Abstract
Cell cycle progression and differentiation are highly coordinated during the development of multicellular organisms. The mechanisms by which these processes are coordinated and how their coordination contributes to normal development are not fully understood. Here, we determine the developmental fate of a population of precursor cells in the developing Drosophila melanogaster retina that arrest in G2 phase of the cell cycle and investigate whether cell cycle phase-specific arrest influences the fate of these cells. We demonstrate that retinal precursor cells that arrest in G2 during larval development are selected as sensory organ precursors (SOPs) during pupal development and undergo two cell divisions to generate the four-cell interommatidial mechanosensory bristles. While G2 arrest is not required for bristle development, preventing G2 arrest results in incorrect bristle positioning in the adult eye. We conclude that G2-arrested cells provide a positional cue during development to ensure proper spacing of bristles in the eye. Our results suggest that the control of cell cycle progression refines cell fate decisions and that the relationship between these two processes is not necessarily deterministic.
Keywords: cell cycle, differentiation, Drosophila, retina, sensory organ precursor, bristle
Introduction
Proliferation and differentiation are two central processes driving development of multicellular organisms. These processes are highly regulated and fine-tuned through numerous overlapping genetic networks. Furthermore, cell cycle progression and differentiation are highly coordinated. Prior to adopting a final differentiated fate, most cells permanently exit the cell cycle (Ruijtenberg and van den Heuvel, 2016). Historically, terminal differentiation has been defined in part by cell cycle exit (Reiner, 1983), and cell cycle exit is often viewed as a precursor to final cellular differentiation.
There are a number of ways cell cycle regulators and regulators of differentiation interact to coordinate developmental processes, both at the level of transcriptional control and protein activity. Activating cell cycle regulators can suppress differentiation programs to keep proliferating precursor cells in an undifferentiated state. For example, in proliferating myoblasts, Cyclin/CDK complexes phosphorylate and inactivate MyoD and Mef2, two transcription factors that drive formation of myotubes (Tintignac et al., 2000; Di Giorgio et al., 2015). Similarly, Cyclin/CDK-dependent phosphorylation of Neurogenin3 (Ngn3) in proliferating precursor cells antagonizes the ability of Ngn3 to stimulate differentiation of pancreatic endocrine cells (Azzarelli et al., 2017; Krentz et al., 2017). Cell cycle inhibitors also can promote differentiation. The retinoblastoma protein (pRb) regulates the cell cycle by binding and inhibiting E2f transcription factors that drive cell cycle progression. pRb can also bind and activate transcription factors that promote differentiation, such as the osteoblast-specific transcription factor CBFA1 (Thomas et al., 2001).
Recent studies have suggested that although cell cycle exit and differentiation often occur concurrently, cell cycle exit is not necessarily required prior to the completion of cell differentiation, as defined by cell functionality and expression of differentiation markers. Under normal development, differentiated hepatocytes in the mammalian liver undergo rounds of S phase followed by either complete or incomplete mitoses, resulting in a heterogeneous population of diploid and polyploid cells (Anatskaya et al., 1994). During liver regeneration, hepatocytes will re-enter the cell cycle and proliferate or undergo endocycles (Gentric et al., 2012). Experimentally, cell cycle exit can be blocked by genetically manipulating cell cycle regulators. Failure to appropriately inhibit E2F and Cyclin/CDK activity in neurons in the developing Drosophila wing and eye results in continued proliferation past normal stages of cell cycle exit that occurs concurrently with expression of markers of differentiated neurons (Du et al., 1996; Firth and Baker, 2005; Buttitta et al., 2007). Similarly, muscle cells in C. elegans expressing ectopic Cyclin/CDK display markers of mitosis while still contracting as functioning myocytes (Korzelius et al., 2011). These experiments and others suggest that cell cycle progression and differentiation are not necessarily incompatible, and there is likely a degree of flexibility depending on the tissue and cell type.
In addition to proliferation versus cell cycle exit, position in the cell cycle can also affect a cell’s receptiveness to differentiation signals. Recently, elegant experiments using human embryonic stem cells (hESCs) that were isolated according to cell cycle phase demonstrated that cells in G1 adopt a differentiated state more frequently than cells in other phases of the cell cycle (Sela et al., 2012; Pauklin and Vallier, 2013). Interestingly, cells in early G1 readily differentiate into endoderm or mesoderm but not neuroectoderm, while cells in late G1 differentiate into neuroectoderm but not endoderm or mesoderm (Pauklin and Vallier, 2013). These results suggest that cell cycle regulators present in early versus late G1 may bias cells to adopt different lineages. Differences in chromatin accessibility during cell cycle phases may also influence a cell’s responsiveness to developmental signals (Ma et al., 2015). In the Drosophila notum, genetic manipulation of a chromatin regulator modulates the receptiveness of bristle cells to Notch signaling during S phase (Remaud et al., 2008; Ma et al., 2015). Whether cell cycle phase directly influences cell fate and differentiation in vivo or is merely correlated with these processes during development is not clear.
An excellent model for studying relationships between cell cycle regulation and differentiation is the developing Drosophila eye (Baker, 2007; Kumar, 2010). The larval eye imaginal disc, an epithelial sheet of cells that metamorphoses into the adult eye during pupation, undergoes a precise pattern of cell cycle progression and differentiation (Fig. 1A). During the first two larval stages, cells in the primordial eye disc undergo asynchronous cell divisions that increase the pool of precursor cells. During the third and final larval stage, a wave of differentiation sweeps across the disc epithelium from posterior to anterior. This wave is associated with apical constriction of cells resulting in an indentation in the disc called the morphogenetic furrow (MF) (Fig. 1A). Cells just anterior to the MF arrest in G1 phase and remain arrested in G1 within the furrow. A subset of G1-arrested cells subsequently begin to differentiate into five of the eight photoreceptors that make up each ommatidium, the photoreception unit of the compound eye (Kumar, 2012). Immediately posterior to the MF, the remaining undifferentiated cells synchronously re-enter the cell cycle. This cell cycle is referred to as the Second Mitotic Wave (SMW) (Wolff and Ready, 1991), and the resulting synchronous wave of S-phase of the SMW is readily visualized by EdU labeling (Fig. 1B). Following S-phase of the SMW, cells enter G2 and the majority subsequently undergo mitosis and become quiescent in G1 phase (Baker and Yu, 2001). Posterior to the SMW, no additional cell cycles occur within the larval disc (Fig. 1B). Undifferentiated cells will either differentiate into the remaining photoreceptors or retinal accessory cells or will be cleared from the retina by apoptosis during pupation, as there are more undifferentiated precursor cells in the disc than is needed to assemble all ~800 ommatidia (Cagan and Ready, 1989). This precise and stereotyped program of cell cycle progression and differentiation results in the highly organized and latticed appearance of the adult eye. Consequently, defects in regulation of cell cycle progression or differentiation are readily apparent as disruptions to the precisely patterned adult eye.
Fig. 1. Assays used to detect a subset of cells in the eye imaginal disc that arrest in G2 after S phase of the Second Mitotic Wave.
