integrin expression was determined through flow cytometry and displayed as mean fluorescence intensity (MFI). (b) MJ or HuT78 cells were cultured in the presence of DMSO, 100 nM ATRA, 2×EC50 RARα, β, or γ agonists for the indicated time. Static cell adhesion assays were assessed on 0.75 μg/ml of the β7 specific ligand MAdCAM-1. (c) MJ cells were cultured with the indicated RAR isotype antagonists at 500 nM for 24 hrs. Cells were further subcultured for an additional 24 hrs in the presence of 200 nM ATRA. Static cell adhesion assays were conducted as described before. Ordinate represents data that have been normalized to adhesion levels obtained with ATRA in the absence of antagonist. (d) Adhesion assays were repeated as previously described in (B) with the non-CTCL cell line, Jurkat. (e) Whole cell lysates (30 μg/lane) of CTCL or non-CTCL (Jurkat) cell lines were examined for the presence and relative abundance of the various RAR receptor isotypes.
We next determined if the β7 expression changes prompted by RAR isotype agonism was functionally relevant. As shown in figure 1b, all three RAR isotype selective agonists were capable of inducing CTCL cell adhesion to the β7-ligand, MAdCAM-1, at the longest exposure time of 72 hrs. However, only the RARα agonist directly mimicked results obtained with all-trans-retinoic acid (ATRA) by increasing β7-mediated adhesion at all time points examined in both MJ and HuT78 lines. These results suggest that while all three RAR isotypes induce β7 integrin expression, RARα activity accounts for β7-dependent functional changes in CTCL lineages achieved with the natural retinoid ATRA. To confirm this conclusion, we conducted experiments with RAR antagonists. ATRA-induced CTCL cell adhesion was exclusively inhibited with a RARα antagonist (Fig. 1c).
This effect was also unique to the CTCL etiology as Jurkat cells, a non-CTCL derived T cell line, did not increase β7-dependent adhesion upon RAR isotype agonism (Fig. 1d). The disparate response to RAR stimulation between CTCL and non-CTCL lineages was not due to a distinct expression pattern of RAR receptors (Fig. 1e). While certain differences were evident (e.g. increased RARγ expression in HuT78), all three isoforms were detected at fairly uniform abundance across cell lines utilized. This also indicates that the absence of receptor subtypes in cells does not likely account for the relative delay/lack of response of CTCL cell lines to RARβ or RARγ agonism.
Concomitant activation of RARα and RXR receptors synergistically induces CTCL cell adhesion
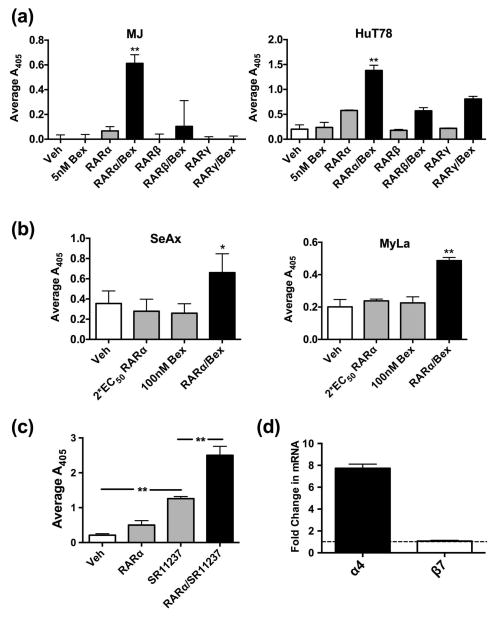
RAR/RXR dual agonism can result in robust, synergistic changes in gene expression [5, 6]. As RAR activation alone yielded changes in integrin expression and function, we explored if CTCL cells exhibit retinoid receptor synergism. By utilizing Bexarotene alone or in combination with a RAR isotype selective agonist at nM levels, we demonstrate that RARα/RXR synergism potently induced β7-mediated adhesion within 24 hrs of exposure (Fig. 2a). This is in contrast to single agents or dual agonism of other RAR isotypes and Bexarotene (Fig. 2a).
Figure 2.
