Abstract
Long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) have contributed substantially to reductions in the burden of malaria in the last 15 years. Building on this foundation, the goal is now to drive malaria towards elimination. Vector control remains central to this goal but there are limitations to what is achievable with the current tools. Here we highlight how a broader appreciation of adult mosquito behavior is yielding a number of supplementary approaches to bolster the vector control tool kit. We emphasize tools that offer new modes of control and could realistically contribute to operational control in the next 5 years. Promoting complementary tools that are close to field-ready is a priority for achieving the global malaria control targets.
Keywords: Malaria, vector control, Anopheles, insecticide resistance, behaviour, Integrated Vector Management
Vector control and malaria
The World Health Organization (WHO) recently published its ‘Global Technical Strategy for Malaria 2016–2030′, which sets out a vision and strategic framework to reduce malaria transmission by at least 90% over the next 15 years, and prevent its re-establishment in countries that are currently free of malaria [1]. Vector control is a central pillar within this Global Technical Strategy, reflecting the fact that wide scale deployment of long-lasting insecticide-treated bed nets (LLINs) [see Glossary] and indoor residual spraying (IRS) with insecticides have contributed to substantial declines in the burden of malaria in the last 15 years [1,2]. However, the robustness and utility of current vector control faces two key biological challenges. First, the negative impacts of insecticide exposure on survival and reproduction impose strong selection for resistance [3]. This problem is exacerbated by the fact that there is a very limited selection of chemical insecticides approved for public health use; at present pyrethroids are the only insecticide class used on a wide scale on bed nets and account for two-thirds of the total product (by area) used in IRS for malaria control [4]. Accordingly, physiological (and to a lesser extent behavioral) resistance is now widespread across mosquito species and populations, threatening the effectiveness of the frontline insecticide-based interventions [1,5]. Second, the current core tools are most effective against Anopheles vectors that feed and rest indoors and exhibit a preference for feeding on human hosts during nighttime [2]. Yet in many locations vectors exhibit more diverse behaviors, feeding on other hosts, feeding and resting outdoors, and/or feeding in the early evening [6–8]. A consequence of both these challenges is that there are limits to how much LLINs and IRS alone can reduce transmission, even with further intensification and optimization [9]. This problem creates a pressing need for supplementary vector control tools.
Exploration of vector control tools is a rich area of research. A recent review commissioned by the President’s Malaria Initiative highlighted examples of 12 broad technologies/approaches for new interventions, including new types of LLINs with resistance breaking properties (http://www.vector-works.org/wp-content/uploads/Vector-Control-Landscape-2015.pdf). Another recent analysis evaluated the evidence for 21 existing and emerging vector control tools excluding LLINs and IRS (http://www.rollbackmalaria.org/files/files/working-groups/VCWG/New challenges%2C new tools in vector control/2_Allison Tatarsky.pdf). Other reviews have focused on more specific strategies, such as biologically based or transgenic approaches [10,11]. Given these recent articles, our aim here is not to conduct an exhaustive review of prospective control tools. Rather, we outline two key criteria that we consider important in prioritizing the development of supplementary vector control tools; a mode of action that is complementary to current tools, and a short-timeline for implementation. Based on these criteria, we highlight a handful of tools/approaches that we feel have greatest immediate potential to add to the malaria vector control tool kit.
Timeline to impact
As described above, the WHO Global Technical Strategy for Malaria aims to reduce malaria transmission by at least 90% over the next 15 years. Similar ambitious targets are set out in the ‘Aspiration to Action’ document prepared by the Bill and Melinda Gates Foundation (https://www.mmv.org/newsroom/publications/aspiration-action-what-will-it-take-end-malaria), which calls for a halving in transmission every 10 years, leading to ultimate eradication by 2040. Inter-country alliances, such as the Asian Pacific Malaria Elimination Network, aim for regional elimination by 2030 (http://aplma.org/upload/resource/files/APLMA_Roadmap_final_EAS_2015.pdf).
The Global Technical Strategy is informed by a modeling analysis, which explores a range of future intervention scenarios that vary in terms of access to vector control (LLINS and IRS) and drug treatments (both seasonal malaria chemo-prevention and first line treatments with artemisinin combination therapy) [9]. The modeling analysis reveals a number of key insights (Figure 1). First, if vector control and drug use remain at current levels, malaria mortality is expected to increase in the next 10–15 years due to changing immunity profile in the population, wherein people born after the current interventions were scaled up are exposed more slowly and acquire their first and subsequent cases at an older age. Second, if effectiveness of existing tools falls (e.g. through evolution of resistance) the rebound in malaria burden will likely be more pronounced. Third, further intensification of existing core tools to 80 or 90% coverage can lead to reductions in malaria burden and even elimination in some settings, but fails to reach the anticipated targets in areas of intense transmission. Finally, only if supplementary tools are forthcoming within the near future is it predicted that the WHO targets can be achieved.
Figure 1. Estimate of historic and projected global deaths due to malaria based on different control scenarios.
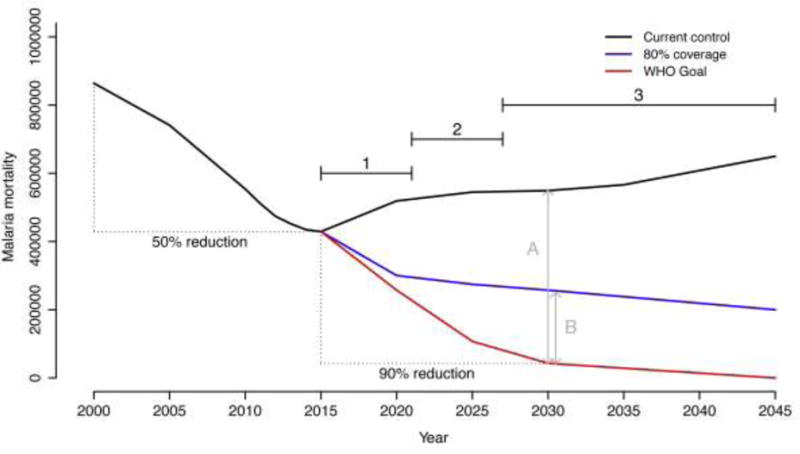
The figure (modified from [9]) shows estimates of global malaria deaths from 2000–2045. The 50% decline in malaria related mortality recorded from 2000–2015 is largely attributable to the wide scale implementation of vector control tools (Long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS)) [1,2]. The future projections are based on a model analysis that considers different scenarios of access to vector control, together with malaria drug treatments [9].
The graph is modified from Figure 1B of Griffin et al. [9] by using data from the 2016 World Malaria Report [89] to convert the original y-axis of ‘deaths per 1000 people per year’ into estimates of overall malaria mortality per year, and adding the target line for future decline in malaria deaths from the WHO Global Technical Strategy. The back line indicates resurgence in malaria deaths if control efforts remain at current levels. The blue line is the predicted decline in deaths assuming coverage of current control tools can be increased to reach 80% of the population at risk. The red line represents the target set out in the WHO Global Technical Strategy [1], which aims for a 90% decline in malaria deaths by 2030 and then ultimate elimination thereafter.
