Abstract
Purpose
The most common GABA-editing approach, MEGA-PRESS, uses J-editing to measure GABA distinct from larger overlapping metabolites but suffers contamination from co-edited macromolecules (MMs) comprising 40–60% of the observed signal. MEGA-SPECIAL is an alternative method with better MM suppression but is not widely used primarily due to relatively poor spatial localization. Our goal was to develop an improved MM-suppressed GABA editing sequence at 3T.
Methods
We modified a single-voxel MEGA-SPECIAL sequence with an oscillating readout gradient for improved spatial localization, and used very selective 30 ms editing pulses for improved suppression of co-edited MMs.
Results
Simulation and in vivo experiments confirmed excellent MMs suppression, insensitive to the range of B0 frequency drifts typically encountered in vivo. Both inter-subject and intra-subject studies showed MMs, suppressed by the improved MEGA-SPECIAL method, contributed approximately 40% to the corresponding MEGA-PRESS measurements. From the inter-subject study, the coefficient-of-variation for GABA+/Cre (MEGA-PRESS) was 11.2% versus 7% for GABA/Cre (improved MEGA-SPECIAL), demonstrating significantly reduced variance (p=0.005) likely coming from co-edited MMs.
Conclusions
This improved MEGA-SPECIAL sequence provides unbiased GABA measurements with reduced variance as compare to conventional MEGA-PRESS. This approach is also relatively insensitive to the range of B0 drifts typically observed in in vivo human studies.
Keywords: GABA, MRS, MEGA-PRESS, MEGA-SPECIAL, macromolecule suppression
INTRODUCTION
In vivo measurement of the human brain’s primary inhibitory neurotransmitter, γ-aminobutyric-acid (GABA), using proton magnetic resonance spectroscopy (MRS) offers valuable information for understanding brain function in health and disease (1–12). Due to its low concentration, complicated spectrum, and overlap with other larger metabolites resonances at 3T, detecting GABA is difficult with conventional single-voxel MRS techniques such as PRESS and STEAM (13, 14). The most widely used sequence for in vivo GABA detection is MEGA-PRESS, a J-difference editing approach whereby spectral editing pulses are sequentially applied to the GABA C3 resonance at 1.9 ppm for the “ON” case and at 7.5 ppm, (symmetric to the water resonance at 4.7 ppm) for the “OFF” case (15). Subtraction of the two spectra yields a 3 ppm GABA signal due to J-coupling between the 1.9 and 3 ppm GABA peaks. Based on the GABA J-coupling constant, maximum signal is achieved using an echo time (TE) of 68 ms (16, 17).
Contamination from co-edited macromolecules (MMs) is the primary limitation for interpreting MEGA-PRESS GABA measurements. Finite echo times limit the duration (and hence spectral selectivity) of the editing pulses, resulting in partial excitation of MM components at 1.7 ppm that, like GABA, have J-coupled partners at 3.0 ppm (18). Indeed, the first reported in vivo 1H MRS GABA studies recognized that 40%–60% of the detected 3 ppm J-edited peak is not from GABA, but rather macromolecules exhibiting similar J-coupling patterns (19). For many published 1H MRS GABA studies, MM contamination is either ignored or assumed invariant across groups or treatments (5), and this limitation is recognized by reporting results as GABA+ = (GABA+MM). However, more detailed studies of brain MM resonances imply this is a problematic approach (20–22). Most troubling is the finding that MM contamination is variable across individuals and contributes a significant component to the observed MEGA-PRESS GABA+ signal variance (23). Regional MM differences have been reported (24), whereas findings with respect to age-dependence are inconsistent (25). Using magnetization transfer methods, metabolite-nulled spectra showed a 38% higher gray versus white matter MM baseline (26), and MRS-derived differences were found by Hoffman et al. (24) but not by Snoussi (27).
Several techniques combining GABA-editing with MM suppression have been proposed, however, none are widely used. Direct measurement of MMs via a second metabolite-suppressed acquisition doubles the already long scan time (5), for which none of the additional scan time is used to improve GABA SNR. An alternative, called “symmetric” or “MM-suppressed” MEGA-PRESS, uses inversion pulses placed symmetrically about the 1.7 ppm MM signal to suppress these unwanted resonances the compromise of the edited GABA signal (5). Editing efficiency and MM suppression can be further improved using longer spectral editing pulses. However, a 68 ms echo time limits the maximum length of the editing pulses in a double spin-echo MEGA-PRESS sequence to approximately 16 ms (28). Edden et al. showed that at 3T the edited GABA signal at 3 ppm is relatively insensitive to modest increases in echo time and proposed 80 ms TE to allow 20 ms editing pulses for improved selectivity (5). However, symmetric MEGA-PRESS, which relies on the assumption that the macromolecule resonances have chemical shifts symmetrical around 1.7 ppm and are thus equally affected by both editing pulses (29), is highly sensitive to variations in the scanner frequency with drifts of ≥1 Hz/min common after gradient intensive scans such as fMRI (30). Thus, a 10 minute GABA editing sequence, as part of a standard brain imaging protocol, could easily experience center frequency drifts on the order of ±10 Hz, resulting in unreliable MM suppression. Indeed, Mikkelsen et al. found GABA+ measurements were only weakly correlated with GABA signals acquired with symmetric MEGA-PRESS, an effect attributed to either sensitivity to frequency drifts or inter-individual MM variability (28). Recently, prospective frequency corrections using interleaved water referencing has been suggested to help correct such B0 frequency drifts (31).