RARα/RXR coactivation induces CTCL cell adhesion in a synergistic manner. (a) MJ or HuT78 cells were cultured in the presence of RAR isotype-specific agonists (1×EC50) alone or in combination with 5 nM Bexarotene for 24 hrs. Cells were then assessed for adhesion to 0.75 μg/ml of MAdCAM-1. (b) CTCL cell line SeAx or MyLa cells were cultured with DMSO, 2×EC50 RARα agonist, 100 nM Bexarotene, or a combination of agonists for 72 hrs. The extent of adhesion was determined on the integrin β7-ligand MAdCAM-1. (c) HuT78 cells were cultured in the presence of an RAR isotype-specific agonist (1×EC50) alone or in combination with 100 nM of the pan-RXR activator SR11237 for 48 hrs. Cell adhesion was then determined as previously described. (d) Real-time quantitative PCR was conducted with primers that amplify regions encoding the human integrin α4 or β7 subunits. Templates were derived from MJ cells cultured with vehicle or RARα/RXR agonists. The ordinate reflects normalized data obtained by dividing values for RARα/RXR agonists with those from vehicle treated samples.
As synergism rapidly induced CTCL responses at low doses, we further explored the power of RARα/RXR synergism. Two CTCL cell lines, SeAx and MyLa, were previously reported to be insensitive to ATRA with respect to augmented β7 expression and function [26]. Notably, the combination of low dose RARα agonist (2 × EC50) and Bexarotene (100 nM) reversed retinoid insensitivity in both CTCL linages (Fig. 2b). These results indicate that synergizing select retinoid receptors surmounts non-responsiveness that may occur in some refractory CTCL variants, and attests to the potential impact of such combinatorial strategies. To further confirm that the robust response was due to genuine RAR/RXR agonism and not a spurious attribute of the synthetic retinoid Bexarotene, another RXR selective pan-agonist, SR11237, was utilized. SR11237 yielded the same result as Bexarotene validating the findings (Fig. 2c).
RA induces α4β7 integrin expression through RARα-mediated transcriptional activation of the Itg-α4 gene in primary murine T cells [30]. Gene activation leads to increased α4-subunit abundance to promote dimerization with previously synthesized pools of β7 [31]. To determine if a similar mechanism of induction accounted for the current responses in human CTCL cells, the mRNA abundance of the respective subunits in templates derived from vehicle treated cells or cells cultured with the RARα and RXR selective activators was determined. Consistent with previous findings, RARα/RXR activation resulted in no detectable increase in β7 transcript levels, but treatment increased α4 transcript abundance (Fig. 2d). These findings provide initial mechanistic insight as to how retinoids govern early cellular changes in CTCL cells.
RARα/RXR coactivation promotes CTCL chemotaxis toward the gut-homing chemokine CCL25