The arrows A and B illustrate the differences between the WHO target and the two control scenarios. Business as usual clearly represents a massive failure (A). Perhaps more notably, even substantial intensification of existing tools still yields a substantial shortfall (B). These gaps in control demonstrate the need for new interventions. The numbered horizontal lines refer to the estimated timelines for implementation of a range of prospective control tools where: (1) refers to tools that are close to field ready (e.g. attractive toxic sugar baits, housing improvement, livestock targets, next generation LLINs and IRS); (2) represents tools that require a few more years for product development (e.g. improved topical repellents, long lasting endectocides for human use); and (3) tools that either for technical and/or regulatory reasons are still far from operational use (e.g. transinfection with Wolbachia, population replacement strategies using genetically modified mosquitoes and gene drive). The fact that the WHO target shows an immediate deviation from the two control scenarios highlights a critical role for tools that can be implemented in the short- and medium-term (1 and 2).
The requirement for supplementary tools to be implemented at scale within the next 5 or so years puts an emphasis on approaches that are close to field-ready, and limits the immediate utility of prospective tools that are still far from operational (see Figure 1). For example, there is considerable interest in the potential of new gene editing technologies for developing transgenic mosquitoes for use in population replacement or population suppression strategies [12–15]. Approaches to reduce vector competence by manipulating elements of the mosquito microbiome [16–18], or via transinfection with endosymbionts such as Wolbachia [19,20], are also being examined. However, given the current exploratory nature of this research (in most cases the research has yet to progress beyond lab-based proof of principle studies), together with the challenges and timelines of regulatory approval, it is questionable whether such technologies will achieve wide scale operational use for malaria control within the next 8–10 years. This argument does not mean that these technologies cannot make valuable contributions somewhere down the line. Nonetheless, it is very difficult to see how they can play a substantial role in averting the present-day insecticide resistance crisis, or in driving down malaria transmission in the next decade (Figure 1).
Complementing existing vector control
Because transmission of malaria is so directly linked to the bite of the mosquito, a lot of research focuses on blood feeding behavior and factors affecting vector competence. Yet the life cycle of the adult mosquito involves much more than taking and digesting a blood meal.
A young adult mosquito emerges from the aquatic larval habitat with a small reserve of energy [21]. Both male and female mosquitoes then consume sugars, mainly obtained from floral and extra-floral nectar, and honeydew [22]. Mating does not occur for a couple of days after the adults emerge. Males form mating swarms and virgin females enter these swarms, locate a male and then exit as a couple to mate [23]. To complete the first gonotrophic cycle, most female mosquitoes must next take a blood meal. The host could be a human or, depending on the feeding behavior, an alternative vertebrate such as a cow [24]. Feeding can take place indoors or outdoors depending on the species and their populations [6]. To digest a blood meal safely and before the onset of searching for an oviposition site, a female will rest for 2–3 days. Resting can take place indoors or outdoors, again depending the species [25]. After blood digestion, a female has to find a suitable oviposition site, which in some cases can be distant and take several days to locate, during which there is likely more demand for sugars [26]. Because human malaria parasites take around 8–12 days to complete the sporozoite cycle within the mosquito under optimum temperatures (and this can be considerably longer under suboptimal conditions) [27,28], female mosquitoes must survive at least three such gonotrophic cycles before being able to transmit malaria [29] (Figure 2).
Figure 2. Diverse behaviors and activities of adult malaria mosquitoes as they progress from emergence through to egg laying over one or more gonotrophic cycles.
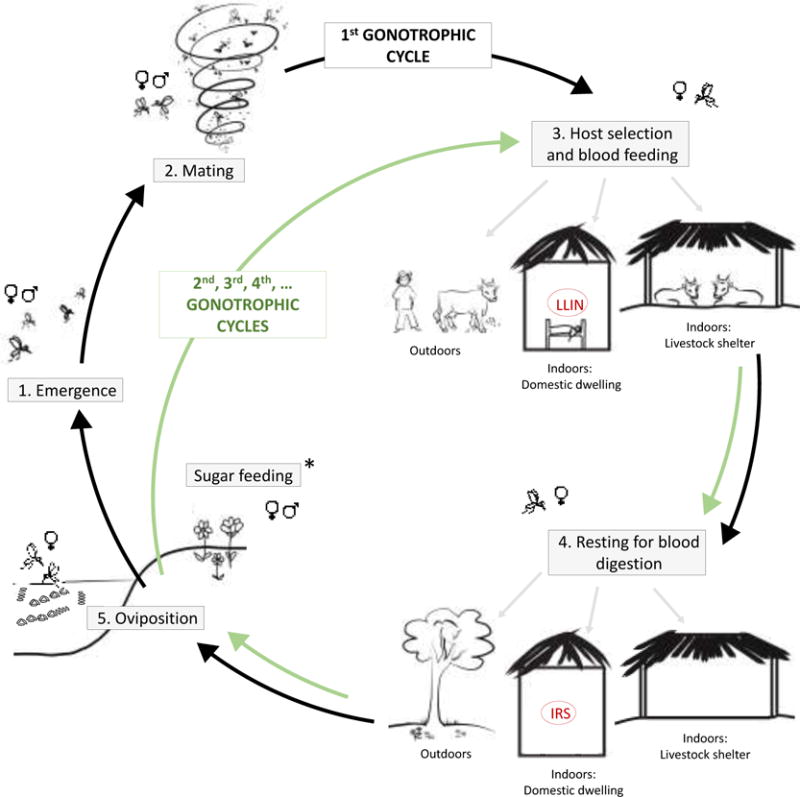
Adult mosquitoes emerge from aquatic habitats (1) and mate within a few days (2), potentially taking a sugar meal for energy (*). Male mosquitoes then tend to die quite quickly, while females go in search of a blood meal (3). Blood feeding could be on a diversity of hosts, either indoors or outdoors. After blood feeding the mosquitoes will tend to rest for 2–4 days while they digest the blood to produce eggs (4). Resting can occur in a range of indoor or outdoor environments. Once the eggs are fully developed the mosquitoes then search for a suitable oviposition site (5), potentially taking another sugar meal (*) to boost energy reserves for flight. Once a suitable aquatic habitat is located and the eggs are laid, female mosquitoes can repeat the blood feeding and egg production process over subsequent days to complete multiple gonotrophic cycles.
Current core vector control tools (Long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS)) target female mosquitoes at just two points in the adult life cycle within domestic dwellings only.
All these mosquito activities and the locations in which they take place provide opportunities for disrupting the adult mosquito life cycle and hence, reducing transmission. LLINs and IRS work by lowering contact rate between humans and vectors, either because the insecticide changes the normal feeding or host-searching behavior (repellency or deterrence) [30], and/or the insecticide causes mosquito death, affecting the age structure of the mosquito population and potentially adult mosquito density [31]. Because of the importance of these core tools and the potential for insecticide resistance to render them less effective, development of next generation LLIN and IRS products comprising novel active ingredients that overcome resistance, is an important ongoing activity [32]. Nevertheless, LLINs and IRS target only mosquitoes inside the domestic dwelling, leaving activities such as sugar feeding, mating, outdoor biting, host searching and house entry, alternate host feeding, outdoor resting etc. untouched (Figure 2). Also, LLINs and IRS generally only impact females. Supplementary tools that target adult mosquitoes more broadly at multiple points across their life cycle are needed complement these established tools and in so doing, address the challenge of residual transmission and create new opportunities for insecticide resistance management.