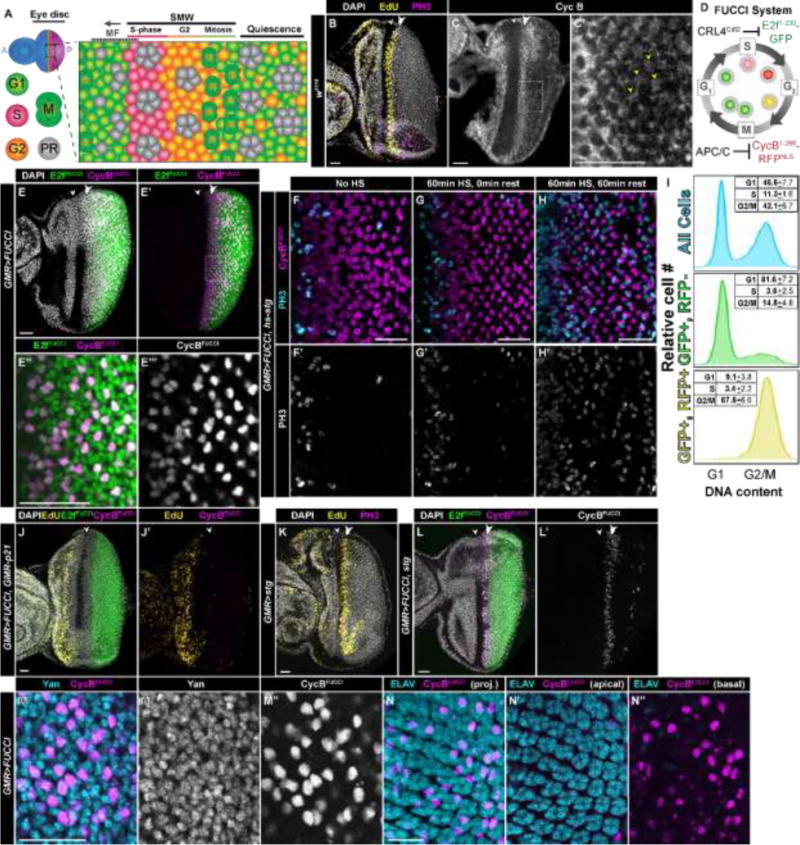
(A) Diagram of the onset of cell differentiation during Drosophila eye development. Cell differentiation during eye development begins during the third larval instar stage and occurs as a wave across the eye disc represented by the morphogenetic furrow (MF), which initiates at the posterior (P) and moves towards the anterior (A). Cells anterior to the furrow proliferate asynchronously (blue in full disc). Cells within the MF arrest in G1 (green cells), and a subset of these differentiate into the first five photoreceptors (PR; gray) while the remaining undifferentiated cells synchronously enter S-phase (red cells) of the second mitotic wave (SMW). The majority of these cells then enter mitosis (M), after which they sequentially differentiate and get recruited into developing ommatidia. Some cells (orange) posterior to the MF remain in G2. Relative area of each region is not to scale. (B–C′) w1118 control larval eye disc stained with DAPI (DNA, gray), EdU (S-phase, yellow), and anti-phospho histone H3 antibodies (PH3, mitosis, magenta). In this and subsequent panels the white arrowhead indicates the MF and the double arrowhead indicates the SMW. (C,C′) w1118 control larval eye disc stained with anti-Cyclin B antibodies (CycB, G2/mitosis, gray; dotted box indicates area of magnification in C′). Post-SMW cells with high levels of CycB are indicated with yellow arrowheads. (D) Diagram of the FUCCI system. GFP-E2f1-230 (E2fFUCCI) is targeted for degradation by CRL4Cdt2 during S phase, and RFP-NLS-CycB1–266 (CycBFUCCI) is degraded by APC/C during mitosis and G1. See (Zielke et al., 2014) for additional details. (E–E‴) GMR-Gal4; UAS-FUCCI (GMR>FUCCI) larval eye discs express both E2fFUCCI (green) and CycBFUCCI (E,E′, magenta; E″, grey) posterior to the MF; DAPI staining in gray (E′), dotted box indicates area of magnification in E″,E‴. (F–H) GMR>FUCCI, hsp70-stg eye discs stained with anti-PH3 antibodies (F–H, cyan; F′–H′, grey) with no heat shock (F), or 60-min 37⁰ C heat shock followed by 0 min (G) or 60 min (H) rest. 60 min after heat shock, cells expressing CycBFUCCI (F–H, magenta) enter mitosis. (I) Flow cytometry histograms of relative cell number versus DNA content for dissociated GMR>FUCCI eye discs. Cell cycle phase peaks (G1 and G2/M) are based on measurements from all cells (top panel, blue). Cells expressing only E2fFUCCI have G1 DNA content (middle panel, green) while cells expressing both E2fFUCCI and CycBFUCCI have G2 DNA content (bottom panel, yellow). n=3 individual flow trials, with 30+ eye discs per trial. (J,J′) GMR>FUCCI, GMR-p21 larval eye disc expressing E2fFUCCI (green) and CycBFUCCI (magenta) and stained with DAPI (J, gray) and EdU (yellow). No SMW is present, and all cells posterior to the MF express E2fFUCCI but do not express CycBRFP. (K) GMR-Gal4, UAS-stg (GMR>stg) eye disc stained with DAPI (gray), EdU (yellow), and anti-PH3 (magenta). (L,L′) GMR>FUCCI, stg eye disc expressing E2fFUCCI (green) and CycBFUCCI (L, magenta; L′, gray). Very few G2 cells (CycBFUCCI+) are present posterior to the MF. (M,M′,M″) GMR>FUCCI eye disc stained with antibodies recognizing Yan (M, cyan; M′, gray), a marker of undifferentiated cells. Yan positive cells expressing CycBFUCCI (M, magenta; M″, gray) are in G2. (N,N′,N″) GMR>FUCCI eye disc stained with antibodies recognizing ELAV (cyan), a marker of differentiated photoreceptors. CycBFUCCI cells (magenta) do not express ELAV. The Z-projection provides reference for the location of G2 cells in relation to ommatidia (N). Apical (N′) and basal (N″) sections are also shown, as photoreceptors and undifferentiated cells reside in different focal plans. All eye discs are from third instar larvae and are oriented with the posterior to the right. Scale bars = 20μM.
While numerous studies have investigated cell cycle arrest and differentiation in the developing Drosophila eye, less attention has been paid specifically to how cell cycle phase might affect differentiation. We were particularly interested in this question because Baker and Yu previously discovered that a small subset of undifferentiated cells posterior to the MF arrest in G2 (Baker and Yu, 2001). The fate of these cells and whether they contribute to a specific cell type in the adult eye is not known. We hypothesized that arrest in G2 might predispose these cells to adopt a certain cell fate or perhaps prevent them from prematurely adopting an inappropriate cell fate. Here, we provide evidence that these G2-arrested cells re-enter the cell cycle during pupal development and become interommatidial mechanosensory bristles, which are evenly spaced throughout the adult eye. We propose a model in which G2 arrest predisposes cells to be selected as a sensory organ precursor cells and thus ensures efficient and precise bristle development.
Materials and Methods
Drosophila culture conditions and stocks
All experiments were carried out at 25⁰ C, except heat shocks, which were carried out at 37⁰ C in a water bath. For experiments with staged pupae, white prepupae were marked (counted as 0hr APF) and aged for the appropriate number of hours. Due to the length of the white prepupal stage (Bainbridge and Bownes, 1981), pupal ages listed may be +20 minutes. Stocks used are as follows (Bloomington Stock numbers are listed where applicable): w1118, GMR-Gal4 [w*;P{GAL4-ninaE.GMR}12] #1104, UAS-E2fFUCCI/UAS-CycBFUCCI [w1118;P{UASp-GFP.E2f1.1-230}64, P{UASp-mRFP1.NLS.CycB.1-266}5] #55111, GMR-p21 [y1 w1118; P{GMR-p21.Ex}3] #8414, UAS-stg [w1118; P{UAS-stg.N}4] #4778, CycB-GFP [y1 w*; P{PTT-GC}CycBCC01846] #51568, hs-stg, (Edgar and O’Farrell, 1990), UAS-p35 [w*; P{UAS-p35.H}BH2] #5073, hs-Flp, FRT19A Ubi-mRFP.nls [P{Ubi-mRFP.nls}1, w*, P{hsFLP}12 P{neoFRT}19A] #31418, FRT19A His2Av-GFP [y1 w* P{His2AvT:Avic\GFP-S65T}1 P{hsFLP}12 P{neoFRT}19A/FM7a] #32045, UAS-p21 (I. Hariharan). Genotypes listed in figures as GMR-p21, GMR>p21, or GMR>stg (except Fig. 1K) also contain UAS-FUCCI. Transgenic genotypes described in all experiments are heterozygous, with the exception of GMR>FUCCI alone (as in Fig. 1E) and CycB-GFP alone (as in Fig. S1A).
Immunostaining
Immunostaining of larval and pupal retinas was performed using standard protocols (Klein, 2008). EdU staining was performed as previously described (Meserve and Duronio, 2015). Primary antibodies: 1/1000 rabbit α-PH3 (Millipore 06-570), 1/50 mouse α-CycB (Developmental Studies Hybridoma Bank (DSHB) F2F4), 1/1000 mouse α-GFP (Abcam ab1218), 1/1000 rabbit α-RFP (Clontech 632496), 1/500 mouse α-Yan (DSHB 8B12H9), 1/100 rat α-ELAV (DSHB 9F8A9), 1/100 mouse α-Cut (DSHB 2B10), 1/200 guinea pig α-Sens (H. Bellen), 1/50 mouse α-Ac (DSHB anti-achaete), 1/1000 rabbit α-Dlg (DSHB 4F3), 1/100 mouse α-Loz (DSHB anti-lozenge). Secondary antibodies (all 1/1000): Oregon Green 488-conjugated goat α-mouse (Invitrogen O6380), Cy3-conjugated goat α-mouse (Jackson ImmunoResearch 115-165-003), Cy5-conjugated goat α-mouse (Jackson ImmunoResearch 115-175-146), Alexa 488-conjugated goat anti-rabbit (Jackson ImmunoResearch 111-545-144), Rhodamine Red-conjugated goat α-rabbit (ThermoFisher Scientific R6394), Cy5-conjugated goat α-rabbit (Abcam ab6564), Cy2-conjugated goat α-rat (Jackson ImmunoResearch 112-225-143), Cy3-conjugated goat α-rat (Jackson ImmunoResearch 112-165-143), Cy5-conjugated goat anti-rat (Jackson ImmunoResearch 712-175-153), Cy5-conjugated donkey α-guinea pig (Jackson ImmunoResearch 706-175-148).