Richardson and colleagues reported that Bexarotene potentially alleviates skin tropism of CTCL cells by dampening CCR4 expression [32]. However, a chief, natural role of vitamin A signaling is to establish and maintain mucosal immunity through populating mucosal structures [33]. Whether retinoids induce chemokine receptors associated with mucosal trafficking in addition to dampening skin trafficking receptors remains unknown.
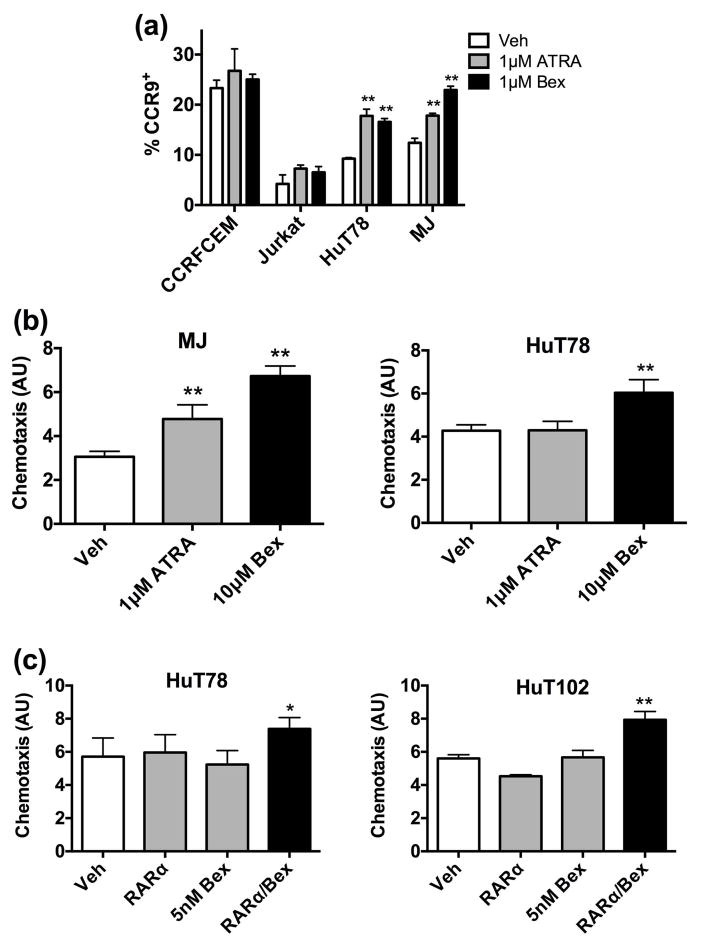
As shown in figure 3a, ATRA and Bexarotene alone induced the expression of the mucosal homing receptor, CCR9, selectively on CTCL cells. Exposure to pharmacological levels of these single agents functionally altered the migratory properties of CTCL cells. MJ and HuT78 migration toward 100 ng/ml of the mucosal homing chemokine ligand, CCL25, increased upon Bexarotene exposure (Fig. 3b). ATRA exposure yielded increased migration in the MJ cell line but not the HuT78 line. As with cell adhesion, RARα/RXR nuclear receptor synergism governed the migratory properties of CTCL. Migratory changes were not detected with CTCL cells exposed to RARα agonist or low dose Bexarotene (5 nM) alone. However, simultaneous activation of RARα and RXR receptors at low doses prompted cell migration (Fig. 3c).
Figure 3.
CCR9-mediated chemotaxis to CCL25 is enhanced by RARα/RXR activity. (a) CTCL cell lines MJ and HuT78 or the non-CTCL T cell lines CCRFCEM and Jurkat were treated with 1 μM ATRA or 1 μM Bexarotene for 72 hrs. Percentage of CCR9+ cells was detected through flow cytometry. (b) MJ and HuT78 cells were cultured with DMSO, 1 μM ATRA or 10 μM Bexarotene for 72 hrs. Directed migration towards 100 ng/ml of CCL25 was determined and displayed on the ordinate in arbitrary units (AU). (c) HuT78 or HuT102 cells were incubated with the vehicle DMSO, 1×EC50 RARα agonist, 5 nM Bexarotene or combination of the two agonists. As described in (b), the relative extent of chemotaxis towards 100 ng/ml of CCL25 was measured.
RARα/RXR synergism induces apoptosis and decreases proliferation of CTCL cells
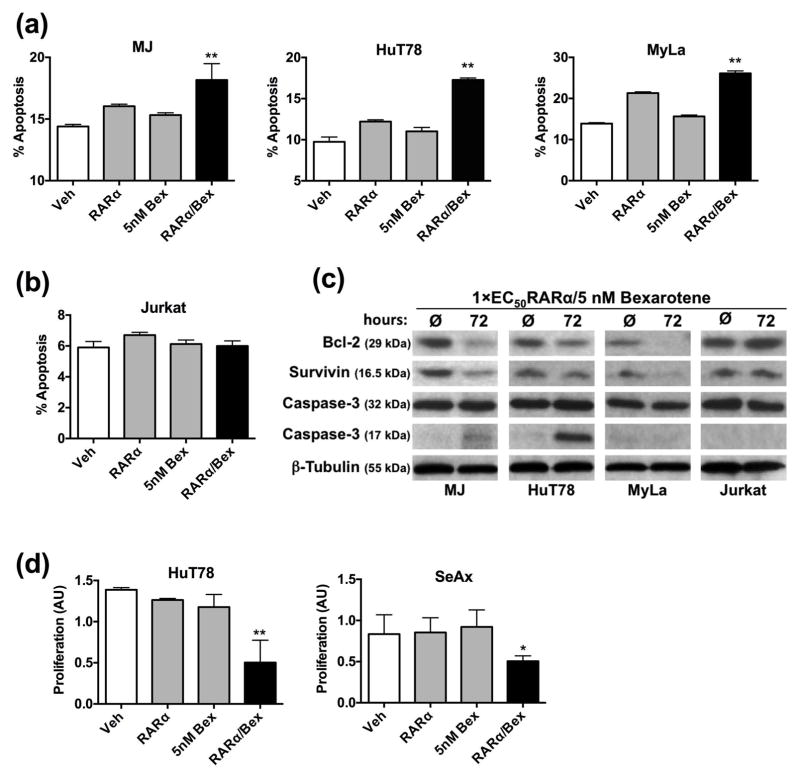
We wanted to determine if the same receptor pairs that induce the early adhesion and migratory changes in CTCL cells also prompt apoptosis and growth arrest. According to previous studies, Bexarotene-induced CTCL apoptosis and growth arrest requires high doses (10 μM) and extended exposure times of at least 96 hrs [13, 14]. In contrast, RARα/RXR coactivation, achieved comparable levels of CTCL cell apoptosis with abbreviated times (48 hrs) at lower dosage (5 nM Bexarotene) in MJ, HuT78, and MyLa cell lines (Fig. 4a). Heightened apoptosis was not observed in a T cell lineage not of CTCL origin (Fig. 4b). To identify the biochemical basis of the RARα/Bex treatment, the expression of multiple protein markers of apoptosis was determined. Dampened expression of the anti-apoptotic bcl-2 and survivin as well augmented cleavage of caspase-3 were induced with RARα/RXR co-activation (Fig. 4c). In addition, the combination of RARα agonists and Bexarotene at only 1×EC50 and 5 nM, respectively, inhibited CTCL cell proliferation as compared to vehicle controls or single agents (Fig. 4d).