Candidate tools
In Table 1 we provide an illustrative (not exhaustive) list of adult vector control tools that are currently being researched (i.e. have been published on in recent years) and assess them according to our criteria of ‘field-ready’ and ‘complementary’. We also outline briefly some of the challenges to move forward to operational use. This assessment is somewhat subjective, but our aim is to highlight technologies that bring something new to the table (Figure 2) and identify a feasible timeline for implementation (Figure 1). We discuss a number of tools/approaches that we feel have greatest immediate utility in more detail below.
Table 1.
Illustrative list of prospective tools/approaches for control of adult malaria vectors, outlining modes of action, whether the tool is ‘field ready’, the estimated to operational use and some of the remaining research and development challenges.
| CONTROL TOOL | COMPLEMENTARY MODE OF ACTION | FIELD-READY TECHNOLOGY | ESIMATED TIME TO USE | CHALLENGES | REFERENCES |
|---|---|---|---|---|---|
| Attractive Toxic Sugar Bait | YES: Targets both sexes of diverse mosquito species; repeated exposure across life time; independent of blood feeding or resting behavior; resistance breaking actives. | YES: Ongoing small- scale field trials; simple technology with available products. | 0–5 years | Short lifespan when used as sprays on vegetation; further work needed to evaluate in different ecological contexts and to optimize within integrated vector management strategies (IVM); non-target evaluation. | [33–42] |
| Swarm sprays | YES: Targets males and also pre- gravid females; independent of blood feeding or resting behavior; resistance breaking actives. | YES: Ongoing small- scale field trials; simple technology with available products. | 0–5 years | Large number of swarm targets; demonstrate impact across diverse species and ecosystems; needs optimization within IVM; cost evaluations and implementation strategies required (who sprays and who pays?). | [43–49] |
| Housing improvement | YES: Prevents house entry and protects users without LLINs; independent of insecticide resistance; potential for resistance breaking actives in Eave Tubes. | YES: Numerous available approaches and new technologies (like Eave Tubes) under large-scale field evaluation; existing field trials and meta analyses support impact; housing improvement is already happening across many disease affected countries. | 0–5 years | Further research required on appropriateness in different socioeconomic settings; need for cost- effectiveness evaluations and exploration of different implementation strategies. | [50–67] |
| Livestock targets | YES: Addresses problem of zoophilic vectors | YES: IRS of livestock structures can use existing technology; numerous topical insecticides and endectocides on market. | 0–5 years | Need for longer lasting endectocides to reduce treatment frequency; not all livestock are treatable or have defined housing structures; cost and effectiveness across different systems and socio-economic contexts. | [68–82] |
| Spatial repellents | YES: Potentially protects users before they go indoors and users without LLINs. | YES: Certain products already commercially available and used. | 0–5 years | Need for improved long lasting products; costs likely prohibitive in certain settings and require financial contribution from end- user (consumer products not covered by normal public health budgets); appropriate targeting and optimizing within IVM. | [83–87] |
| Next generation LLINs | NO: Resistance breaking but same limitations as conventional LLINs. | YES: Certain nets are available now and more are under development. | 0–5 years | Current next generation nets cost 2–3 times as much as standard LLINs; not clear that they completely restore efficacy and improve control in all locations; resistance can still evolve. | [90–93] |
| Next generation IRS | NO: Resistance breaking but same limitations as conventional IRS. | YES: Certain new products are available and more are under development. | 0–5 years | New IRS products cost more than existing IRS so either more money needs to be made available or fewer houses are sprayed; resistance can still evolve; many countries don’t use IRS. | [94–96] |
| Sterile Insect Technique via irradiation | YES: Targets all females and subsequent offspring; independent of blood feeding or resting behavior; independent of insecticide resistance. | YES/NO: Small-scale field trials with An. arabiensis but not yet applicable to other species. | 4–8 years | Fitness costs of irradiation; challenges of mass rearing and sorting of males; mating competition with wildtypes; dispersal constraints; mixed species complexes; public acceptance. | [10,11] |
| Topical repellents | YES: Independent of blood feeding or resting behavior. | YES/NO: Products do exist but little demonstrated protection against malaria infection (both field trials and metaanalysis). | 4–8 years | Need clearer efficacy data; costs; short duration products needing repeat application; user acceptance; potential for resistance | [97–99] |
| Endectocide for humans | YES: Targets mosquitoes whenever they feed on humans, including outdoor biters. | YES/NO: Some products readily available and undergoing fieldtesting but persistence issues possibly limit current utility. | 4–8 years | Need for longer lasting formulations; need better understanding of mode of action; need more efficacy data on lethal and sub-lethal effects; safety constraints; public acceptance; safety monitoring. | [78,81,100–102] |
| Transinfection with Wolbachia | YES: Population replacement or suppression approaches work irrespective of blood feeding or resting behavior, and insecticide resistance. | NO: Laboratory proof of principle only. | 8–10 years | Development of technology (stable transinfection in only one Anopheles species so far); works best with low density populations; potential fitness costs affecting dispersal and mating behaviors; effectiveness across environments; mass rearing; species complexes; environmental and ethical safety; regulation; public acceptance. | [10,11,19,20] |
| Population suppression strategies via genetic modification | YES: Targets all females and subsequent offspring; independent of blood feeding or resting behavior, and independent of insecticide resistance. | NO: Laboratory proof of principle only. | 8–10 years | Development of technology (there is no clear product as yet for malaria vectors); potential fitness costs; effectiveness across environments; challenges of mass rearing; mating competition with wildtypes; dispersal constraints; mixed species complexes; environmental and ethical safety; regulation; public acceptance. | [10–18] |
| Population replacement strategies using genetically modified mosquitoes and gene drive | YES: Many possible modes of action independent of blood feeding or resting behavior, and independent of insecticide resistance. | NO: Laboratory proof of principle only. | 8–10 years | Development of technology (there is no clear product as yet); potential fitness costs; effectiveness across environments; dispersal constraints; mixed species complexes; resistance evolution against transgene or gene drive; environmental and ethical safety; regulation; public acceptance. | [10–18] |
Tools highlighted in bold are those that have modes of action that complement current tools (i.e. target different mosquito behaviors or different segments of the mosquito population than conventional LLINs and IRS) and are sufficiently advanced that they could be implemented in the near term (i.e. either are, or are close to being, ‘field ready’).