Flow cytometry
Eye discs were prepared for flow analysis as previously described (de la Cruz and Edgar, 2008). Briefly, eye discs from 15–20 larvae were dissected in Grace’s media, rinsed with 1× PBS, and dissociated in a trypsin/Hoescht solution for ~3 hrs. GFP expression and DNA content (Hoescht) were measured using a Becton Dickinson LSR II flow cytometer. Histograms were made using FlowJo software with conservative gating for fluorescent transgenes. Cell cycle phase percentages were calculated by manually gating G1, S, and G2/M based on histograms of all cells, then applying these gates to fluorescent sub-populations.
Results
Assays for visualizing and manipulating eye disc cells that undergo S-phase of the SMW and remain arrested in G2 throughout larval development
To visualize G2-arrested cells posterior to the MF in the developing eye disc, we used immunofluorescence to detect Cyclin B (CycB) (Fig. 1C,C’) and combined FACS analysis with previously developed Fluorescent Ubiquitylation Cell Cycle Indicators (FUCCI) (Sakaue-Sawano et al., 2008), to identify cells in different phases of the cell cycle. The FUCCI system recently developed for use in Drosophila tissues including the eye disc (Zielke et al., 2014) is comprised of two parts: 1) a transgene expressing a functionally inert fragment of CycB fused to RFP that is targeted for destruction during mitosis and G1 by the Anaphase-Promoting Complex (APC/C); and 2) a transgene expressing a functionally inert fragment of E2f1 fused to GFP that is targeted for destruction during S phase by CRL4Cdt2. The co-expression of these two fluorescent indicators (E2fFUCCI and CycBFUCCI) allows cytological visualization of cell cycle position (Fig. 1D). Cells expressing both fluorescent proteins are presumed to be in G2. When we express UAS-FUCCI transgenes under control of GMR-Gal4 (GMR>FUCCI), which drives expression posterior to the MF in the eye disc (Hay et al., 1994), we observe E2fFUCCI and CycBFUCCI double-positive cells posterior of the MF (Zielke et al., 2014). A number of these cells persist to the posterior edge of the disc and do not undergo mitosis during larval development (Fig. 1E–E‴). These double positive cells enter mitosis upon expression of hs-stg (Fig. F–H), as previously demonstrated for CycB-expressing cells (Baker and Yu, 2001). Flow analysis indicates that cells expressing both CycBFUCCI and E2fFUCCI using GMR-Gal4 have 4C DNA content, as expected of cells in G2 (Fig. 1I). We also analyzed a line containing a GFP protein trap insertion into the endogenous CycB locus (Buszczak et al., 2007). CycB-GFP expression in eye discs from this line mirrors the endogenous CycB expression pattern (compare Fig. 1C to Fig. S1A) that has been reported previously (Thomas et al., 1994; Thomas et al., 1997; Baker and Yu, 2001). CycB-GFP expressing cells have 4C DNA content based on flow analysis (Fig. S1B) and enter mitosis following hs-stg expression (Fig. S1C–E). These data support an abundance of approximately one G2 cell per ommatidium, based on calculations of FUCCI double positive cells and ELAV staining (Fig. 1N), similar to G2 cell numbers described previously by Baker and Yu (2001).
Genetic manipulation of cell cycle regulators can eliminate the population of G2 arrested cells posterior to the MF, either by preventing S phase entry in the SMW or by preventing G2 arrest posterior to the MF. GMR-driven expression of the human CDK inhibitor, p21, completely ablates S phase of the SMW while not affecting S phases anterior to the MF (Fig. 1J,J’) (de Nooij and Hariharan, 1995). Accordingly, no E2fFUCCI/CycBFUCCI double-positive G2 cells are observed posterior to the MF in GMR>FUCCI, GMR-p21 eye discs (Fig. 1J,J’). Ectopic UAS-stg expression using GMR-Gal4 bypasses the G2 arrest, and GMR>stg eye discs have very few G2-arrested cells posterior to the MF (Fig. 1K,L,L’), similar to the result with heat shock expressed stg (Baker and Yu, 2001). Finally, the undifferentiated G2-arrested cells can be distinguished from photoreceptors and other differentiated retinal cell types that arrest in G1 (Buttitta et al., 2007) by staining GMR>FUCCI discs with a marker for undifferentiated cells (Yan) (Rohrbaugh et al., 2002) and a marker for photoreceptors (ELAV) (Robinow and White, 1991). All the undifferentiated G2-arrested cells express Yan (Fig. 1M–M″) but not ELAV (Fig. 1N–N″). We used these assays and genetic tools to determine the fate of the G2 arrested cells during pupal development.
G2 cells express markers of SOPs and divide during pupation to produce interommatidial mechanosensory bristles
We hypothesized that these G2-arrested eye precursor cells adopt a specific fate(s). We therefore performed a series of experiments to follow these G2-arrested cells through pupal development and determine whether they contribute to the adult eye. During the first ~12 hours after puparium formation (APF), the MF and SMW continue traversing the disc. When the MF reaches the anterior of the disc and the SMW concludes, a subset of undifferentiated cells across the disc epithelium re-enter the cell cycle and divide (Cagan and Ready, 1989). These cell divisions begin centrally in the disc and radiate outwards, continuing for the next 12 hours of development. Cagan and Ready (1989) previously showed that all proliferating cells which incorporate BrdU injected during pupal development become part of the interommatidial mechanosensory bristles, which are positioned at three vertices of each ommatidium and cover the adult eye (see Fig. 4A,B). These bristles are comprised of four cells: a neuron, a glial cell, a socket cell, and a shaft cell. Each bristle group is derived from a single precursor cell called a sensory organ precursor (SOP) (Fig. 2A). By 24 hours APF, the bristle group divisions have concluded, and the vast majority of retinal cells arrest in G1 based on flow cytometry (Buttitta et al., 2007) and expression of FUCCI transgenes (Fig. S2A–F’). The absence of G2 cells is not a result of apoptosis, a common phenomenon during pupal retinal development (Cagan and Ready, 1989), as retinas expressing the p35 caspase inhibitor also contain very few cells expressing CycBFUCCI at 24hrs APF (Fig. S2G,G’; see Fig. S2H for retina at 48hrs APF where p35 expression results in excess numbers of cells that would normally be cleared by apoptosis).
Fig. 4. Stg expression disrupts but does not block bristle development.
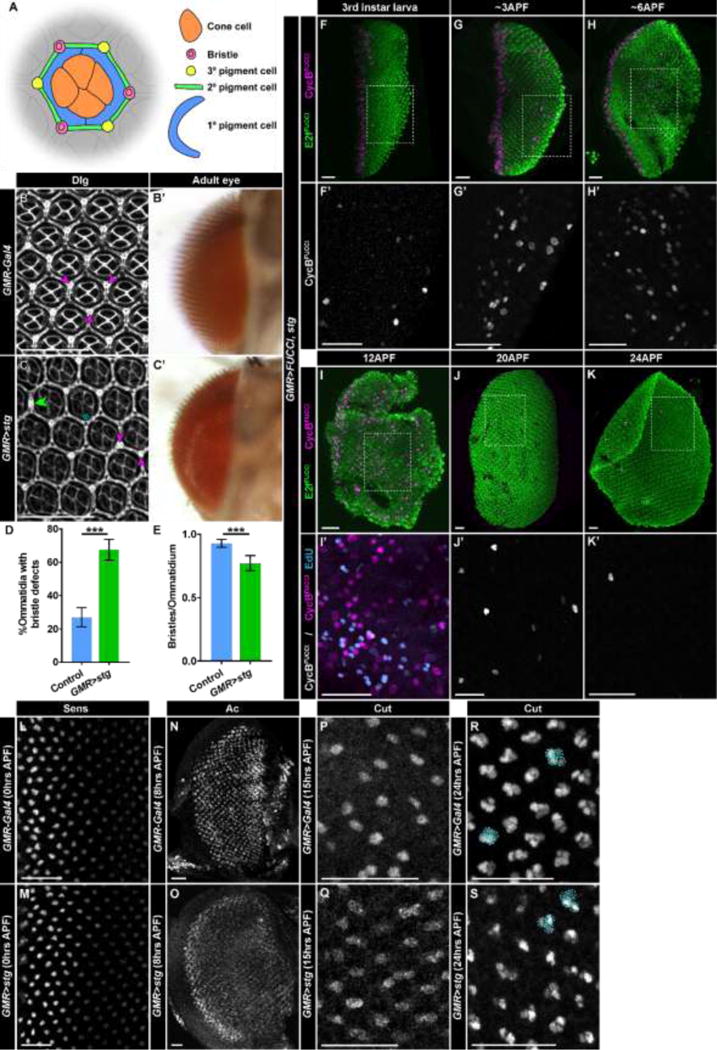
(A) Diagram of cell types in an ommatidium visible in apical sections of the retina at 48hrs APF. The morphological organization of the ommatidium allows identification of each cell type using membrane staining. (B,B′) Retinas from control (GMR-Gal4) flies. (B) Retina at 48hrs APF stained with antibodies recognizing Dlg, which marks cells membranes. Bristles (purple arrows) are normally located at alternating vertices of the hexagonal ommatidia, generating a regular pattern in the adult eye (B′). (C,C′) Retinas from GMR>stg flies. (C) In a retina stained with anti-Dlg at 48hrs APF, bristles are misplaced. Examples include bristles located at neighboring vertices (purple arrows), absent bristles (blue asterisk), and bristles located in between two vertices (green double arrow). Bristles are visible in adult eyes (C′). (D) Quantification of ommatidia per retina with bristle defects (less than or more than three bristles; bristles at neighboring vertices; bristles in between two vertices). Control is GMR-Gal4/CyO. p<0.0001; n>5 retinas, with >180 ommatidia counted/retina. (E) Quantification of average number of bristles per ommatidia for control (GMR-Gal4/CyO) and GMR>stg. p<0.001 n>5 retinas, with >180 ommatidia counted/retina. (F–K′) GMR>FUCCI, stg retinas expressing E2fFUCCI (green) and CycBFUCCI (F–K,I′, magenta; F′–G′,J′,K′ grey) analyzed at the indicated times after pupal formation (APF). (I′) Retina at 12APF stained with EdU (blue). Dotted boxes indicate areas of magnification in E′–J′. (L,M) Retinas at 0hrs APF stained with anti-Sens antibodies in (L) control and (M) GMR>stg. (N,O) Retinas at 8hrs APF stained with anti-Achaete/Ac antibodies in (N) control and (O) GMR>stg. (P,Q) Retinas at 15hrs APF stained with anti-Cut antibodies in (P) control and (Q) GMR>stg. (R,S) Retinas at 24hrs APF stained with anti-Sens antibodies in (R) control and (S) GMR>stg. Four cell Cut-expressing groups (circled) are present in both genotypes; groups that appear to have less than four cells have additional cells outside the plane. Scale bars = 20μM.