An alternative approach uses the MEGA-SPECIAL technique, which replaces the double-spin-echo PRESS acquisition with a single spin-echo acquisition in combination with a preceding slice-selective inversion pulse. This allows for longer more-selective editing pulses, which in turn reduces MM signal contamination (32, 33). Unfortunately, MEGA-SPECIAL localization along the inversion direction is relatively poor, as this 1D ISIS approach is susceptible to subtraction artifacts from out-of-voxel signals (34, 35). Furthermore, MM-suppressed spectroscopic imaging of GABA has yet to be achieved (18, 36), and conventional single-voxel MEGA-PRESS remains the current method of choice. Indeed, Mullins et al., in a recent MEGA-PRESS review paper (16), state that “This widespread failure to account for MM … stands as the greatest single limitation of this area to date and should be acknowledged as such.”
Here we propose to address this concern via an improved MEGA-SPECIAL J-editing pulse sequence, employing an echo-planar (EP) readout gradient to improve spatial localization in ISIS direction and very selective editing pulses to improve MM suppression.
METHODS
The improved MEGA-SPECIAL pulse sequence, shown in Figure 1(a), was implemented on a 3T GE MR750 scanner (Waukesha, WI, USA) with a 32 channel Nova receive head coil. GE standard CHESS water suppression and slice-selective outer-volume-suppression pre-excitation RF pulses were used in the sequence. An adiabatic hyperbolic secant inversion pulse with a bandwidth of 5000 Hz was used for the 1D ISIS localization in the Z direction. A 3.6 ms slice-selective 90° RF pulse with a bandwidth of 2366 Hz was used for excitation while a 180° 5.2 ms slice-selective RF pulse with a bandwidth of 1384 Hz was used for refocusing. With 1.5 ms crusher gradients surrounding the refocusing RF pulse, two 30 ms Gaussian weighed sinc pulses, shown in Figure 1(b), were incorporated into the 80 ms TE sequence and applied at 1.9 ppm and 7.5 ppm for GABA editing.
Figure 1.
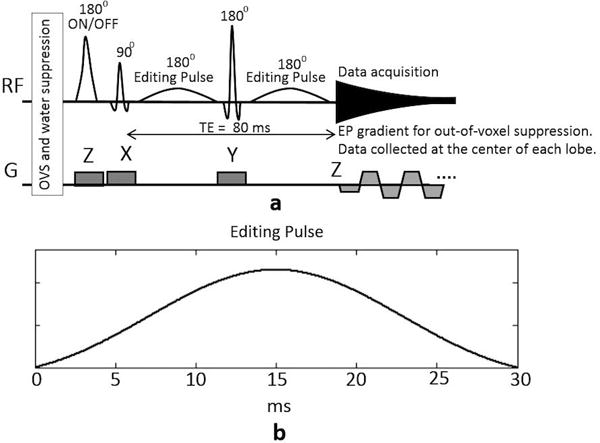
(a) Schematic of the proposed improved MEGA-SPECIAL sequence for GABA editing with MM suppression. An echo-planar readout gradient is used for out-of-voxel artifact suppression. The data points are only acquired at the center of each readout lobe. (b) The waveform of the 30 ms Gaussian weighed sinc editing pulse.
To suppress unwanted out-of-voxel signals in the ISIS direction, an echo-planar (EP) readout gradient was added. The data sampling rate and corresponding spectral bandwidth was kept unchanged from that for a standard spectroscopic gradient-free readout with the acquisition of each data point set to occur at the center of each gradient lobe (crossings of the z-axis in k-space), and the EP gradient strength were determined by the prescribed voxel thickness in the ISIS (Z) direction. This readout gradient can be viewed as an echo-planar version of the Spectroscopic Imaging Mode (SIAM) method for out-of-voxel artifact suppression (37, 38), a 1D implementation of the sensitive point method (39), or a proton echo-planar spectroscopic imaging (PEPSI) acquisition for which only data from the central slice is reconstructed (40).
Specifically, this approach exploits the scanner’s built-in digital low-pass filter. In contrast to conventional EPSI, where a high-bandwidth receiver filter is used and localization is achieved in the EP direction via post-processing, a narrow-bandwidth low-pass filter as would be used for a conventional spectroscopic readout in the absence of the EP gradient is retained. This narrow-bandwidth low-pass filter suppresses all out-of-slice signals, which resonance outside of the filter bandwidth. For iso-center voxels, the EP gradients cause out-of-slice signals to resonate beyond the cut-off frequency of the receiver’s low-pass filter. To accommodate differences in the low-pass filter passband, EP gradient selected slice, and the RF-selected slice profile, the EP gradient amplitude is set to provide a slice slightly larger (10%) than the corresponding RF-selected slice in the ISIS (Z) direction. For voxels out of the iso-center slice, demodulation of the acquired signal with the frequency determined by the gradient amplitude and the distance from the voxel center to the gradient iso-center is needed. In practice, this can be achieved by dynamically providing the receiver a demodulating frequency calculated from the same waveform as the EP gradient. This approach also works for oblique voxels as the EP gradient is played in the logical ISIS (Z) direction and thus rotation corresponding to the voxel orientation is applied to obtain the gradient waveforms in all three physical directions. For this application, each FID was acquired with 1024 data points at an acquisition bandwidth of 2500 Hz, i.e., 0.4 ms dwell time, resulting in a 409.6 ms readout. Accordingly, there were 1024 EP gradient lobes for each readout with a 0.4 ms duration of each lobe.