Sugar Feeding
Attractive toxic sugar baits (ATSBs), which utilize a mix of an oral toxin, natural sugars, and floral attractants to lure mosquitoes [33,34], take advantage of the natural propensity of both male and female mosquitoes to sugar feed. ATSBs can be used in outdoor bait stations, indoor bait stations, or can be sprayed directly onto non flowering vegetation [35–37]. The products appear inexpensive and require minimal change in user behavior [38]. Moreover, the wide choice of candidate stomach toxins creates options for control of mosquitoes resistant to the currently used contact insecticides [39].
A small-scale field trial in Mali showed that ATSBs sprayed onto vegetation reduced the population of Anopheles gambiae s.l. by 90% [40]. A similar study in the Rift Valley showed a 95% reduction in female Anopheles sergentii populations, while completely eradicating males [41]. Even with indoor bait stations, both males and female mosquitoes were attracted to and fed from this source, with more than 90% reduction in populations [38]. Moreover, these studies report changes in population age structure towards younger mosquitoes; an important result as it is the old mosquitoes that are responsible for transmission. A recent modeling study showed that ATSBs could substantially reduce An. gambiae populations and associated entomological inoculation rates (EIR) to near zero, in both sugar-resource-rich and sugar-resource-poor environments [42]. Evaluating this prediction empirically, and exploring the full range and potential usage of ATSBs in future integrated vector control strategies more generally, are key next steps.
Swarm sprays
Another underexploited target for vector control is swarming behavior [43]. The locations of mating swarms are stable over the seasons [44] and appear linked to swarm markers on the ground such as wells, wood piles or the limits between footpaths and grass [45,46]. These markers seem to provide visual cues for the males [43]. The proposed strategy for targeting these swarms is to use field observations and Geographic Information Systems (GIS) [43,47] to map swarm locations and then spray swarms with insecticide when they start forming, just after sunset. The swarms are generally accessible, as they are only 1 to 3m above the ground, depending on the swarm markers [45,46].
A recent field trial conducted in Burkina Faso recruited a team of 20 volunteers from a village and targeted 300 swarm locations, spraying swarms with aerosols as they appeared over a 9- day period. These spray treatments reduced mosquito (An. gambiae s.l.) density by 80% over a period of 10 days compared with a control village, and also caused a significant reduction in female insemination rate [48]. Other similar studies show equivalent results [43]. As with ATSB, further work is required to fully evaluate and optimize the spraying pattern and frequency across a wider range of settings, and to determine cost effectiveness. However, swarm spraying requires little specialist equipment and all the major African malaria vectors, as well as certain Asian and Latin American species [49], illicit swarming behavior, suggesting considerable potential for the approach. Importantly, swarm sprays target males and pre blood-fed females so any impact is independent of the blood feeding and resting behavior that can affect LLINs and IRS [43].
House entry
Houses are not the only location where malaria transmission occurs, but they remain the most important transmission environment in many endemic areas [50–52]. Even with outdoor biting and transmission, there is evidence that a mosquito is likely to enter a house at some point during its life prior to delivering an infective bite [53]. Accordingly, one complementary vector control intervention is to modify the house to limit mosquito entry.
Modern houses tend to be more protective against malaria than traditional houses made of natural materials that leave multiple gaps through which mosquitoes can enter [54], and in some settings offer protection equivalent to LLINs [55]. What constitutes modern housing is context dependent, but generally includes a shift in the type of building materials from thatch roofs to metal, and from mud walls to brick or concrete. Houses might also include finished flooring, ceilings, improved doors, window screening, and closed eaves. All these changes help to make a house more mosquito-proof and can reduce malaria in the inhabitants [56–59].
None of the standard house modifications require new technology per se, but there is a recent innovation that could add to the impact by combining house improvements with targeted insecticide treatment and effectively turning the house into a lethal lure. Open eaves are an important source of host attractant cues and a key entry point for An. gambiae s.l. in Africa [60,61]. Closing the eaves is, therefore, an important mosquito prevention measure. Eave tubes are pieces of PVC pipe that can be fitted to partially re-open the eaves. The eave tubes contain an insert comprising insecticide treated netting that kills mosquitoes as they attempt to enter the house through the tubes [62,63]. An electrostatic coating on the insert screening allows for the use of powder formulations of insecticides, a delivery method that is highly effective even against resistant mosquitoes [64]. One benefit of the lethal house lure approach is that it is a passive technology that protects everyone sleeping in the house (IRS is a household level intervention but generally does not prevent house entry; LLINS provide personal protection but rarely does everyone in a house use a net). As coverage of eave tubes increases, a community-wide mass action effect is also predicted [65].
Eave tubes require only small quantities of insecticide per house, enabling use of insecticide products that might be too expensive for use in IRS. Replacement of inserts is also very easy, potentially providing a method to deliver insecticides with rapid turnover that would not be appropriate for IRS or LLINs. Beyond diversifying the active ingredients available for vector control, the flexibility and potential for rapid turnover could provide a real opportunity to implement insecticide resistance management strategies that use insecticide rotations, mosaics, or mixtures [66]. Other house modifications such as insecticide treated eave and window screening [67], or insecticide treated eave baffles [68] could offer similar opportunities, and increase options for extending the ‘lethal house lure’ approach to a broader array of house types (note, however, that eave baffles are designed to allow mosquitoes to enter the house and so, like IRS, do not necessarily provide direct protection against biting). The cost effectiveness of any of these approaches requires further research, and will likely depend strongly on the nature of the local housing. However, leveraging private and public investment in housing improvement could provide a means to improve public health without adding burden to existing public health budgets.
Targeting livestock
Certain key malaria vectors are strongly anthropophilic. However, there are many vector species or populations that exhibit more diverse behavior, feeding on livestock (zoophilic behavior) as well as humans. While feeds on non-human hosts represent ‘wasted bites’ in terms of acquiring or passing on the malaria parasite, they allow the mosquito to escape the effects of interventions like IRS and LLINs that center on the human host. Targeting these mosquitoes with livestock-based interventions could play an important role in reducing residual transmission [7,8,69].
Mosquitoes feeding on livestock could be targeted through treatment of livestock structures (e.g. IRS of cattle sheds). This approach is attractive as the technology exists, livestock structures tend to be less numerous than households (e.g. [70]), and many of the challenges that apply to conventional IRS (such as inconvenience of householders having to be available to grant access and remove furniture, concerns over odors or staining of walls etc.) are less relevant [7]. In addition, it might well be possible to use different chemical products than those approved for use in domestic dwellings, providing opportunities for resistance management [7]. Where structures don’t exist, livestock-baited tents [71,72] and use of LLIN fences as livestock enclosures [73] have been shown to kill mosquitoes and reduce mosquito numbers indoors.
Direct treatment of cattle with insecticides by dipping, sponging, or spraying has also been shown to kill mosquitoes [74,75] and to reduce malaria in the human population [76]. One of the challenges in this approach is that many of the candidate insecticides are pyrethroid-based [72,77], and the pyrethroid resistance in Anopheles populations is particularly wide spread in Africa. An alternative is the use of systemic veterinary insecticides (referred to as endectocides) that affect the mosquitoes upon blood feeding. Ivermectin has been successfully tested in cattle and demonstrated to both kill mosquitoes and shorten lifespan of survivors [78,79]. Other candidate endectocides are also being explored [80,81], as well as slow release formulations that could reduce frequency of retreatment [82].