Fig. 2. Cells arrested in G2 in larval eye discs divide during pupation.
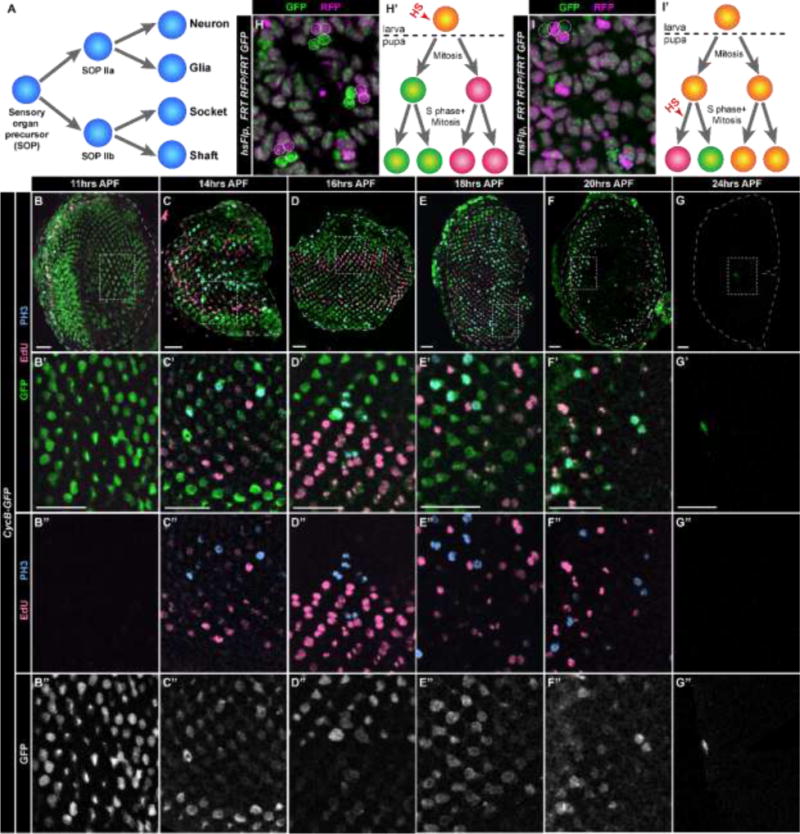
(A) Diagram of SOP divisions and bristle group lineage in the pupal retina. (B–G″) CycB-GFP retinas dissected at various times after pupal formation (APF) and stained with EdU (pink, S-phase) and anti-PH3 (blue, mitosis). Cells in G2/M express GFP (B–G′, green; B″–G″, grey). (H,H′) hs-Flp, FRT19A Ubi-mRFP.nls/FRT19A His2Av-GFP retina at 24hrs APF in which mitotic recombination was induced at the 3rd instar larval stage to produce GFP expressing (green) and RFP expressing (magenta) clones in the posterior. Dotted circles indicate clones with two cells expressing GFP and two cells expressing RFP. Such four cell-clones indicate mitotic recombination in a precursor cell that subsequently underwent two divisions (H′). (I,I′) Clones as in panel H resulting from mitotic recombination induced at 19hrs APF and analyzed at 29hrs APF. Two cell-clones (dashed circles) with one cell expressing GFP (green) and one cell expressing RFP (magenta) result from mitotic recombination in a precursor cell that subsequently underwent one division (I′). Four cell-clones as in panel H are also apparent (dotted circles). Scale bars = 20μM.
Previous models for mechanosensory bristle development have presumed the SOP is arrested in G1 and undergoes two cycles of S phase and cell division to provide the four cells for each bristle group (Cagan and Ready, 1989). Instead, we hypothesized that retinal SOPs are arrested in G2 and first enter mitosis, followed by one round of S phase and mitosis to make the four cell bristle groups. Therefore, we predicted these G2-arrested cells undergo mitosis during the first 24 hours of pupal development when SOP divisions occurs. By 12hrs APF, we observed S phase and mitosis throughout the disc, beginning interiorly and radiating outward over the next 12 hours (Fig. 2B–G″). By 24hrs APF, the majority of divisions have ceased (Fig. 2G–G″). Cells in G2, marked by CycB-GFP (Fig. 2B–G″) or CycBFUCCI expression (Fig. S2A–F’), also progressively disappear between 12 and 24hrs APF. To determine whether the G2-arrested cells themselves undergo mitosis and S-phase, we used the Flp/FRT system (Xu and Rubin, 1993) to generate cell clones by mitotic recombination, which occurs between homologous chromosomes in G2 after DNA replication. When the FLP recombinase is induced by heat shock in third instar larval eye discs, recombination within the posterior of the disc will only occur in cells arrested in G2. The products of recombination will only be observed if these G2-arrested cells divide and segregate genetically marked homologous chromosomes (in this experiment, His2Av-GFP and Ubi-mRFP.nls) into their daughter cells. When larvae were heat shocked and pupal retinas were dissected at 24hrs APF, we observed four-cell clones in the posterior of the retina in which two cells express GFP and two cells express RFP (Fig. 2H). These data indicate that cells in G2 in larval discs divide twice during pupation (Fig. 2H′). When we heat shocked pupae at 19hrs APF and dissected retinas 10 hours later, we observed four cell clones as well as two cell clones containing one cell expressing GFP and one cell expressing RFP (Fig. 2I). This result is consistent with cell division of the SOPs occurring asynchronously (Cagan and Ready, 1989), such that at the time of heat shock, mitotic recombination was induced in an SOP daughter that divided once, generating the two-cell clone (Fig. 2I′). These data indicate that G2-arrested cells are not eliminated from the developing retina and undergo two rounds of mitosis during the first 24 hours of pupal development.
Based on their morphological location and composition, we hypothesized that these clones were derived from SOP cells and composed the bristle groups. To test this hypothesis, we induced clones in 3rd instar larvae and subsequently stained 24hrs APF retinas with antibodies recognizing Cut, which is expressed in all cells of the bristle group at this stage (Frankfort et al., 2004). Of the 221 clones analyzed that were located in the posterior of the retina, 100% contained four cells total and all expressed Cut (Fig. 3A–A″). In contrast, clones in the anterior marked all cell lineages as they arose in asynchronously proliferating precursor cells anterior to the MF that have the potential to contribute to every retinal cell type (Fig. S3A–C). Although 100% of posteriorly located clones were Cut+, only 27% (221/809) of bristle groups were present in marked clones. This is likely a result of mitotic recombination not being induced in all G2-arrested cells because the FLP recombinase is not 100% efficient (Golic, 1991). This result could also indicate that some bristle groups are derived from cells in G1. We therefore analyzed younger pupal retinas to determine if cells in G2 express markers of SOPs. By 6hrs APF, SOPs in wild type retinas express Sens (Frankfort et al., 2004). We observed in CycB-GFP line that ~80% of cells in G2 express Sens (Fig. 3B–C). The ~20% of G2-cells that do not express Sens are always in contact with a Sens+ G2 cells (Fig. 3B) and likely undergo developmental apoptosis (see Discussion). An equal population of Sens-expressing cells are in G1 and express ELAV (Fig. 3C). These are the R8 photoreceptors, which have been previously shown to express Sens (Frankfort et al., 2004). There are also a small number of Sens-expressing G1 cells that don’t express ELAV (Fig. 3C). As Sens expression is typically weak in these cells, they were likely part of the proneural cluster but not selected as SOPs, as described previously (Frankfort et al., 2004). These data indicate that G2-arrested cells are selected as SOPs and divide twice to become the bristle group.