Data reconstruction is thus identical to that for data acquired without the oscillatory EP gradient readout. The net effect of this additional oscillatory readout gradient is to provide additional localization in the Z-direction to complement that provided by the, often imperfect, ISIS localization in the original MEGA-SPECIAL approach. Because a four cycle acquisition (A (inversion off, editing off); B (inversion on, editing off); C (inversion off, editing on); and D (inversion on, editing on)) was employed, localized FIDs with editing off and editing on were first obtained from A–B and C–D, respectively (32). Localized FIDs from each individual coil were phase corrected frame-by-frame using the dominant signal of residual water before averaging and coil combination (41, 42). Fourier transform was then applied after 4 Hz line broadening. Finally, spectrum with editing on was subtracted from that with editing off to obtain the localized GABA-edited spectrum.
To evaluate the sensitivity of the MM suppression to B0 inhomogeneities, we simulated the residual MM signal from (1) MEGA-PRESS, (2) symmetric MEGA-PRESS, and (3) the improved MEGA-SPECIAL. MMs were modeled as J-coupled peaks at 1.7 and 3.0 ppm with a 4 Hz linewidth. The MEGA-PRESS and symmetric MEGA-PRESS utilized 14 ms 180° Gaussian-weighed sinc editing pulses as compared to 30 ms 180° Gaussian-weighted sinc editing pulses for the improved MEGA-SPECIAL. Residual MM signals were computed for ±10 Hz B0 frequency drift. Ideal hard RF pulses were assumed for the spatial excitation and refocusing RF pulses.
The effects of the oscillatory readout gradient were next assessed by comparing in vivo data from an occipital lobe voxel of a healthy adult acquired with and without the EP gradient. To best visualize out-of-voxel signals, which would normally fold into the final spectrum, 16 phase encodes were applied in the ISIS (Z) direction, and a total of 256 transients were acquired with TE/TR = 80/2000 ms.
Finally, GABA measurements with the new improved MEGA-SPECIAL sequence were directly compared with those obtained using conventional MEGA-PRESS in intra- and inter-subject repeatability and variability studies. For the intra-subject study, data were collected from the same healthy subject in five sessions (with subject removal and repositioning performed between scans). The inter-subject study comprised data from ten healthy subjects. All in vivo data were acquired in a period of two months, and all human studies were approved by the local Institution Review Board with informed consent obtained from each participant. The editing pulses for MEGA-PRESS were 14 ms 180° Gaussian weighed sinc pulses and were applied at 1.9/7.5ppm with TE/TR=68ms/2s. For the improved MEGA-SPECIAL, the editing pulse were 30ms Gaussian weighed sinc pulse and applied at 1.9/7.5ppm with TE/TR=80ms/2s. GABA editing was performed on a 30×30×30mm voxel in the occipital lobe. For both MEGA-PRESS and the improved MEGA-SPECIAL, 256 transients were acquired with an 8:40 minute scan time. GABA levels were estimated by integrating the edited peak from 2.85 to 3.15ppm and referenced to Cre for quantification.
RESULTS
Figure 2(a) shows simulated spectral profiles of the 14 ms 180° Gaussian-weighed sinc editing pulse for conventional MEGA-PRESS (and symmetric MEGA-PRESS) and 30 ms 180° Gaussian-weighed sinc editing pulse for the improved MEGA-SPECIAL. The editing pulses are centered at 1.9/1.5 ppm for the ON/OFF cases for symmetric MEGA-PRESS and at 1.9/7.5 ppm for MEGA-PRESS and the improved MEGA-SPECIAL. The transition bandwidths, measured from 5% to 95% transition, for the 14 ms and 30 ms editing pulses were 73 and 32 Hz, respectively. Simulated coedited MM signals in the presence of a ±10 Hz B0 frequency drift for MEGA-PRESS, symmetric MEGA-PRESS and the improved MEGA-SPECIAL are shown in Figure 2(b). The MM levels were normalized to 1.0 for on-resonance MEGA-PRESS. Compared to MEGA-PRESS, symmetric MEGA-PRESS achieves perfect MM suppression on-resonance but is highly sensitive to B0 frequency drifts as its ON and OFF cases are affected by B0 frequency drifts in opposite directions. In contrast, the improved MEGA-SPECIAL has substantial MM suppression (8% of MM contamination as obtained using on-resonance MEGA-PRESS), and is less sensitive to B0 frequency shifts as compared to symmetric MEGA-PRESS.
Figure 2.
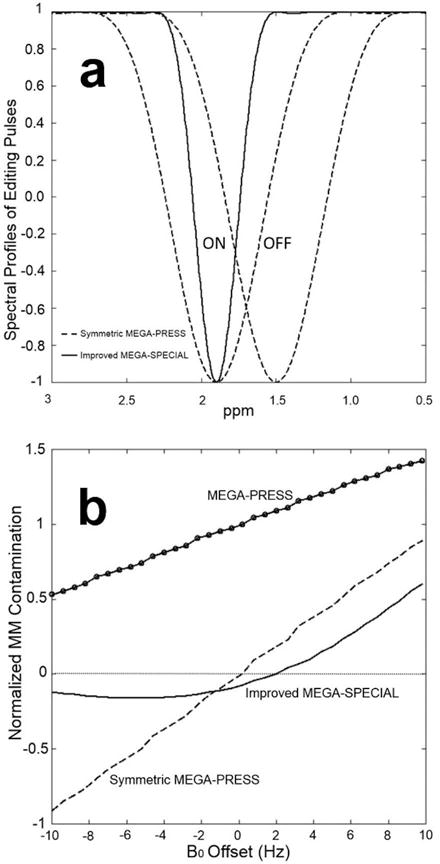
(a) Simulated spectral profiles of the 14 ms 180° editing pulse for MEGA-PRESS and symmetric MEGA-PRESS and 30 ms 180° editing pulse for the improved MEGA-SPECIAL. The editing pulses are centered at 1.9/1.5 ppm for the ON/OFF cases for symmetric MEGA-PRESS and at 1.9/7.5 ppm for MEGA-PRESS and the improved MEGA-SPECIAL. (b) Simulated coedited MM using the MEGA-PRESS, symmetric MEGA-PRESS and the improved MEGA-SPECIAL under ±10 Hz B0 frequency drift. All coedited MM was normalized to that using MEGA-PRESS on resonance.