Spatial repellents
Spatial repellents (i.e. an airborne chemical that reduces human-vector contact by eliciting one or more changes in insect behavior) have been researched for many years and shown to have potential to reduce transmission [see [83] for overview], including randomized controlled trials demonstrating epidemiological impact of commercially available products [84,85]. A feature of spatial repellents is that they can potentially provide protection in the evening before householders go to sleep and so could be complementary to LLINS [84]. They might also be utilized where LLIN or IRS use is minimal [86]. One of the operational challenges, and subject of ongoing research, is development of long lasting formulations or delivery systems to increase user acceptance and cost-effectiveness [83,87]. However, use of available consumer products (coils, vaporizers, impregnated mats etc.) has been correlated with lower risk of malaria at the household level, depending on transmission environment and socio-economic status [86]. As such, these tools already appear to be contributing, albeit with little strategic integration into control programs.
Concluding remarks
Increasing the coverage and overall effectiveness of vector control is key to achieving the targets of the WHO Global Technical Strategy for malaria, and the broader goals of elimination and eradication. The current tools, LLINs and IRS, provide the foundation and intensifying their use is a priority. To maintain the effectiveness of these core tools moving forward there is a need for novel chemical actives that circumvent insecticide resistance [but see Outstanding Questions box]. However, to supplement existing vector control, target behavioral as well as physiological resistance, and address the challenges of residual transmission, requires supplementary methods that target mosquitoes more broadly. Moreover, in order to avert an anticipated rebound in malaria due to waning natural immunity and potential impacts of insecticide resistance, it is essential that new tools enter into operational use within the next 5 or so years.
Outstanding Questions Box.
How much will emerging pyrethroid resistance reduce the effectiveness of core vector control tools? Better understanding the effect size of resistance on malaria transmission would help define the magnitude of the ‘control gap’ that needs to be filled by supplementary tools.
How good does a novel control tool need to be in order to justify implementation? Determining the value of a technology not only depends on local ecology and socio-economic context but also becomes increasingly complex when multiple tools are deployed together. Integrated strategies might well deliver better overall control but it is almost inevitable that there will be some redundancy between tools.
How do we best combine tools to develop locally effective and sustainable integrated vector management strategies and how should these integrated strategies be evaluated? Conventional randomized controlled trials are extremely challenging when there are multiple factorial combinations of treatments and when effect sizes become small.
Can we leverage mechanisms outside the traditional public health sector (such as consumer products and housing improvement) to promote technologies and help bridge funding shortfalls for malaria control?
Can regulatory and approval mechanisms be streamlined to facilitate adoption of new tools without compromising necessary data on safety and efficacy?
The tools we have highlighted here (ATSB, swarm sprays, housing improvements, livestock treatments, spatial repellents) are among those that both complement existing control and have the potential to be implemented at scale in the near future. In order to make this a reality, a number of interrelated challenges remain [see Outstanding Questions box]. First, each of the candidate technologies needs further research to evaluate impact and achieve relevant regulatory approvals. Most crucially, there is a need to demonstrate epidemiological impact, as this is the current gold standard for evaluation. Large-scale epidemiological trials are underway for some tools, but further efforts (and hence funding) are required to build the evidence base. One uncertainty here is what constitutes a sufficient body of evidence given both the urgent need for supplementary tools, and the diversity of malaria transmission ecologies and socioeconomic settings. Once proof of principle has been demonstrated in a single epidemiological trial, it might be better to focus efforts on challenges of implementation, rather than conducting further trials in the hope of satisfying the notion of generality. Second, there is a need for economic evaluation and analysis of factors that influence the potential for scale up, such as user acceptance, supply chains and distribution networks, costs and willingness to pay across different market sectors etc. Third, there is a need to develop appropriate implementation strategies so that individual technologies can be tailored to local ecological and socio-economic contexts, and combined into optimum integrated vector management strategies [88]. The emergence of supplementary technologies creates new challenges for operational control. For example, should a particular national malaria control program choose ATSBs, or endectocides, or eave tubes, or is there a benefit in combing all three? Answering such questions empirically through the classical approach of randomized controlled trials is extremely challenging. However, if this is the evidence that is required, such trials will need supporting. Progress to address these challenges over the next 5 years will maximize the chances that these tools can help sustain the downward trajectory in malaria burden and provide the platform for the next generation of tools (e.g. transgenic mosquitoes) and approaches (e.g. combined vector, drug and vaccine strategies) to deliver on the ultimate goals of elimination and eradication.
Trend box.
-
➢
The last decade has seen a dramatic decline in the burden of malaria, with vector control playing a central role. The aim is now to build on this recent success and progress towards elimination.
-
➢
Current core vector controls tools alone are insufficient to achieve this goal, as they fail to target all adult mosquitoes and emerging insecticide resistance is their effectiveness.
-
➢
By considering the full range of adult mosquito behaviors, a number of supplementary tools are now under development that complement the core tools and create opportunities for tackling resistance and improving overall control.
Glossary
- Anthropophilic
a preference for feeding on humans and resting in and around domestic dwellings
- Behavioral resistance
changes in vector feeding or resting behavior that reduce insecticide exposure
- Community-wide effect
a reduction in transmission risk at community level even though only a certain proportion of the community are protected directly by an intervention. It occurs, for example, when an intervention kills the mosquito and so reduces the vector-human contact for the whole community
- Entomological inoculation rates (EIR)
a measure of human exposure to infectious mosquitoes defined as the number of infectious bites received by a person over a given time period (usually per year)
- Gonotrophic cycle
describes a life cycle of alternate feeding and laying eggs. The duration of the gonotrophic cycle is defined as the number of days that gravid mosquitoes take to lay their eggs after taking a blood meal
- Integrated Vector Management (IVM)
the optimal use of diverse tools, tactics, and resources to reduce transmission of disease by vectors
- Indoor Residual Sprays (IRS)
spraying the walls and other surfaces of a house with a residual insecticide that is designed to kill mosquitoes as they rest after blood feeding
- Long lasting Insecticide-treated Net (LLIN)
a bed net coated or impregnated with insecticide that is designed to remain effective for 3–5 years and 20 washes
- Physiological insecticide-resistance
reduced susceptibility to an insecticide by changes in basic physiology, including target site mutations that reduce neuronal sensitivity, and metabolic mechanisms that enhance detoxification
- Residual transmission
malaria transmission that persists after full operational coverage with effective LLIN and/or IRS interventions has been achieved
- Vector competence
physiological and behavioral characteristics that shape a vector’s capability to transmit a pathogen (i.e. become infected following an infectious blood meal, successfully harbor the parasite as it develops, and pass the parasite on to a susceptible host in a later blood meal)
- Zoophilic
a preference for feeding on non-human hosts (such as cattle) and potentially resting in and around livestock structures such as cattle sheds
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
All authors contributed to the manuscript.