Fig. 3. G2 arrested cells express SOP markers and divide to become the bristle groups.
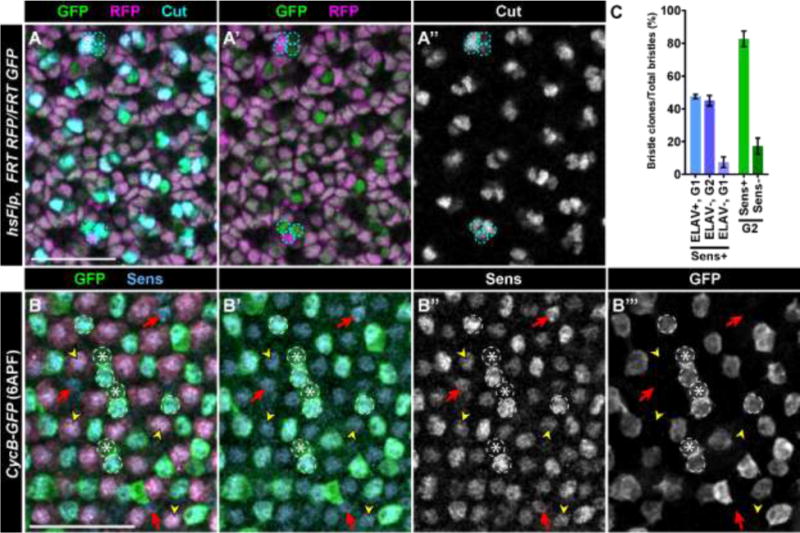
(A–A″) hs-Flp, FRT19A Ubi-mRFP.nls/FRT19A His2Av-GFP retina at 24 hrs APF stained with antibodies recognizing Cut (cyan) in which mitotic recombination was induced at the 3rd instar larval stage to produce GFP expressing (green) and RFP expressing (magenta) clones in the posterior. Four cell clones (dashed circles) are Cut+. Some of the clones, including the three cells marked in the lower section of the panel, appear to have three cells because the fourth cell is out of the focal plane. (B–B″) CycB-GFP retinas at 8hrs APF stained with antibodies recognizing Senseless/Sens (B, blue; B″, grey); most cells expressing GFP (B, green; B′, grey) also express Sens (arrowheads; quantified in C). Scale bars = 20μM.
Disruption of G2 arrest disrupts bristle development
Our observations thus far raise two new questions regarding development of interommatidial mechanosensory bristles: 1) is G2 arrest of SOPs necessary for bristle formation? and 2) are the cell divisions of the SOP and its daughters necessary for bristle formation? Although asymmetric divisions are important for many neuronal lineages (Sousa-Nunes and Somers, 2013; Paridaen and Huttner, 2014) and have been demonstrated to be important for bristle development in other tissues as well (Bellaïche et al., 2001), the bristle cells are the only cells in the retina that are clonally derived. All other cells in an ommatidium (for example, the four cone cells and eight photoreceptors) are derived independently (Ready et al., 1976). Therefore, it seemed possible that bristles could develop in the absence of SOP divisions by recruitment of additional undifferentiated cells. To test whether cell division in the pupal retina is required for bristle development, we blocked S phase entry by expressing p21. In GMR-p21 flies in which the SMW and bristle divisions are ablated, retinas are missing bristles at 48hrs APF (Fig. S4A; compare to control Fig. 4A,B) and adult eyes display only 13% of the normal number of bristles (Fig. S4A’) (de Nooij and Hariharan, 1995). The striking reduction of bristles in this genotype has previously been attributed to a reduction in precursor cell number resulting from loss of the SMW as other non-bristle cell types are also missing (de Nooij and Hariharan, 1995). Indeed, GMR-p21 retinas are disorganized and appear to be missing several cell types (Fig. S4A). However, we hypothesized that bristle defects observed in GMR-p21 retinas are due to disruption of SOP divisions and not merely a consequence of reduction of precursor cell number. To test this prediction, we expressed UAS-p21 under control of GMR-Gal4 (GMR>p21) rather than expressing p21 directly from the GMR promoter. GMR>p21 eye discs retain the SMW, likely due to delayed accumulation of p21 in this genotype (Fig. S4C,C’). Therefore, GMR>p21 is predicted to disrupt divisions of the SOP lineage during pupation without reducing precursor cell number (Fig. S4D,D’). Retinas from GMR>p21 pupae at 48hrs APF appear relatively normal, but the majority of bristle groups are missing and appear, based on morphology, to be replaced in the ommatidia by tertiary pigment cells (Fig. S4B). Consequently, adults lack most interommatidial bristles (Fig. S4B’). We conclude that SOP divisions during pupation are required for the full complement of bristles in the adult eye.
We next investigated whether G2 arrest preceding SOP selection is required for bristle development. We explored this question by expressing UAS-stg under control of GMR-Gal4 (GMR>stg), which precociously drives G2-arrested cells into mitosis following the SMW. Although GMR>FUCCI, stg larvae lack a G2-arrested cell population (Fig. 1I,I’), adults of this genotype have qualitatively normal interommatidial bristles compared to control flies (Fig. 4B’,C’). However, we observe bristle placement defects in GMR>stg retinas at 48hr APF (Fig. 4C,D). Normally, bristle groups appear at alternating vertices of the ommatidia, with tertiary pigment cells occupying the other vertices (Fig. 4A,B). In GMR>stg retinas, we often observe bristle groups at neighboring vertices, missing from an ommatidium, or occurring between two vertices (Fig. 4C). Overall, the numbers of bristles in GMR>stg retinas are significantly, though not drastically, reduced (Fig. 4E). These results suggest that while G2 arrest is not essential for bristle development, cells arrested in G2 may provide positional information to ensure proper bristle patterning and quantity in the retina.
We predicted that division of the SOP cell and its daughters still occur in GMR>stg retinas prior to bristle development. To monitor this process in GMR>stg retinas, we assessed cell cycle progression and the appearance of SOP markers at various time points during the first 24hrs of pupal development (Fig. 4F–K’). Although almost all cells post-SMW arrest in G1 in GMR>FUCCI, stg larval eye discs, a subset of these cells re-enter the cell cycle during early pupal development. By ~3hrs APF, a small number of CycBFUCCI+ cells appear in the posterior region of the eye disc (Fig. 4G,G’). CycBFUCCI+ cells increase in number as pupal development continues, and by 12hrs APF, widespread mitoses and S phases are observed throughout the retina (Fig. 4I,I’; compare to Fig. 2D). These data suggest that cells can re-enter the cell cycle during pupation in the absence of a preceding G2 arrest, though this cell cycle re-entry in GMR>stg retinas occurs earlier than in wild type. Although cell cycle re-entry occurs prematurely, SOP selection does not appear to occur precociously; at 0hrs APF in GMR>stg retinas, no Sens-expressing proneural clusters are observed (Fig. 4L,M) and no cells posterior to the furrow express the proneural gene Achaete (Ac) (data not shown). By 8hrs APF, the proneural gene Ac is observed throughout the retina (Fig. 4O), although expression appears somewhat lower than in control (Fig. 4M). Cells expressing Cut appear at 15hrs APF (Fig. 4P,Q) and 24hrs APF (Fig. 4R,S). Therefore, G2 arrest preceding SOP selection is not absolutely essential for bristle development but contributes to the normal pattern of bristles in the eye.
Discussion
The complex interactions between cell cycle control and differentiation programs are still not fully understood, particularly when considering in vivo systems. Here, we report that G2-phase cell cycle arrest contributes to the development of interommatidial bristles of the compound Drosophila eye.