Figure 4.
RARα/RXR cooperatively induce apoptosis and inhibit CTCL cell proliferation. (a) HuT78, MJ cells, MyLa cells or (b) the non-CTCL cell line Jurkat were treated with DMSO, 1×EC50 RARα agonist, 5 nM Bexarotene or combination thereof for 48 hrs. Data shown are the percentage of Annexin V positive cells. (c) Total cell lysates (40 μg/lane) from CTCL or non-CTCL cell lines were assessed for the indicated apoptotic protein marker. Lysates were derived from untreated cells or cells treated with RARα/RXR agonists for 72 hrs. (d) HuT78 or SeAx cells were incubated with DMSO or retinoids as described above for 48 hrs or 72 hrs, respectively. BrdU cell proliferation assays were conducted and the relative extent of proliferation is shown as arbitrary units (AU).
Discussion
Discovery of retinoid nuclear receptors revolutionized the understanding of retinoid action in both normal and aberrant states [3, 34, 35]. These receptors comprise unique molecular drug targets for multiple cancers including CTCL. The current findings delineate that CTCL cells transduce retinoid exposure into multiple responses through RARα receptor activity. While select responses were achieved with RARβ and RARγ agonists, a longer exposure time was required to prompt changes as compared to RARα agonists. As all three RAR protein isoforms were detectable in CTCL cells, we cannot definitively rule out the contribution of RARβ and RARγ to the CTCL responses reported here. To minimize cross reactivity of the agonists, the agents were employed at concentrations between 1−2×EC50 [21–24]. In addition, it is evident from the current work that RARα predominately drives CTCL changes as we confirmed the functional requirement of RARα by antagonists, and RARα but not RARβ or RARγ exhibited synergism with Bexarotene.
It is appreciated that skin-derived chemokine signals are pivotal in CTCL establishment, survival, and expansion [36]. RARα/RXR receptor synergism reprogramed the expression and function of homing receptors on CTCL lineages to respond to mucosal-derived trafficking signals. Importantly, function of the same RARα/RXR receptor subset induced apoptosis and dampened proliferation of CTCL cells. Although this is not causative proof linking retinoid-induced adhesion and migratory changes to apoptosis, these data are the first evidence that the same subset of nuclear receptors participate in each of these respective responses. Despite the heterogeneity of CTCL, we show that manipulation of the same receptor(s) is effective across multiple variants of CTCL. Of note, synergism resulted in all responses being achieved at substantially lower retinoid doses (200–2,000 fold) and shorter exposure times than previously reported [13, 14, 26, 32]. This finding is of particular relevance considering the high doses of retinoids currently required to achieve clinical end points in CTCL.