The out-of-voxel artifact suppression using the oscillating readout gradient is demonstrated in Figure 3. By adding phase-encoding in the ISIS (Z) direction (16 steps), out-of-voxel signals can be visualized in the stack plots of the edited spectra from each slice with (Fig. 3c) and without (Fig. 3d) the oscillating readout gradient. Sum spectra, as would be obtained by a conventional single-voxel acquisition without additional z-phase encoding, are shown in Figure 3(b). In addition to using x, y, and z selective RF pulses to define the targeted voxel, all spectra were acquired using conventional slice-selective outer-volume-suppression (OVS) pulses. Such OVS pulses are typically sufficient for most 1H MRS applications, but the small residual signals can be important for highly SNR sensitive acquisitions such as GABA-editing. As shown in Figure 3(d), without oscillating readout gradient, significant water and lipids signals exist in the out-of-voxel slices, affecting the baseline of non-localized edited spectrum.
Figure 3.
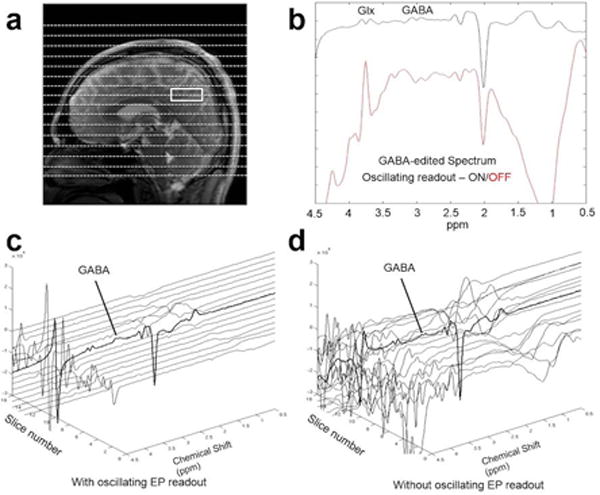
Beneficial effects of an oscillating readout gradient. (a) MRI image showing selected single voxel. (b) improved MEGA-SPECIAL spectra w/wo the addition of an oscillatory z-readout gradient for suppression of out-of-voxel (OVS) signals (along the z-axis) that adversely contribute to single-voxel spectra. OVS signals are visualized by adding z phase encoding (16 slices), and stack plots of the edited spectra from each slice with (c) and without (d) the oscillating readout gradient show the achievable artifact reduction. The spectrum from slice centered on the excited voxel is highlighted in BOLD. In addition to using x, y, and z selective RF pulses to define the targeted voxel, all spectra were acquired using conventional slice-selective OVS MRS suppression pulses, demonstrating the added level of suppression achievable with an oscillatory readout gradient. Parameters: editing at 1.7/7.5 ppm, TE/TR = 80/2000 ms, 256 transients, 8.5 min acquisition.
Figure 4 shows results from an inter-subject variability study comparing MEGA-PRESS and the improved MEGA-SPECIAL for ten healthy adult subjects. All GABA measurements were quantified relative to the creatine (Cre) signal. The mean ± standard deviation of GABA+/Cre was 0.12 ± 0.013 using MEGA-PRESS as compared to 0.07 ± 0.005 for GABA/Cre obtained with the improved MEGA-SPECIAL. The coefficient-of-variation for GABA+/Cre was 11.2% compared to 7% for GABA/Cre. Furthermore, the variance of the GABA/Cr measurements using the improved MEGA-SPECIAL was significantly less than that for GABA+/Cr measurements using MEGA-PRESS (f-test: f(9,9) = 6.72, p = 0.005). Based on the relative means, MMs contributed approximately 38% to the MEGA-PRESS measurements.
Figure 4.
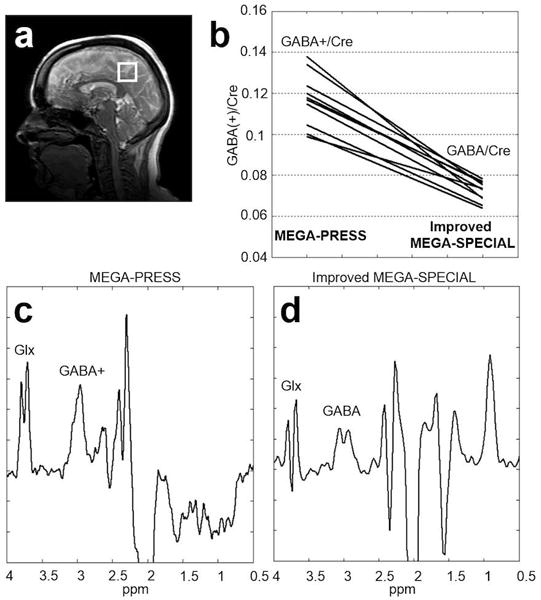
Inter-subject variability study comparing MEGA-PRESS GABA+ versus improved MEGA-SPECIAL GABA estimates from (a) a 27 cc voxel in the occipital lobe of normal adults (N = 10). (b) GABA measurements were quantified relative to the creatine (Cre) signal. GABA+/Cre = 0.12 ± 0.013 (mean ± sd) and GABA/Cre = 0.07 ± 0.005. Acquisition parameters: 256 transients, 8.5 min acquisition. The coefficient-of-variation for GABA+/Cre was 11.2% compared to 7% for GABA/Cre. (c) Representative edited spectrum using MEGA-PRESS, and (d) representative edited spectrum using the improved MEGA-SPECIAL.