Coordination of cell cycle progression and differentiation in the Drosophila eye
The Drosophila retina has long been used as a model to study both differentiation and cell cycle progression (Wolff and Ready, 1991; de Nooij and Hariharan, 1995; Baker, 2007). Decades of research have elucidated the many signaling pathways that underlie development of the precisely patterned retina. These pathways often direct both cell cycle progression and differentiation (Kumar, 2010), resulting in these two processes being intimately linked. This relationship is apparent during MF progression as signaling promotes cell cycle exit and specification of the first photoreceptor, R8, which in turn directs specification of the other photoreceptors in the five-cell precluster (Roignant and Treisman, 2009). All cells in the eye disc that do not differentiate in the MF subsequently enter S phase of the SMW (Wolff and Ready, 1991). These cells will contribute to the remaining photoreceptors as well as the accessory cells, i.e. cone, pigment, and bristle cells. In the larva and early pupa, cell specification of cone cells and primary pigment cells is driven largely by short range signaling, including EGFR and Notch, from differentiated cells (Treisman, 2013). The remaining undifferentiated cells will either be selected as SOPs or will become secondary or tertiary pigment cells.
Pigment cell fate is often considered a default differentiation path in the pupal retina, as inhibiting apoptosis results in excess numbers of secondary and tertiary pigment cells while other cell types appear in relatively normal numbers (Hay et al., 1994; Kumar, 2012). Integration of pigment cells into ommatidia is driven by significant cell movement and contacts with other retinal cells (Larson et al., 2008), which is required to give the retina its characteristic lattice appearance. Bristle cells, on the other hand, do not make stereotyped contacts with differentiated cells during their early development (Cagan and Ready, 1989). Furthermore, the bristle precursor SOPs are the only retinal cells outside the SMW to divide during pupation, and both SOP divisions and differentiation occur radially in the retina, unlike the posterior to anterior pattern observed for all other retinal cell types (Cagan and Ready, 1989). What then is the distinction between undifferentiated cells that will be selected as SOPs and undergo this unique development and undifferentiated cells that differentiate into other accessory cell types, such as the tertiary pigment cells that occupy a seemingly equivalent position in the ommatidia? The data presented in this paper and discussed below suggest that undifferentiated cells in the pupae are divided into G2-arrested cells, which are selected as SOPs, and G1-arrested cells, which differentiate into pigment cells.
G2 arrest refines SOP selection
Although G1 arrest often precedes terminal differentiation during development, arrest in G2 phase allows cells to “pause” without committing to a specific lineage. In some instances, this “pause” preceding mitosis is required for normal cell movement during morphogenesis. For example, cells in the Drosophila embryo temporarily arrest in G2 during mesoderm invagination, and precocious entry into mitosis disrupts their movements (Edgar and O’Farrell, 1989; Grosshans and Wieschaus, 2000; Mata et al., 2000; Seher and Leptin, 2000). Similarly, horizontal cell progenitors arrest in G2 as they migrate through the developing chick retina (Boije et al., 2009). G2 arrest may also synchronize cell division with developmental signals to properly coordinate cell cycle exit and differentiation. Our data demonstrate that during bristle development in the Drosophila retina, a subset of cells from the SMW arrest in G2 and become selected as SOPs during pupation. Although precocious entry of G2-arrested cells into mitosis does not eliminate bristles from the eye, it does disrupt bristle organization. This disruption in patterning may be a result of SOPs not being properly selected within the field of undifferentiated cells in the pupal retina. While tertiary pigment cells, which occupy alternating vertices with bristles in the ommatidium, are properly positioned by cell-cell contacts, bristles do not appear to make these same cell-cell contacts (Cagan and Ready, 1989). Consequently, it is possible that the mechanism responsible for the pattern of G2-arrested cells during larval development is important for the final positions of bristles in the mature retina. If SOP divisions conclude earlier than in wild type, as in GMR>stg retinas, precocious bristle development and integration into the ommatidia may also affect positioning. Our results are similar to previous results in the developing Drosophila wing, where a subset of cells in the larval wing disc arrest in G2 and are subsequently selected as SOPs, which undergo divisions during pupal development and become bristles of the adult wing (Phillips and Whittle, 1993; Johnston and Edgar, 1998). Ectopic expression of Stg in the developing wing induces G2-arrested SOPs to prematurely enter mitosis, resulting in adult flies with bristles that are reduced in number, disorganized, and shorter than in wildtype (Nègre et al., 2003). Similar phenotypes occur in bristles of the thorax when progenitors precociously undergo mitosis (Ayeni et al., 2016). These results suggest that arrest in G2 is not absolutely required for bristle development but likely helps refine the differentiation program.
What regulates G2 arrest during development? A major regulator of the G2/M transition in Drosophila is Stg. The stg locus has a very large and complex collection of cis-regulatory modules that are acted upon by many transcription factors (Lehman et al., 1999; Lopes and Casares, 2015). The Stg protein is also relatively unstable (Edgar and Datar, 1996; Bernardi et al., 2000). During Drosophila development, transcriptional regulation of stg typically determines whether cells arrest in G2 and when they subsequently enter mitosis. G2 arrest in presumptive SOPs of the wing results from Wg-dependent expression of the transcription factors Achaete and Scute, which downregulate stg (Johnston and Edgar, 1998). In the eye disc, Ac is not expressed at the time in which cells arrest in G2 ((Garcia-Alonso et al., 1995) and data not shown), and Wg signaling inhibits bristle development (Cadigan et al., 2002). What then regulates stg expression in the SMW? Baker and Yu (2001) demonstrated that EGF receptor signaling is required for G2➔M progression following the SMW. EGF signaling depends on differentiation of the photoreceptor preclusters, which secrete the EGF ligand Spitz. Cells that receive Spitz upregulate Stg, allowing entry into mitosis. As Spitz is a short-range signal, the authors hypothesized that cells not directly in contact with preclusters do not receive a signal to transcribe stg and therefore remain arrested in G2. This hypothesis is supported by the observation that roughly 1–2 cells per ommatidium do not contact the precluster, which is similar to the number of cells arrested in G2 (Baker and Yu, 2001). This spatially constrained mechanism may ensure the proper number of cells at the appropriate positions arrest in G2.
Our data support this hypothesis as inhibition of EGF signaling results in increased numbers of G2 cells in GMR>FUCCI eye discs and G2 cells in the posterior of eye discs lack stg mRNA (data not shown). While we predict excess cells in G2 in Egfr mutant retinas would result in excess bristles, EGFR signaling controls other aspects of eye development and adult eyes are generally disrupted in these mutant genotypes (Baker and Rubin, 1989), even with p35 expression (not shown), precluding bristle analysis. Other mechanisms likely also contribute to G2 arrest. For example, mutations in roughex, a Cyclin/CDK inhibitor, result in inappropriate mitoses within the posterior eye disc, including in photoreceptors (Ruggiero et al., 2012). The Cyclin/CDK inhibitor Dacapo is also required for proper cell cycle arrest following the SMW; dacapo mutants in a GMR>p35 background have drastically increased numbers of interommatidial bristles (Sukhanova and Du, 2008). These processes likely coordinate an arrest of the majority of cells in G1 following the SMW, while allowing a subset to arrest in G2 and subsequently proliferate during pupation.
How does G2 arrest inform the developmental program of sensory organs? We hypothesize that G2 arrest may provide a cell an advantage in being selected as an SOP. A recent model proposed by Troost et al (2015) suggests SOP selection in the developing notum depends on differential expression of Extramacrochaetae (Emc) throughout the tissue. Emc inhibits Ac and Sc, such that cells with lower levels of Emc are more likely to be selected as SOPs. Notch activity further refines SOP selection. However, Emc levels and Notch signaling alone cannot explain how an SOP is selected, and the authors suggest an unknown, “pre-selecting mechanism” drives SOP selection. Our results suggest that G2 arrest may drive this pre-selection, ensuring properly selected SOPs, as abolishing G2 arrest results in improperly selected SOPs, which we infer from misplaced bristles. G2 arrest may affect this selection directly through interaction of cell cycle machinery with proneural gene pathways. Alternatively, the mode of signaling from preclusters may promote development into non-SOP cell types such that cells not receiving short range signals like the EGFR ligand Spitz, which drives cells through mitosis (Baker and Yu, 2001), will both arrest in G2 and be predisposed to become SOPs. In this model, Emc levels and Notch signaling may be sufficient for SOP selection in a field of cells in which no cells are arrested in G2 (as in the GMR>stg retinas), although final bristle placement will not be precise. Further research is required to investigate this model and what factors may predispose G2-arrested cells to adopt an SOP fate.
G2 arrest likely also ensures the proper number of cell divisions of the SOP lineage. In both the thorax (Ayeni et al., 2016) and the eye (Fig. 4G), driving G2-arrested cells precociously through mitosis results in premature pupal divisions. G2 arrest may ensure that SOP selection and mitotic entry are coordinated. To induce cell cycle re-entry in Drosophila, Stg and Cyclin E are upregulated by developmental signals to control the G2/M and G1/S transitions, respectively (Swanhart et al., 2005). SOPs in the notum transition rapidly from mitosis into S phase without an intervening G1 phase due to high levels of accumulated Cyclin E (Audibert et al., 2005). In the absence of G2 arrest, high levels of Cyclin E may drive selected SOPs prematurely into S phase, as in our GMR>stg experiments. G2-arrested cells may be induced to enter mitosis at a specific point in development by coupling Stg expression with levels of the hormone ecdysone. In abdominal histoblasts, a pulse of ecdysone during pupation induces stg transcription and subsequent entry into mitosis (Ninov et al, 2009). Developmental signals such as ecdysone may trigger upregulation of Cyclin E and Stg in undifferentiated cells, thereby allowing coordination of SOP selection and subsequent cell cycle re-entry.