References
- 1.World Health Organization. Global technical strategy for malaria 2016–2030. 2015. [Google Scholar]
- 2.Bhatt S, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–11. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alout H, et al. Interactive cost of Plasmodium infection and insecticide resistance in the malaria vector Anopheles gambiae. Sci Rep. 2016 doi: 10.1038/srep29755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Berg H, et al. Global trends in the use of insecticides to control vector- borne diseases. Environ Health Perspect. 2012;120:577–582. doi: 10.1289/ehp.1104340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mnzava AP, et al. Implementation of the global plan for insecticide resistance management in malaria vectors: progress, challenges and the way forward. Malar J. 2015;14:173. doi: 10.1186/s12936-015-0693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durnez L, Coosemans M. Residual transmission of malaria: an old issue for new approaches. In: Manguin S, editor. Anopheles mosquitoes - New insights into malaria vectors. 2013. pp. 671–704. InTech. [Google Scholar]
- 7.Waite JL, et al. Increasing the potential for malaria elimination by targeting zoophilic vectors. Sci Rep. 2017;7:40551. doi: 10.1038/srep40551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Killeen GF, et al. Going beyond personal protection against mosquito bites to eliminate malaria transmission: population suppression of malaria vectors that exploit both human and animal blood. BMJ Glob Heal. 2017;2:e000198. doi: 10.1136/bmjgh-2016-000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffin JT, et al. Potential for reduction of burden and local elimination of malaria by reducing Plasmodium falciparum malaria transmission: a mathematical modelling study. Lancet Infect Dis. 2016;16:465–472. doi: 10.1016/S1473-3099(15)00423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGraw Ea, O’Neill SL. Beyond insecticides: new thinking on an ancient problem. Nat Rev Microbiol. 2013;11:181–93. doi: 10.1038/nrmicro2968. [DOI] [PubMed] [Google Scholar]
- 11.Benelli G, et al. Biological control of mosquito vectors: Past, present, and future. Insects. 2016;7:52. doi: 10.3390/insects7040052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammond A, et al. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat Biotechnol. 2015;34:78–83. doi: 10.1038/nbt.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gantz VM, et al. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc Natl Acad Sci U S A. 2015;112:E6736–43. doi: 10.1073/pnas.1521077112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckhoff PA, et al. Impact of mosquito gene drive on malaria elimination in a computational model with explicit spatial and temporal dynamics. Proc Natl Acad Sci U S A. 2017;114:E255–E264. doi: 10.1073/pnas.1611064114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beaghton A, et al. Requirements for driving anti-pathogen effector genes into populations of disease vectors by homing. Genetics. 2017;205:1587–1596. doi: 10.1534/genetics.116.197632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cirimotich CM, et al. Low- and high-tech approaches to control Plasmodium parasite transmission by anopheles mosquitoes. J Trop Med. 2011;2011:891342. doi: 10.1155/2011/891342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang G, et al. Anopheles midgut FREP1 mediates Plasmodium invasion. J Biol Chem. 2015;290:16490–16501. doi: 10.1074/jbc.M114.623165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandiford SL, et al. Cytoplasmic cctin is an extracellular insect immune factor which is secreted upon immune challenge and mediates phagocytosis and direct killing of bacteria, and is a Plasmodium antagonist. PLOS Pathog. 2015;11:e1004631. doi: 10.1371/journal.ppat.1004631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bian G, et al. Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science. 2013;340:748–751. doi: 10.1126/science.1236192. [DOI] [PubMed] [Google Scholar]
- 20.Joshi D, et al. Wolbachia strain wAlbB confers both fitness costs and benefit on Anopheles stephensi. Parasit Vectors. 2014;7:336. doi: 10.1186/1756-3305-7-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Briegel H. Fecundity, metabolism, and body size in Anopheles (Diptera: Culicidae), vectors of malaria. J Med Entomol. 1990;27:839–850. doi: 10.1093/jmedent/27.5.839. [DOI] [PubMed] [Google Scholar]
- 22.Kessler S, et al. Sugar-sensitive neurone responses and sugar feeding preferences influence lifespan and biting behaviours of the Afrotropical malaria mosquito, Anopheles gambiae. J Comp Physiol A Neuroethol Sensory, Neural, Behav Physiol. 2015;201:317–329. doi: 10.1007/s00359-015-0978-7. [DOI] [PubMed] [Google Scholar]
- 23.Assogba BS, et al. Studies on the breeding swarms of Anopheles gambiae complex in malaria control perspective. Malar J. 2010;9:O1. [Google Scholar]
- 24.Takken W, Verhulst NO. Host preferences of blood-feeding mosquitoes. Annu Rev Entomol. 2013;58:433–453. doi: 10.1146/annurev-ento-120811-153618. [DOI] [PubMed] [Google Scholar]
- 25.Paaijmans KP, Thomas MB. The influence of mosquito resting behaviour and associated microclimate for malaria risk. Malar J. 2011;10:183. doi: 10.1186/1475-2875-10-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Day JF. Mosquito oviposition behavior and vector control. Insects. 2016;7:65. doi: 10.3390/insects7040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Detinova TS. Age-grouping methods in Diptera of medical importance with special reference to some vectors of malaria. Monogr Ser World Health Organ. 1962;47:13–191. [PubMed] [Google Scholar]
- 28.Paaijmans KP, et al. Understanding the link between malaria risk and climate. Proc Natl Acad Sci U S A. 2009;106:13844–13849. doi: 10.1073/pnas.0903423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Killeen GF, et al. A simplified model for predicting malaria entomologic inoculation rates based on entomologic and parasitologic parameters relevant to control. Am J Trop Med Hyg. 2000;62:535–544. doi: 10.4269/ajtmh.2000.62.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tainchum K, et al. Behavioral responses of Anopheles species (Culicidae: Diptera) with varying surface exposure to pyrethroid-treated netting in an excito-repellency test system. J Vector Ecol. 2016;41:254–264. doi: 10.1111/jvec.12220. [DOI] [PubMed] [Google Scholar]
- 31.Hoi AG, Roitberg BD. Mosquito behaviour and disease control. Evol Med public Heal. 2014;162 doi: 10.1093/emph/eou030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hemingway J, et al. Tools and strategies for malaria control and elimination: what do we need to achieve a grand convergence in malaria? PLOS Biol. 2016;14:e1002380. doi: 10.1371/journal.pbio.1002380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fikrig K, et al. Assessment of synthetic floral-based attractants and sugar baits to capture male and female Aedes aegypti (Diptera: Culicidae) Parasit Vectors. 10:32. doi: 10.1186/s13071-016-1946-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Oppen S, et al. A floral-derived attractant for Aedes aegypti mosquitoes. Entomol Exp Appl. 2015;155:184–192. [Google Scholar]
- 35.Xue RD, et al. Effect of application rate and persistence of boric acid sugar baits applied to plants for control of Aedes Albopictus. J Am Mosq Control Assoc. 2011;27:56–60. doi: 10.2987/10-6069.1. [DOI] [PubMed] [Google Scholar]