Five edited spectra using the improved MEGA-SPECIAL from the intra-subject repeatability study are shown in Figure 5. The summary statistics were as follows: MEGA-PRESS GABA+/Cre = 0.11 ± 0.007 (mean ± sd) and the improved MEGA-SPECIAL GABA/Cre = 0.07 ± 0.004. The coefficient-of-variation was 6.0% for both the GABA+/Cre and GABA/Cre estimates. The estimated MMs contribution to the MEGA-PRESS measurements was 40%.
Figure 5.
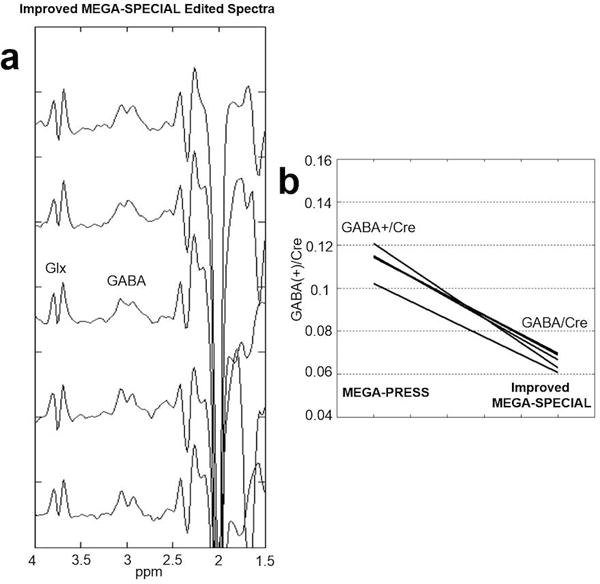
Intra-subject repeatability. (a) GABA-edited improved MEGA-SPECIAL spectra from a 27 cc voxel in the occipital lobe of a normal volunteer scanned five times (with subject removal from scanner followed by repositioning repeated after each scan). Acquisition parameters: 256 transients, 8.5 min acquisition. (b) Summary data comparing MEGA-PRESS GABA+/Cre = 0.11 ± 0.007 (mean ± sd) versus improved MEGA-SPECIAL GABA/Cre = 0.07 ± 0.004. The coefficient-of-variation was 6.0% for both the GABA+/Cre and GABA/Cre estimates.
DISCUSSION
Compared to MEGA-PRESS, the 1D ISIS-based MEGA-SPECIAL sequence has the advantage of using only a single spin-echo, thus allowing significantly longer editing pulses. This advantage, however, comes with the drawback of the out-of-voxel signal artifacts in the ISIS direction due to imperfect subtraction. If not suppressed, these signals can greatly compromise the quality of the edited spectra, significantly hampering visualization and quantification of the edited GABA peak. Using the low-pass filter on the scanner’s data acquisition board, the out-of-voxel signals in the ISIS direction can be suppressed by acquiring data with a train of echo planar readout gradient lobes. The amplitude and length of the EP gradient lobes are set according to the acquisition bandwidth and the slice thickness in the ISIS direction while the number of the EP gradient lobes are determined by the number of data points acquired for each FID. In practice, eddy currents can cause imperfect gradient lobes. As a result, precise timing is required to ensure the acquisition of each data point occurs at the z-axis crossing of the k-space. For this application on the GE 750 scanner, the built-in low-pass filter averages 100 data points for each 0.4 ms gradient lobe, effectively suppressing the out-of-voxel signals in the ISIS direction. Compared with the conventional data acquisition without the oscillating EP gradient, this artifact suppression module maintains the same FID data formats and thus requires no changes to the reconstruction and post-processing routine.
With MEGA-PRESS, MM suppression can be achieved by placing the editing pulse at 1.9 and 1.5 ppm, symmetric to the MM resonance at 1.7 ppm (29). Although effective, MM suppression using this method is highly sensitive to center frequency variations. As center frequency temporal drifts on the order of 5 to 10 Hz are common during a 10-min scan, insufficient and unreliable MM suppression can occur (30). By using very selective editing pulses with the improved MEGA-SPECIAL sequence, coedited MM signals are reduced at the stop band of the editing pulse, decreasing sensitivity to such B0 drifts. However, even with the selectivity of the 30 ms editing pulses, coediting at 1.7 ppm cannot be completed avoided. In this simulation, MM signal is modeled as a single J-coupled spin pair at 1.7 ppm and 3 ppm. With the very selective editing pulses applied at 1.9 ppm and ±10 Hz B0 frequency drift, the flip angle at 1.7 ppm can be well below 180°. At such flip angels, the center of the 3ppm peaks for the editing ON is significantly lower than that for the editing OFF. The coedited 3ppm peak, obtained by subtracting the editing OFF spectrum from the editing ON spectrum, can therefore have a negative peak area as shown in Figure 2(b). Nevertheless, over the entire ±10 Hz B0 frequency drift, coedited MM signal using the improved MEGA-SPECIAL is significantly less strong than using the MEGA-PRESS. On the other hand, because the editing pulse is very selective, GABA editing efficiency is more sensitive to B0 drift than using less selective editing pulses. However, even with ±10 Hz B0 frequency drift, the very selective editing pulses achieve over 85% inversion of the 1.9 ppm GABA spins while simultaneously suppressing significantly more MM than those using conventional or symmetrical MEGA-PRESS. Although only single-voxel data is presented in this study, the B0-frequency-drift insensitivity of the improved MEGA-SPECIAL may make it a good choice for multi-voxel studies, where both spatial and temporal B0 variations are even more problematic.