Supplementary Material
Fig. 5. Model of interommatidial development.
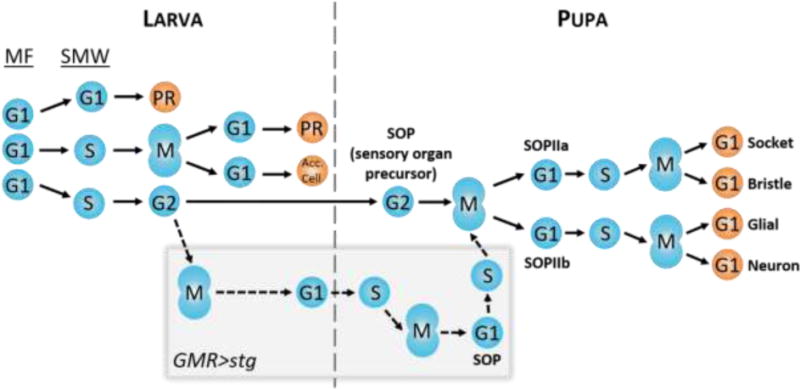
As the larva develops, the MF initiates cell cycle synchronization by promoting G1 arrest in all cells. These cells will subsequently take one of three paths: 1) arrest permanently in G1 and undergo differentiation into photoreceptors R2/3/4/5/8 (PR); 2) undergo S phase of the SMW, followed by mitosis, permanent arrest in G1, and differentiation into photoreceptors R1/7/6 and accessory cells (Acc. Cell; i.e. cone cells and pigment cells); 3) undergo S phase of the SMW and arrest in G2 for the remainder of larval development, followed by selection as an SOP during pupal development and two divisions to make up the four G1-arrested cells of the bristle. Prematurely driving cells through mitosis, such as with GMR>stg (gray box), does not prevent SOP selection and bristle differentiation (dotted lines). Differential timing of SOP divisions between wild type and GMR>stg (not shown in this model) may contribute to bristle positioning defects observed in GMR>stg adult eyes.
HIGHLIGHTS.
G2-arrested cells are selected as mechanosensory organ precursors (SOPs) giving rise to bristles of the compound Drosophila eye
G2 arrest of SOPs is necessary for correct bristle placement in the eye
Cell cycle phase influences but is not deterministic for specific cell fates
Acknowledgments
We thank the Bloomington Drosophila Stock Center, supported by P40-OD018537, for stocks used in this study and the UNC Flow Cytometry Core Facility, supported by P30-CA016086. We also thank the labs of Bruce Edgar, Hugo Bellen, Pat O’Farrell, and Iswar Hariharan for stocks and reagents used in this study.
FUNDING
This work was supported by the National Institutes of Health (F31-AG044957-02 to JHM and GM057859 and GM58921 to RJD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anatskaya OV, Vinogradov AE, Kudryavtsev BN. Hepatocyte polyploidy and metabolism/life-history traits: hypotheses testing. J Theor Biol. 1994;168:191–199. doi: 10.1006/jtbi.1994.1098. [DOI] [PubMed] [Google Scholar]
- Audibert A, Simon F, Gho M. Cell cycle diversity involves differential regulation of Cyclin E activity in the Drosophila bristle cell lineage. Development. 2005;132:2287–2297. doi: 10.1242/dev.01797. [DOI] [PubMed] [Google Scholar]
- Ayeni JO, Audibert A, Fichelson P, Srayko M, Gho M, Campbell SD. G2 phase arrest prevents bristle progenitor self-renewal and synchronizes cell division with cell fate differentiation. Development. 2016;143:1160–1169. doi: 10.1242/dev.134270. [DOI] [PubMed] [Google Scholar]
- Azzarelli R, Hurley C, Sznurkowska MK, Rulands S, Hardwick L, Gamper I, Ali F, McCracken L, Hindley C, McDuff F, Nestorowa S, Kemp R, Jones K, Gottgens B, Huch M, Evan G, Simons BD, Winton D, Philpott A. Multi-site Neurogenin3 Phosphorylation Controls Pancreatic Endocrine Differentiation. Dev Cell. 2017;41:274–286 e275. doi: 10.1016/j.devcel.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge SP, Bownes M. Staging the metamorphosis of Drosophila melanogaster. J Embryol Exp Morphol. 1981;66:57–80. [PubMed] [Google Scholar]
- Baker NE. Patterning signals and proliferation in Drosophila imaginal discs. Curr Opin Genet Dev. 2007;17:287–293. doi: 10.1016/j.gde.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Baker NE, Rubin GM. Effect on Eye Development of Dominant Mutations in Drosophila Homolog of the Egf Receptor. Nature. 1989;340:150–153. doi: 10.1038/340150a0. [DOI] [PubMed] [Google Scholar]
- Baker NE, Yu SY. The EGF receptor defines domains of cell cycle progression and survival to regulate cell number in the developing Drosophila eye. Cell. 2001;104:699–708. doi: 10.1016/s0092-8674(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Bellaïche Y, Gho M, Kaltschmidt JA, Brand AH, Schweisguth F. Frizzled regulates localization of cell-fate determinants and mitotic spindle rotation during asymmetric cell division. Nat Cell Biol. 2001;3:50–57. doi: 10.1038/35050558. [DOI] [PubMed] [Google Scholar]
- Bernardi R, Liebermann DA, Hoffman B. Cdc25A stability is controlled by the ubiquitin-proteasome pathway during cell cycle progression and terminal differentiation. Oncogene. 2000;19 doi: 10.1038/sj.onc.1203564. [DOI] [PubMed] [Google Scholar]
- Boije H, Edqvist PH, Hallbook F. Horizontal cell progenitors arrest in G2-phase and undergo terminal mitosis on the vitreal side of the chick retina. Dev Biol. 2009;330:105–113. doi: 10.1016/j.ydbio.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Buszczak M, Paterno S, Lighthouse D, Bachman J, Planck J, Owen S, Skora AD, Nystul TG, Ohlstein B, Allen A, Wilhelm JE, Murphy TD, Levis RW, Matunis E, Srivali N, Hoskins RA, Spradling AC. The carnegie protein trap library: a versatile tool for Drosophila developmental studies. Genetics. 2007;175:1505–1531. doi: 10.1534/genetics.106.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttitta LA, Katzaroff AJ, Perez CL, de la Cruz A, Edgar BA. A double-assurance mechanism controls cell cycle exit upon terminal differentiation in Drosophila. Dev Cell. 2007;12:631–643. doi: 10.1016/j.devcel.2007.02.020. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Jou AD, Nusse R. Wingless blocks bristle formation and morphogenetic furrow progression in the eye through repression of Daughterless. Development. 2002;129:3393–3402. doi: 10.1242/dev.129.14.3393. [DOI] [PubMed] [Google Scholar]
- Cagan RL, Ready DF. The emergence of order in the Drosophila pupal retina. Dev Biol. 1989;136:346–362. doi: 10.1016/0012-1606(89)90261-3. [DOI] [PubMed] [Google Scholar]
- de la Cruz AF, Edgar BA. Flow cytometric analysis of Drosophila cells. Methods Mol Biol. 2008;420:373–389. doi: 10.1007/978-1-59745-583-1_24. [DOI] [PubMed] [Google Scholar]
- de Nooij JC, Hariharan IK. Uncoupling Cell Fate Determination from Patterned Cell-Division in the Drosophila Eye. Science. 1995;270:983–985. doi: 10.1126/science.270.5238.983. [DOI] [PubMed] [Google Scholar]
- Di Giorgio E, Gagliostro E, Clocchiatti A, Brancolini C. The control operated by the cell cycle machinery on MEF2 stability contributes to the downregulation of CDKN1A and entry into S phase. Mol Cell Biol. 2015;35:1633–1647. doi: 10.1128/MCB.01461-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W, Xie JE, Dyson N. Ectopic expression of dE2F and dDP induces cell proliferation and death in the Drosophila eye. EMBO J. 1996;15:3684–3692. [PMC free article] [PubMed] [Google Scholar]
- Edgar BA, Datar SA. Zygotic degradation of two maternal Cdc25 mRNAs terminates Drosophila’s early cell cycle program. Genes Dev. 1996;10:1966–1977. doi: 10.1101/gad.10.15.1966. [DOI] [PubMed] [Google Scholar]
- Edgar BA, O’Farrell PH. Genetic control of cell division patterns in the Drosophila embryo. Cell. 1989;57:177–187. doi: 10.1016/0092-8674(89)90183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar BA, O’Farrell PH. The three postblastoderm cell cycles of Drosophila embryogenesis are regulated in G2 by string. Cell. 1990;62:469–480. doi: 10.1016/0092-8674(90)90012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth LC, Baker NE. Extracellular signals responsible for spatially regulated proliferation in the differentiating Drosophila eye. Dev Cell. 2005;8:541–551. doi: 10.1016/j.devcel.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Frankfort BJ, Pepple KL, Mamlouk M, Rose MF, Mardon G. Senseless is required for pupal retinal development in Drosophila. Genesis. 2004;38:182–194. doi: 10.1002/gene.20018. [DOI] [PubMed] [Google Scholar]