- 36.Revay EE, et al. Formulation of attractive toxic sugar bait (ATSB) with safe EPA- exempt substance significantly diminishes the Anopheles sergentii population in a desert oasis. Acta Trop. 2015;150:29–34. doi: 10.1016/j.actatropica.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qualls WA, et al. Evaluation of attractive toxic sugar bait (ATSB) —Barrier for control of vector and nuisance mosquitoes and its effect on non-target organisms in subtropical environments in Florida. Acta Trop. 2014;131:104–110. doi: 10.1016/j.actatropica.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qualls WA, et al. Indoor use of attractive toxic sugar bait (ATSB) to effectively control malaria vectors in Mali, West Africa. Malar J. 2015;14:301. doi: 10.1186/s12936-015-0819-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart ZP, et al. Indoor application of attractive toxic sugar bait (ATSB) in combination with mosquito nets for control of pyrethroid-resistant mosquitoes. PLoS One. 2013;8:e84168. doi: 10.1371/journal.pone.0084168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller GC, et al. Successful field trial of attractive toxic sugar bait (ATSB) plantspraying methods against malaria vectors in the Anopheles gambiae complex in Mali, West Africa. Malar J. 2010;9:210. doi: 10.1186/1475-2875-9-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beier JC, et al. Attractive toxic sugar bait (ATSB) methods decimate populations of Anopheles malaria vectors in arid environments regardless of the local availability of favoured sugar-source blossoms. Malar J. 2012;11:31. doi: 10.1186/1475-2875-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu L, et al. Modelling optimum use of attractive toxic sugar bait stations for effective malaria vector control in Africa. Malar J. 2015;14:492. doi: 10.1186/s12936-015-1012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diabaté A, et al. Targeting male mosquito mating behaviour for malaria control. Parasit Vectors. 2015;8:347. doi: 10.1186/s13071-015-0961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diabate A, et al. Spatial distribution and male mating success of Anopheles gambiae swarms. BMC Evol Biol. 2011;11:184. doi: 10.1186/1471-2148-11-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manoukis NC, et al. Structure and dynamics of male swarms of Anopheles gambiae. J Med Entomol. 2009;46:227–35. doi: 10.1603/033.046.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Assogba BS, et al. Characterization of swarming and mating behaviour between Anopheles coluzzii and Anopheles melas in a sympatry area of Benin. Acta Trop. 2014;132:53–63. doi: 10.1016/j.actatropica.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Manoukis NC, et al. Stereoscopic video analysis of Anopheles gambiae behavior in the field: challenges and opportunities. Acta Trop. 2014;132 doi: 10.1016/j.actatropica.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sawadogo SP, et al. Targeting male mosquito swarms to control malaria vector density. PLoS One. 2017;12:e0173273. doi: 10.1371/journal.pone.0173273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Howell PI, Knols BG. Male mating biology. Malar J. 2009;8:S8. doi: 10.1186/1475-2875-8-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huho B, et al. Consistently high estimates for the proportion of human exposure to malaria vector populations occurring indoors in rural Africa. Int J Epidemiol. 2013;42:235–247. doi: 10.1093/ije/dys214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moiroux N, et al. Human exposure to early morning Anopheles funestus biting behavior and personal protection provided by long-lasting insecticidal nets. PLoS One. 2014;9:e104967. doi: 10.1371/journal.pone.0104967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bayoh M, et al. Persistently high estimates of late night, indoor exposure to malaria vectors despite high coverage of insecticide treated nets. Parasit Vectors. 2014;7:380. doi: 10.1186/1756-3305-7-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Killeen GF, et al. Most outdoor malaria transmission by behaviourally-resistant Anopheles arabiensis is mediated by mosquitoes that have previously been inside houses. Malar J. 2016;15:225. doi: 10.1186/s12936-016-1280-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tusting LS, et al. The evidence for improving housing to reduce malaria: a systematic review and meta-analysis. Malar J. 2015;14:209. doi: 10.1186/s12936-015-0724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tusting LS, et al. Housing improvements and malaria risk in Sub-Saharan Africa: a multi-country analysis of survey data. PLOS Med. 2017;14:e1002234. doi: 10.1371/journal.pmed.1002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kirby MJ, et al. Effect of two different house screening interventions on exposure to malaria vectors and on anaemia in children in The Gambia: a randomised controlled trial. Lancet. 2009;374:998–1009. doi: 10.1016/S0140-6736(09)60871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ogoma SB, et al. Window screening, ceilings and closed eaves as sustainable ways to control malaria in Dar es Salaam, Tanzania. Malar J. 2009;8:221. doi: 10.1186/1475-2875-8-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu JX, et al. Is housing quality associated with malaria incidence among young children and mosquito vector numbers? Evidence from Korogwe, Tanzania. PLoS One. 2014;9:e87358. doi: 10.1371/journal.pone.0087358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lindsay SW, et al. Reducing malaria by mosquito-proofing houses. Trends Parasitol. 2002;18:510–4. doi: 10.1016/s1471-4922(02)02382-6. [DOI] [PubMed] [Google Scholar]
- 60.Njie M, et al. Importance of eaves to house entry by anopheline, but not culicine, mosquitoes. J Med Entomol. 2009;46:505–10. doi: 10.1603/033.046.0314. [DOI] [PubMed] [Google Scholar]
- 61.Spitzen J, et al. Visualization of house-entry behaviour of malaria mosquitoes. Malar J. 2016;15:233. doi: 10.1186/s12936-016-1293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knols BGJ, et al. Eave tubes for malaria control in Africa: an introduction. Malar J. 2016;15:404. doi: 10.1186/s12936-016-1452-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sternberg ED, et al. Eave tubes for malaria control in Africa: initial development and semi-field evaluations in Tanzania. Malar J. 2016;15:447. doi: 10.1186/s12936-016-1499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andriessen R, et al. Electrostatic coating enhances bioavailability of insecticides and breaks pyrethroid resistance in mosquitoes. Proc Natl Acad Sci. 2015;112:12081–12086. doi: 10.1073/pnas.1510801112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Waite JL, et al. Eave tubes for malaria control in Africa: a modelling assessment of potential impact on transmission. Malar J. 2016;15:44. doi: 10.1186/s12936-016-1505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.World Health Organization. Global plan for insecticide resistance management in malaria vectors. 2015. [Google Scholar]