Cho and Cr subtraction errors are typically manifested as residual peaks at 3.2 and 3.0 ppm respectively. While any residual 3.0 ppm Cr cannot be distinguished from 3.0 ppm edited-GABA signal, we observed no residual peak at 3.2 ppm in any of our data, suggesting such subtraction artifacts were minimal in these studies.
In our comparison studies, a MEGA-PRESS sequence with TE=68 ms and 14 ms editing pulses was chosen due to the widespread use of these parameters. A TE=80ms MEGA-PRESS implementation would likely yield MM suppression intermediate between the improved MEGA-SPECIAL and TE=68ms MEGA-PRESS.
For both intra-subject and inter-subject repeatability studies, the mean of the GABA/Cre (improved MEGA-SPECIAL) were approximately 40% less than the GABA+/Cre (MEGA-PRESS), demonstrating significant MM suppression achieved using the improved MEGA-SPECIAL sequence. The coefficient-of-variation for the GABA+/Cre (MEGA-PRESS) and GABA/Cre (improved MEGA-SPECIAL) were both 6.0% for the intra-subject repeatability study while they were 11.2% versus 7% respectively, for the inter-subject repeatability study. Variations of coedited MMs across subjects likely causes a higher coefficient-of-variation for the GABA+/Cre (MEGA-PRESS) in the inter-subject repeatability study than that in the intra-subject repeatability study. Hence, in contrast to the intra-subject data, the significant reduction of the coefficient-of-variation in the inter-subject study using the improved MEGA-SPECIAL compared with MEGA-PRESS likely arises from MM suppression and the corresponding elimination of MM differences across subjects as a source of variance.
CONCLUSIONS
In this work, we propose a new 1H-MRS editing pulse sequence, improved MEGA-SPECIAL, providing GABA measurements with MM-suppression insensitive to the B0 temporal drifts seen in typical in vivo human brain studies. The method extends the previously proposed MEGA-SPECIAL method via the addition of an echo-planar readout gradient to suppress out-of-voxel artifacts. In vivo 3T data demonstrate reduced spectral baseline artifacts and both intra- and inter-subject studies demonstrated MM-suppressed GABA measurements with less variance than using conventional MEGA-PRESS.
Acknowledgments
The Lucas Foundation, GE Healthcare, NIH P41 EB015891, NIH R01 HD084214, NIH fellowship F32 EY02229
List of abbreviations
- GABA
γ-aminobutyric-acid
- MM
Macromolecule
- ISIS
Image-Selected In vivo Spectroscopy
- MEGA-PRESS
MEGA-Point-RESolved Spectroscopy
- MEGA-SPECIAL
MEGA-SPin-ECho-full-Intensity-Acquired-Localized spectroscopy
- EP
Echo-planar
- SIAM
Spectroscopic Imaging Acquisition Mode
- MRS
magnetic resonance spectroscopy
References
- 1.Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Boorman E, P MM, P JC. Low GABA concentrations in occipital cortex and anterior cingulate cortex in medication-free, recovered depressed patients. Int J Neuropsychopharmacol. 2008;11(2):255–60. doi: 10.1017/S1461145707007924. Epub 2007/07/13. doi: S1461145707007924 [pii] 10.1017/S1461145707007924. [DOI] [PubMed] [Google Scholar]
- 2.Boy F, Evans CJ, Edden RA, Lawrence AD, Singh KD, Husain M, Sumner P. Dorsolateral prefrontal gamma-aminobutyric acid in men predicts individual differences in rash impulsivity. Biol Psychiatry. 2011;70(9):866–72. doi: 10.1016/j.biopsych.2011.05.030. Epub 2011/07/16. doi: S0006-3223(11)00590-7 [pii] 10.1016/j.biopsych.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boy F, Evans CJ, Edden RA, Singh KD, Husain M, Sumner P. Individual differences in subconscious motor control predicted by GABA concentration in SMA. Curr Biol. 2010;20(19):1779–85. doi: 10.1016/j.cub.2010.09.003. Epub 2010/10/05. doi: S0960-9822(10)01084-5 [pii] 10.1016/j.cub.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brady RO, Jr, McCarthy JM, Prescot AP, Jensen JE, Cooper AJ, Cohen BM, Renshaw PF, Ongur D. Brain gamma-aminobutyric acid (GABA) abnormalities in bipolar disorder. Bipolar Disord. 2013;15(4):434–9. doi: 10.1111/bdi.12074. Epub 2013/05/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edden RA, Crocetti D, Zhu H, Gilbert DL, Mostofsky SH. Reduced GABA concentration in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2012;69(7):750–3. doi: 10.1001/archgenpsychiatry.2011.2280. Epub 2012/07/04. doi: 1211983 [pii] 10.1001/archgenpsychiatry.2011.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foerster BR, Petrou M, Edden RA, Sundgren PC, Schmidt-Wilcke T, Lowe SE, Harte SE, Clauw DJ, Harris RE. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum. 2012;64(2):579–83. doi: 10.1002/art.33339. Epub 2011/09/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaetz W, Bloy L, Wang DJ, Port RG, Blaskey L, Levy SE, Roberts TP. GABA estimation in the brains of children on the autism spectrum: measurement precision and regional cortical variation. Neuroimage. 