Receptor synergism may also be a viable approach for refractory situations [15]. The CTCL derived cell lines, SeAx and MyLa, are marginally responsive to completely resistant to single retinoid treatment [26], but concomitant activation of RARα and RXR receptors overcame this insensitivity. In addition, the MJ cell line is resistant to apoptotic induction when exposed to Bexarotene alone [14]. However, synergism induced apoptosis with MJ cells. With respect to apoptotic protein markers, pharmacological doses of Bexarotene achieve changes of survivin in multiple CTCL lines but failed to decrease bcl-2 abundance [14]. In comparison, RARα/RXR synergism induced changes in both survivin and bcl-2 at a 2,000 fold lower retinoid dose with a shorter exposure.
Investigations providing the molecular resolution necessary to attribute specific cellular responses to the activity of receptor subtypes are lacking. Preliminary reports attest to the potential clinical benefit of such tailored strategies. The antiproliferative ability of RARβ and RARγ agonists in premalignant and malignant human bronchial epithelial cells was enhanced upon RXR coactivation [37]. In addition, coactivation of RARγ and to a lesser extent RARα with RXRs induces apoptosis and inhibits proliferation of embryonic carcinoma cells [38]. However, these outcomes required μM levels of RAR and RXR agents. Here we demonstrate that CTCL cells respond in at least four functional ways namely, adhesion, migration, apoptosis, and proliferation to nM levels of retinoid when select receptors are costimulated.
Overall, the importance of the current findings is that i) CTCL cellular responses were achieved with substantially lower dosages and shortened exposure time of retinoid compared to previous reports, ii) activation of the same receptor isotype or receptor pair accounted for multiple clinically relevant outcomes in CTCL including apoptosis, and iii) combinatorial activation of RARα and RXR receptors yielded detectable responses from refractory CTCL cells. The current findings suggest that Bexarotene therapy can be augmented with RARα agonism to induce synergistic receptor activation in CTCL. Isotype specific activation coupled with receptor synergism described in the current work embodies a strategy whereby a desired cellular response could be specified and amplified to such a degree that deleterious side effect associated with current retinoid dosing regimens might be circumvented.
Acknowledgments
The authors are grateful to Drs. Phillip Pekala and Ross Longley for their guidance in the preparation of this manuscript. This work was supported by new faculty start-up funds from the Division of Research and Graduate studies at the Brody School of Medicine (LCB), the faculty enrichment fund at the Arkansas Colleges of Health Education (LCB), and NIH 1R01AT008375 (SRS).
Footnotes
Abbreviations: ATRA, all-trans-retinoic acid; Bex, Bexarotene; CCR, chemokine (C-C motif) receptor; CTCL, cutaneous T cell lymphoma; mAb, monoclonal antibody; MAdCAM, mucosal address in cell adhesion molecule; MFI, mean fluorescence intensity; PPAR, peroxisome proliferator-activated receptor; RA, retinoic acid; RAR, retinoic acid receptor; and RXR, retinoid X receptor
Author contributions
LCB, LW, and SSD conceived the study and wrote the paper. LW performed expression analysis, chemotaxis measurements, BrdU proliferation assays, select adhesion studies, and the majority of apoptosis analysis. SSD initiated and optimized the use of all RAR selective agonists, Bexarotene, and exposure times for adhesion measurements. MSS performed the control experiments of the Jurkat cell line. ALM and MJC contributed to the apoptosis data collection and analysis. JC developed protocols for culturing cells and generated the protocol for the antagonist study. BMS designed the RTPCR experiments. CMP and SRS provided vital expertise to comprise the Discussion with SRS also providing critical flow cytometry expertise. All authors analyzed the results and approved the final version of the manuscript.
Conflict of Interest
Authors declare no conflict of interest related to this work.