- 67.Odhiambo MTO, et al. Supplementary effect and durability of prototype insecticide-treated eave curtains on indoor resting mosquitoes in Kadibo division, Western Kenya. MWJ. 2016;7:11. doi: 10.5281/zenodo.10818166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Killeen GF, et al. Control of malaria vector mosquitoes by insecticide-treated combinations of window screens and eave baffles. Emerg Infect Dis. 2017;23:782–789. doi: 10.3201/eid2305.160662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Al-Eryani SMA, et al. Entomological aspects and the role of human behaviour in malaria transmission in a highland region of the Republic of Yemen. Malar J. 2016;15:130. doi: 10.1186/s12936-016-1179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thomas S, et al. Resting and feeding preferences of Anopheles stephensi in an urban setting, perennial for malaria. Malar J. 2017;16:111. doi: 10.1186/s12936-017-1764-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.St Laurent B, et al. Cow-baited tents are highly effective in sampling diverse Anopheles malaria vectors in Cambodia. Malar J. 2016;15:440. doi: 10.1186/s12936-016-1488-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Njoroge MM, et al. Exploring the potential of using cattle for malaria vector surveillance and control: a pilot study in western Kenya. Parasit Vectors. 2017;10:18. doi: 10.1186/s13071-016-1957-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maia MF, et al. The effect of deltamethrin-treated net fencing around cattle enclosures on outdoor-biting mosquitoes in Kumasi, Ghana. PLoS One. 2012;7:e45794. doi: 10.1371/journal.pone.0045794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hewitt S, Rowland M. Control of zoophilic malaria vectors by applying pyrethroid insecticides to cattle. Trop Med Int Health. 1999;4:481–6. doi: 10.1046/j.1365-3156.1999.00433.x. [DOI] [PubMed] [Google Scholar]
- 75.Mahande AM, et al. Role of cattle treated with deltamethrine in areas with a high population of Anopheles arabiensis in Moshi, Northern Tanzania. Malar J. 2007;6:109. doi: 10.1186/1475-2875-6-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rowland M, et al. Control of malaria in Pakistan by applying deltamethrin insecticide to cattle: a community-randomised trial. Lancet. 2001;357:1837–1841. doi: 10.1016/S0140-6736(00)04955-2. [DOI] [PubMed] [Google Scholar]
- 77.Massebo F, et al. Zoophagic behaviour of anopheline mosquitoes in southwest Ethiopia: opportunity for malaria vector control. Parasit Vectors. 2015;8:645. doi: 10.1186/s13071-015-1264-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chaccour CJ, et al. Establishment of the ivermectin research for malaria elimination network: updating the research agenda. Malar J. 2015;14:243. doi: 10.1186/s12936-015-0691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Naz S, et al. Efficacy of ivermectin for control of zoophilic malaria vectors in Pakistan. Pakistan J Zool. 2013;45:1585–1591. [Google Scholar]
- 80.Lozano-Fuentes S, et al. Evaluation of a topical formulation of eprinomectin against Anopheles arabiensis when administered to Zebu cattle (Bos indicus) under field conditions. Malar J. 2016;15:324. doi: 10.1186/s12936-016-1361-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Poché RM, et al. Treatment of livestock with systemic insecticides for control of Anopheles arabiensis in western Kenya. Malar J. 2015;14:351. doi: 10.1186/s12936-015-0883-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chaccour C, et al. Pilot study of a slow-release ivermectin formulation for malaria control in a pig model. Antimicrob Agents Chemother. 2017;61:e02104–16. doi: 10.1128/AAC.02104-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Achee NL, et al. Spatial repellents: from discovery and development to evidence- based validation. Malar J. 2012;11:164. doi: 10.1186/1475-2875-11-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hill N, et al. A household randomized, controlled trial of the efficacy of 0.03% transfluthrin coils alone and in combination with long-lasting insecticidal nets on the incidence of Plasmodium falciparum and Plasmodium vivax malaria in Western Yunnan Province, China. Malar J. 2014;13:208. doi: 10.1186/1475-2875-13-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Syafruddin D, et al. Impact of a spatial repellent on malaria incidence in two villages in Sumba, Indonesia. Am J Trop Med Hyg. 2014;91:1079–87. doi: 10.4269/ajtmh.13-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van Eijk AM, et al. The use of mosquito repellents at three sites in India with declining malaria transmission: surveys in the community and clinic. Parasit Vectors. 2016;9:418. doi: 10.1186/s13071-016-1709-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ogoma SB, et al. The mode of action of spatial repellents and their impact on vectorial capacity of Anopheles gambiae sensu stricto. PLoS One. 2014;9:e110433. doi: 10.1371/journal.pone.0110433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thomas MB, et al. Lessons from agriculture for the sustainable management of malaria vectors. PLoS Med. 2012;9:e1001262. doi: 10.1371/journal.pmed.1001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.World malaria report. World Health Organization; 2016. [Google Scholar]
- 90.Churcher TS, et al. The impact of pyrethroid resistance on the efficacy and effectiveness of bednets for malaria control in Africa. Elife. 2016;5:e16090. doi: 10.7554/eLife.16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koffi AA, et al. Efficacy of Olyset® Duo, a permethrin and pyriproxyfen mixture net against wild pyrethroid-resistant Anopheles gambiae s.s. from Côte d’Ivoire: an experimental hut trial. Parasite. 2015;22:28. doi: 10.1051/parasite/2015028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Djènontin A, et al. Insecticidal and sterilizing effect of Olyset Duo ®, a permethrin and pyriproxyfen mixture net against pyrethroid-susceptible and -resistant strains of Anopheles gambiae s.s. : a release-recapture assay in experimental huts. Parasite. 2015;22:27. doi: 10.1051/parasite/2015027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bhatt RM, et al. Effectiveness and durability of Interceptor(R) long-lasting insecticidal nets in a malaria endemic area of central India. Malar J. 2012;11:189. doi: 10.1186/1475-2875-11-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oxborough RM, et al. Long-lasting control of Anopheles arabiensis by a single spray application of micro-encapsulated pirimiphos-methyl (Actellic® 300 CS) Malar J. 2014;13:37. doi: 10.1186/1475-2875-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Macdonald M. New ways of approaching indoor residual spraying for malaria. Glob Heal Sci Pract. 2016;4:511–513. doi: 10.9745/GHSP-D-16-00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kanyangarara M, et al. Reduction in malaria incidence following indoor residual spraying with Actellic 300 CS in a setting with pyrethroid resistance: Mutasa District, Zimbabwe. PLoS One. 2016;11:e0151971. doi: 10.1371/journal.pone.0151971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maia MF, et al. Do topical repellents divert mosquitoes within a community? – Health equity implications of topical repellents as a mosquito bite prevention Tool. PLoS One. 2013;8:e84875. doi: 10.1371/journal.pone.0084875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gryseels C, et al. Factors influencing the use of topical repellents: implications for the effectiveness of malaria elimination strategies. Sci Rep. 2015;5:16847. doi: 10.1038/srep16847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Heng S, et al. Safety of a topical insect repellent (picaridin) during community mass use for malaria control in rural Cambodia. PLoS One. 2017;12:e0172566. doi: 10.1371/journal.pone.0172566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chaccour C, et al. Effect of ivermectin on Anopheles gambiae mosquitoes fed on humans: the potential of oral insecticides in malaria control. J Infect Dis. 2010;202:113–116. doi: 10.1086/653208. [DOI] [PubMed] [Google Scholar]
- 101.Chaccour CJ, et al. Ivermectin to reduce malaria transmission: a research agenda for a promising new tool for elimination. Malar J. 2013;12:153. doi: 10.1186/1475-2875-12-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Omura S, Crump A. Ivermectin and malaria control. Malar J. 2017;16:172. doi: 10.1186/s12936-017-1825-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