2014;86:1–9. doi: 10.1016/j.neuroimage.2013.05.068. Epub 2013/05/28. doi: S1053-8119(13)00568-5 [pii] 10.1016/j.neuroimage.2013.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goto N, Yoshimura R, Moriya J, Kakeda S, Ueda N, Ikenouchi-Sugita A, Umene-Nakano W, Hayashi K, Oonari N, Korogi Y, Nakamura J. Reduction of brain gamma-aminobutyric acid (GABA) concentrations in early-stage schizophrenia patients: 3T Proton MRS study. Schizophr Res. 2009;112(1–3):192–3. doi: 10.1016/j.schres.2009.04.026. Epub 2009/05/26. doi: S0920-9964(09)00214-X [pii] 10.1016/j.schres.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 9.Petroff OA. GABA and glutamate in the human brain. Neuroscientist. 2002;8(6):562–73. doi: 10.1177/1073858402238515. Epub 2002/12/07. [DOI] [PubMed] [Google Scholar]
- 10.Rowland LM, Kontson K, West J, Edden RA, Zhu H, Wijtenburg SA, Holcomb HH, Barker PB. In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophr Bull. 2013;39(5):1096–104. doi: 10.1093/schbul/sbs092. Epub 2012/10/20. doi: sbs092 [pii] 10.1093/schbul/sbs092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry. 2002;159(4):663–5. doi: 10.1176/appi.ajp.159.4.663. Epub 2002/04/02. [DOI] [PubMed] [Google Scholar]
- 12.Simpson HB, Shungu DC, Bender J, Jr, Mao X, Xu X, Slifstein M, Kegeles LS. Investigation of cortical glutamate-glutamine and gamma-aminobutyric acid in obsessive-compulsive disorder by proton magnetic resonance spectroscopy. Neuropsychopharmacology. 2012;37(12):2684–92. doi: 10.1038/npp.2012.132. Epub 2012/08/02. doi: npp2012132 [pii] 10.1038/npp.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann N Y Acad Sci. 1987;508:333–48. doi: 10.1111/j.1749-6632.1987.tb32915.x. Epub 1987/01/01. [DOI] [PubMed] [Google Scholar]
- 14.Frahm J, Bruhn H, Gyngell ML, Merboldt KD, Hanicke W, Sauter R. Localized high-resolution proton NMR spectroscopy using stimulated echoes: initial applications to human brain in vivo. Magn Reson Med. 1989;9(1):79–93. doi: 10.1002/mrm.1910090110. Epub 1989/01/01. [DOI] [PubMed] [Google Scholar]
- 15.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11(6):266–72. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. Epub 1998/11/05. doi: 10.1002/(SICI)1099-1492(199810)11:6<266::AID-NBM530>3.0.CO;2-J [pii] [DOI] [PubMed] [Google Scholar]
- 16.Mullins PG, McGonigle DJ, O’Gorman RL, Puts NA, Vidyasagar R, Evans CJ, Edden RA. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage. 2014;86:43–52. doi: 10.1016/j.neuroimage.2012.12.004. Epub 2012/12/19. doi: S1053-8119(12)01177-9 [pii] 10.1016/j.neuroimage.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci U S A. 1993;90(12):5662–6. doi: 10.1073/pnas.90.12.5662. Epub 1993/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu H, Edden RA, Ouwerkerk R, Barker PB. High resolution spectroscopic imaging of GABA at 3 Tesla. Magn Reson Med. 2011;65(3):603–9. doi: 10.1002/mrm.22671. Epub 2011/02/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramadan S, Lin A, Stanwell P. Glutamate and glutamine: a review of in vivo MRS in the human brain. NMR Biomed. 2013;26(12):1630–46. doi: 10.1002/nbm.3045. Epub 2013/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Behar KL, Ogino T. Characterization of macromolecule resonances in the 1H NMR spectrum of rat brain. Magn Reson Med. 1993;30(1):38–44. doi: 10.1002/mrm.1910300107. Epub 1993/07/01. [DOI] [PubMed] [Google Scholar]
- 21.Behar KL, Rothman DL, Spencer DD, Petroff OA. Analysis of macromolecule resonances in 1H NMR spectra of human brain. Magn Reson Med. 1994;32(3):294–302. doi: 10.1002/mrm.1910320304. Epub 1994/09/01. [DOI] [PubMed] [Google Scholar]
- 22.Bhattacharyya PK. Macromolecule contamination in GABA editing using MEGA-PRESS should be properly accounted for. Neuroimage. 2014;84:1111–2. doi: 10.1016/j.neuroimage.2013.08.050. Epub 2013/09/06. doi: S1053-8119(13)00913-0 [pii] 10.1016/j.neuroimage.2013.08.050. [DOI] [PubMed] [Google Scholar]
- 23.Terpstra M, Ugurbil K, Gruetter R. Direct in vivo measurement of human cerebral GABA concentration using MEGA-editing at 7 Tesla. Magn Reson Med. 2002;47(5):1009–12. doi: 10.1002/mrm.10146. Epub 2002/04/30. [DOI] [PubMed] [Google Scholar]
- 24.Hofmann L, Slotboom J, Boesch C, Kreis R. Characterization of the macromolecule baseline in localized (1)H-MR spectra of human brain. Magn Reson Med. 2001;46(5):855–63. doi: 10.1002/mrm.1269. Epub 2001/10/25. doi: 10.1002/mrm.1269 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Aufhaus E, Weber-Fahr W, Sack M, Tunc-Skarka N, Oberthuer G, Hoerst M, Meyer-Lindenberg A, Boettcher U, Ende G. Absence of changes in GABA concentrations with age and gender in the human anterior cingulate cortex: a MEGA-PRESS study with symmetric editing pulse frequencies for macromolecule suppression. Magn Reson Med. 2013;69(2):317–20. doi: 10.1002/mrm.24257. Epub 2012/04/11. [DOI] [PubMed] [Google Scholar]