References
- 1.Sporn MB, Roberts AB, Goodman DS. The Retinoids Biology, Chemistry, and Medicine. New York: Ravens Press; 1994. [Google Scholar]
- 2.Benbrook DM, Chambon P, Rochette-Egly C, et al. History of retinoic acid receptors. Sub-cellular biochemistry. 2014;70:1–20. doi: 10.1007/978-94-017-9050-5_1. [DOI] [PubMed] [Google Scholar]
- 3.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1996;10:940–954. [PubMed] [Google Scholar]
- 4.de Lera AR, Bourguet W, Altucci L, et al. Design of selective nuclear receptor modulators: RAR and RXR as a case study. Nature reviews Drug discovery. 2007;6:811–820. doi: 10.1038/nrd2398. [DOI] [PubMed] [Google Scholar]
- 5.Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiological reviews. 2001;81:1269–1304. doi: 10.1152/physrev.2001.81.3.1269. [DOI] [PubMed] [Google Scholar]
- 6.Howe LR. Rexinoids and breast cancer prevention. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:5983–5987. doi: 10.1158/1078-0432.CCR-07-1065. [DOI] [PubMed] [Google Scholar]
- 7.Uray IP, Dmitrovsky E, Brown PH. Retinoids and rexinoids in cancer prevention: from laboratory to clinic. Seminars in oncology. 2016;43:49–64. doi: 10.1053/j.seminoncol.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nature reviews Cancer. 2001;1:181–193. doi: 10.1038/35106036. [DOI] [PubMed] [Google Scholar]
- 9.Sanz MA, Grimwade D, Tallman MS, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113:1875–1891. doi: 10.1182/blood-2008-04-150250. [DOI] [PubMed] [Google Scholar]
- 10.Altucci L, Leibowitz MD, Ogilvie KM, et al. RAR and RXR modulation in cancer and metabolic disease. Nature reviews Drug discovery. 2007;6:793–810. doi: 10.1038/nrd2397. [DOI] [PubMed] [Google Scholar]
- 11.Duvic M, Edelson R. Cutaneous T-cell lymphoma. Journal of the American Academy of Dermatology. 2004;51:S43–45. doi: 10.1016/j.jaad.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 12.Sidiropoulos KG, Martinez-Escala ME, Yelamos O, et al. Primary cutaneous T-cell lymphomas: a review. Journal of clinical pathology. 2015;68:1003–1010. doi: 10.1136/jclinpath-2015-203133. [DOI] [PubMed] [Google Scholar]
- 13.Zhang C, Hazarika P, Ni X, et al. Induction of apoptosis by bexarotene in cutaneous T-cell lymphoma cells: relevance to mechanism of therapeutic action. Clinical cancer research : an official journal of the American Association for Cancer Research. 2002;8:1234–1240. [PubMed] [Google Scholar]
- 14.Nieto-Rementeria N, Perez-Yarza G, Boyano MD, et al. Bexarotene activates the p53/p73 pathway in human cutaneous T-cell lymphoma. The British journal of dermatology. 2009;160:519–526. doi: 10.1111/j.1365-2133.2008.08931.x. [DOI] [PubMed] [Google Scholar]
- 15.Budgin JB, Richardson SK, Newton SB, et al. Biological effects of bexarotene in cutaneous T-cell lymphoma. Archives of dermatology. 2005;141:315–321. doi: 10.1001/archderm.141.3.315. [DOI] [PubMed] [Google Scholar]
- 16.Duvic M, Hymes K, Heald P, et al. Bexarotene is effective and safe for treatment of refractory advanced-stage cutaneous T-cell lymphoma: multinational phase II–III trial results. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19:2456–2471. doi: 10.1200/JCO.2001.19.9.2456. [DOI] [PubMed] [Google Scholar]
- 17.Zhang C, Duvic M. Treatment of cutaneous T-cell lymphoma with retinoids. Dermatologic therapy. 2006;19:264–271. doi: 10.1111/j.1529-8019.2006.00083.x. [DOI] [PubMed] [Google Scholar]
- 18.Schultz JR, Tu H, Luk A, et al. Role of LXRs in control of lipogenesis. Genes & development. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duvic M, Martin AG, Kim Y, et al. Phase 2 and 3 clinical trial of oral bexarotene (Targretin capsules) for the treatment of refractory or persistent early-stage cutaneous T-cell lymphoma. Archives of dermatology. 2001;137:581–593. [PubMed] [Google Scholar]
- 20.Assaf C, Bagot M, Dummer R, et al. Minimizing adverse side-effects of oral bexarotene in cutaneous T-cell lymphoma: an expert opinion. The British journal of dermatology. 2006;155:261–266. doi: 10.1111/j.1365-2133.2006.07329.x. [DOI] [PubMed] [Google Scholar]
- 21.Schneider SM, Offterdinger M, Huber H, et al. Activation of retinoic acid receptor alpha is sufficient for full induction of retinoid responses in SK-BR-3 and T47D human breast cancer cells. Cancer research. 2000;60:5479–5487. [PubMed] [Google Scholar]
- 22.Sun SY, Yue P, Mao L, et al. Identification of receptor-selective retinoids that are potent inhibitors of the growth of human head and neck squamous cell carcinoma cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2000;6:1563–1573. [PubMed] [Google Scholar]
- 23.Szondy Z, Reichert U, Bernardon JM, et al. Inhibition of activation-induced apoptosis of thymocytes by all-trans- and 9-cis-retinoic acid is mediated via retinoic acid receptor alpha. The Biochemical journal. 1998;331(Pt 3):767–774. doi: 10.1042/bj3310767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taneja R, Roy B, Plassat JL, et al. Cell-type and promoter-context dependent retinoic acid receptor (RAR) redundancies for RAR beta 2 and Hoxa-1 activation in F9 and P19 cells can be artefactually generated by gene knockouts. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:6197–6202. doi: 10.1073/pnas.93.12.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faull RJ, Kovach NL, Harlan JM, et al. Affinity modulation of integrin alpha 5 beta 1: regulation of the functional response by soluble fibronectin. The Journal of cell biology. 1993;121:155–162. doi: 10.1083/jcb.121.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, DeMarco SS, Chen J, et al. Retinoids Bias Integrin Expression and Function in Cutaneous T-Cell Lymphoma. The Journal of investigative dermatology. 2015;135:2102–2108. doi: 10.1038/jid.2015.122. [DOI] [PubMed] [Google Scholar]
- 27.Perez E, Bourguet W, Gronemeyer H, et al. Modulation of RXR function through ligand design. Biochimica et biophysica acta. 2012;1821:57–69. doi: 10.1016/j.bbalip.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Pileri A, Delfino C, Grandi V, et al. Role of bexarotene in the treatment of cutaneous T-cell lymphoma: the clinical and immunological sides. Immunotherapy. 2013;5:427–433. doi: 10.2217/imt.13.15. [DOI] [PubMed] [Google Scholar]
- 29.Hwang ST, Janik JE, Jaffe ES, et al. Mycosis fungoides and Sezary syndrome. Lancet. 2008;371:945–957. doi: 10.1016/S0140-6736(08)60420-1. [DOI] [PubMed] [Google Scholar]
- 30.Kang SG, Park J, Cho JY, et al. Complementary roles of retinoic acid and TGF-beta1 in coordinated expression of mucosal integrins by T cells. Mucosal immunology. 2011;4:66–82. doi: 10.1038/mi.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeNucci CC, Pagan AJ, Mitchell JS, et al. Control of alpha4beta7 integrin expression and CD4 T cell homing by the beta1 integrin subunit. J Immunol. 2010;184:2458–2467. doi: 10.4049/jimmunol.0902407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson SK, Newton SB, Bach TL, et al. Bexarotene blunts malignant T-cell chemotaxis in Sezary syndrome: reduction of chemokine receptor 4-positive lymphocytes and decreased chemotaxis to thymus and activation-regulated chemokine. American journal of hematology. 2007;82:792–797. doi: 10.1002/ajh.20952. [DOI] [PubMed] [Google Scholar]
- 33.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nature reviews Immunology. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petkovich M, Brand NJ, Krust A, et al. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987;330:444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- 35.Giguere V, Ong ES, Segui P, et al. Identification of a receptor for the morphogen retinoic acid. Nature. 1987;330:624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- 36.Wu XS, Lonsdorf AS, Hwang ST. Cutaneous T-cell lymphoma: roles for chemokines and chemokine receptors. The Journal of investigative dermatology. 2009;129:1115–1119. doi: 10.1038/jid.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun SY, Kurie JM, Yue P, et al. Differential responses of normal, premalignant, and malignant human bronchial epithelial cells to receptor-selective retinoids. Clinical cancer research : an official journal of the American Association for Cancer Research. 1999;5:431–437. [PubMed] [Google Scholar]
- 38.Chiba H, Clifford J, Metzger D, et al. Specific and redundant functions of retinoid X Receptor/Retinoic acid receptor heterodimers in differentiation, proliferation, and apoptosis of F9 embryonal carcinoma cells. The Journal of cell biology. 1997;139:735–747. doi: 10.1083/jcb.139.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]