- 26.McLean MA, Barker GJ. Concentrations and magnetization transfer ratios of metabolites in gray and white matter. Magn Reson Med. 2006;56(6):1365–70. doi: 10.1002/mrm.21070. Epub 2006/10/20. [DOI] [PubMed] [Google Scholar]
- 27.Snoussi K, Gillen JS, Horska A, Puts NA, Pradhan S, Edden RA, Barker PB. Comparison of brain gray and white matter macromolecule resonances at 3 and 7 Tesla. Magn Reson Med. 2015;74(3):607–13. doi: 10.1002/mrm.25468. Epub 2014/09/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mikkelsen M, Singh KD, Sumner P, Evans CJ. Comparison of the repeatability of GABA-edited magnetic resonance spectroscopy with and without macromolecule suppression. Magn Reson Med. 2015 doi: 10.1002/mrm.25699. Epub 2015/04/30. [DOI] [PubMed] [Google Scholar]
- 29.Henry PG, Dautry C, Hantraye P, Bloch G. Brain GABA editing without macromolecule contamination. Magn Reson Med. 2001;45(3):517–20. doi: 10.1002/1522-2594(200103)45:3<517::aid-mrm1068>3.0.co;2-6. Epub 2001/03/10. doi: 10.1002/1522-2594(200103)45:3<517::AID-MRM1068>3.0.CO;2-6 [pii] [DOI] [PubMed] [Google Scholar]
- 30.Harris AD, Glaubitz B, Near J, John Evans C, Puts NA, Schmidt-Wilcke T, Tegenthoff M, Barker PB, Edden RA. Impact of frequency drift on gamma-aminobutyric acid-edited MR spectroscopy. Magn Reson Med. 2014;72(4):941–8. doi: 10.1002/mrm.25009. Epub 2014/01/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edden RA, Oeltzschner G, Harris AD, Puts NA, Chan KL, Boer VO, Schar M, Barker PB. Prospective frequency correction for macromolecule-suppressed GABA editing at 3T. J Magn Reson Imaging. 2016 doi: 10.1002/jmri.25304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Near J, Simpson R, Cowen P, Jezzard P. Efficient gamma-aminobutyric acid editing at 3T without macromolecule contamination: MEGA-SPECIAL. NMR Biomed. 2011;24(10):1277–85. doi: 10.1002/nbm.1688. Epub 2011/03/10. [DOI] [PubMed] [Google Scholar]
- 33.Ordidge R, Connelly A, Lohman J. Image-selected invivo spectroscopy (ISIS)—a new technique for spatially selective NMR-spectroscopy. J Magn Reson. 1986;66:283–94. [Google Scholar]
- 34.Keevil SF, Newbold MC. The performance of volume selection sequences for in vivo NMR spectroscopy: implications for quantitative MRS. Magn Reson Imaging. 2001;19(9):1217–26. doi: 10.1016/s0730-725x(01)00449-0. Epub 2002/01/05. doi: S0730725X01004490 [pii] [DOI] [PubMed] [Google Scholar]
- 35.Lawry TJ, Karczmar GS, Weiner MW, Matson GB. Computer simulation of MRS localization techniques: an analysis of ISIS. Magn Reson Med. 1989;9(3):299–314. doi: 10.1002/mrm.1910090302. Epub 1989/03/01. [DOI] [PubMed] [Google Scholar]
- 36.Bogner W, Gagoski B, Hess AT, Bhat H, Tisdall MD, van der Kouwe AJ, Strasser B, Marjanska M, Trattnig S, Grant E, Rosen B, Andronesi OC. 3D GABA imaging with real-time motion correction, shim update and reacquisition of adiabatic spiral MRSI. Neuroimage. 2014;103:290–302. doi: 10.1016/j.neuroimage.2014.09.032. Epub 2014/09/27. doi: S1053-8119(14)00774-5 [pii] 10.1016/j.neuroimage.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gu M, Hurd R, Noeske R, Rokem A, Baltusis L, Spielman D. Macromolecule Suppressed GABA Editing with Single Spin-Echo and Out-Of-Voxel Artifact Suppression. Proceedings of the 23rd Annual Meeting of the International Society for Magnetic Resonance and Medicine; Toronto, Canada. 2015. p. 4692. [Google Scholar]
- 38.Hurd R, Sailasuta N. Elimination of artifacts in short echo proton spectroscopy. Proceedings of the Fifth Annual Meeting of the International Society for Magnetic Resonance and Medicine; Vancouver, Canada. 1997. p. 1453. [Google Scholar]
- 39.Hinshaw WS. Spin Mapping: The Application of Moving Gradients to NMR. PHYSICS LETTERS. 1974;48A(2):87–8. [Google Scholar]
- 40.Posse S, Tedeschi G, Risinger R, Ogg R, Le Bihan D. High speed 1H spectroscopic imaging in human brain by echo planar spatial-spectral encoding. Magn Reson Med. 1995;33(1):34–40. doi: 10.1002/mrm.1910330106. [DOI] [PubMed] [Google Scholar]
- 41.Zhu G, Gheorghiu D, Allen PS. Motional degradation of metabolite signal strengths when using STEAM: a correction method. NMR Biomed. 1992;5(4):209–11. doi: 10.1002/nbm.1940050408. [DOI] [PubMed] [Google Scholar]
- 42.Mullins PG, McGonigle DJ, O’Gorman RL, Puts NA, Vidyasagar R, Evans CJ, Cardiff Symposium on MRSoG. Edden RA. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage. 2014;86:43–52. doi: 10.1016/j.neuroimage.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