- Garcia-Alonso L, VanBerkum MF, Grenningloh G, Schuster C, Goodman CS. Fasciclin II controls proneural gene expression in Drosophila. Proc Natl Acad Sci U S A. 1995;92:10501–10505. doi: 10.1073/pnas.92.23.10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentric G, Celton-Morizur S, Desdouets C. Polyploidy and liver proliferation. Clin Res Hepatol Gastroenterol. 2012;36:29–34. doi: 10.1016/j.clinre.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Golic KG. Site-specific recombination between homologous chromosomes in Drosophila. Science. 1991;252:958–961. doi: 10.1126/science.2035025. [DOI] [PubMed] [Google Scholar]
- Grosshans J, Wieschaus E. A genetic link between morphogenesis and cell division during formation of the ventral furrow in Drosophila. Cell. 2000;101:523–531. doi: 10.1016/s0092-8674(00)80862-4. [DOI] [PubMed] [Google Scholar]
- Hay BA, Wolff T, Rubin GM. Expression of baculovirus P35 prevents cell death in Drosophila. Development. 1994;120:2121–2129. doi: 10.1242/dev.120.8.2121. [DOI] [PubMed] [Google Scholar]
- Johnston LA, Edgar BA. Wingless and Notch regulate cell-cycle arrest in the developing Drosophila wing. Nature. 1998;394:82–84. doi: 10.1038/27925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T. Immunolabeling of imaginal discs. Methods Mol Biol. 2008;420:253–263. doi: 10.1007/978-1-59745-583-1_15. [DOI] [PubMed] [Google Scholar]
- Korzelius J, The I, Ruijtenberg S, Prinsen MB, Portegijs V, Middelkoop TC, Groot Koerkamp MJ, Holstege FC, Boxem M, van den Heuvel S. Caenorhabditis elegans cyclin D/CDK4 and cyclin E/CDK2 induce distinct cell cycle reentry programs in differentiated muscle cells. PLoS Genet. 2011;7:e1002362. doi: 10.1371/journal.pgen.1002362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentz NAJ, van Hoof D, Li Z, Watanabe A, Tang M, Nian C, German MS, Lynn FC. Phosphorylation of NEUROG3 Links Endocrine Differentiation to the Cell Cycle in Pancreatic Progenitors. Dev Cell. 2017;41:129–142 e126. doi: 10.1016/j.devcel.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar JP. Retinal determination the beginning of eye development. Curr Top Dev Biol. 2010;93:1–28. doi: 10.1016/B978-0-12-385044-7.00001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar JP. Building an ommatidium one cell at a time. Dev Dyn. 2012;241:136–149. doi: 10.1002/dvdy.23707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson DE, Liberman Z, Cagan RL. Cellular behavior in the developing Drosophila pupal retina. Mech Dev. 2008;125:223–232. doi: 10.1016/j.mod.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman DA, Patterson B, Johnston LA, Balzer T, Britton JS, Saint R, Edgar BA. Cis-regulatory elements of the mitotic regulator, string/Cdc25. Development. 1999;126:1793–1803. doi: 10.1242/dev.126.9.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes CS, Casares F. Eye selector logic for a coordinated cell cycle exit. PLoS Genet. 2015;11:e1004981. doi: 10.1371/journal.pgen.1004981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Kanakousaki K, Buttitta L. How the cell cycle impacts chromatin architecture and influences cell fate. Front Genet. 2015;6:19. doi: 10.3389/fgene.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata J, Curado S, Ephrussi A, Rorth P. Tribbles coordinates mitosis and morphogenesis in Drosophila by regulating string/CDC25 proteolysis. Cell. 2000;101:511–522. doi: 10.1016/s0092-8674(00)80861-2. [DOI] [PubMed] [Google Scholar]
- Meserve JH, Duronio RJ. Scalloped and Yorkie are required for cell cycle reentry of quiescent cells after tissue damage. Development. 2015;142:2740–2751. doi: 10.1242/dev.119339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nègre N, Ghysen A, Martinez A-M. Mitotic G2-arrest is required for neural cell fate determination in Drosophila. Mech Dev. 2003;120:253–265. doi: 10.1016/s0925-4773(02)00419-7. [DOI] [PubMed] [Google Scholar]
- Paridaen JT, Huttner WB. Neurogenesis during development of the vertebrate central nervous system. EMBO Rep. 2014;15:351–364. doi: 10.1002/embr.201438447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauklin S, Vallier L. The cell-cycle state of stem cells determines cell fate propensity. Cell. 2013;155:135–147. doi: 10.1016/j.cell.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, Whittle JR. wingless expression mediates determination of peripheral nervous system elements in late stages of Drosophila wing disc development. Development. 1993;118:427–438. doi: 10.1242/dev.118.2.427. [DOI] [PubMed] [Google Scholar]
- Ready DF, Hanson TE, Benzer S. Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol. 1976;53:217–240. doi: 10.1016/0012-1606(76)90225-6. [DOI] [PubMed] [Google Scholar]
- Reiner JM. Differentiation, ageing, and terminal differentiation: a semantic analysis. J Theor Biol. 1983;105:545–552. doi: 10.1016/0022-5193(83)90218-7. [DOI] [PubMed] [Google Scholar]
- Remaud S, Audibert A, Gho M. S-phase favours notch cell responsiveness in the Drosophila bristle lineage. PLoS One. 2008;3:e3646. doi: 10.1371/journal.pone.0003646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinow S, White K. Characterization and spatial distribution of the ELAV protein during Drosophila melanogaster development. J Neurobiol. 1991;22:443–461. doi: 10.1002/neu.480220503. [DOI] [PubMed] [Google Scholar]
- Rohrbaugh M, Ramos E, Nguyen D, Price M, Wen Y, Lai ZC. Notch activation of yan expression is antagonized by RTK/pointed signaling in the Drosophila eye. Curr Biol. 2002;12:576–581. doi: 10.1016/s0960-9822(02)00743-1. [DOI] [PubMed] [Google Scholar]
- Roignant JY, Treisman JE. Pattern formation in the Drosophila eye disc. Int J Dev Biol. 2009;53:795–804. doi: 10.1387/ijdb.072483jr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero R, Kale A, Thomas B, Baker NE. Mitosis in neurons: Roughex and APC/C maintain cell cycle exit to prevent cytokinetic and axonal defects in Drosophila photoreceptor neurons. PLoS Genet. 2012;8:e1003049. doi: 10.1371/journal.pgen.1003049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruijtenberg S, van den Heuvel S. Coordinating cell proliferation and differentiation: Antagonism between cell cycle regulators and cell type-specific gene expression. Cell Cycle. 2016;15:196–212. doi: 10.1080/15384101.2015.1120925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaue-Sawano A, Kurokawa H, Morimura T, Hanyu A, Hama H, Osawa H, Kashiwagi S, Fukami K, Miyata T, Miyoshi H, Imamura T, Ogawa M, Masai H, Miyawaki A. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell. 2008;132:487–498. doi: 10.1016/j.cell.2007.12.033. [DOI] [PubMed] [Google Scholar]
- Seher TC, Leptin M. Tribbles, a cell-cycle brake that coordinates proliferation and morphogenesis during Drosophila gastrulation. Curr Biol. 2000;10 doi: 10.1016/s0960-9822(00)00502-9. [DOI] [PubMed] [Google Scholar]
- Sela Y, Molotski N, Golan S, Itskovitz-Eldor J, Soen Y. Human embryonic stem cells exhibit increased propensity to differentiate during the G1 phase prior to phosphorylation of retinoblastoma protein. Stem Cells. 2012;30:1097–1108. doi: 10.1002/stem.1078. [DOI] [PubMed] [Google Scholar]
- Sousa-Nunes R, Somers WG. Mechanisms of asymmetric progenitor divisions in the Drosophila central nervous system. Adv Exp Med Biol. 2013;786:79–102. doi: 10.1007/978-94-007-6621-1_6. [DOI] [PubMed] [Google Scholar]
- Sukhanova MJ, Du W. Control of cell cycle entry and exiting from the second mitotic wave in the Drosophila developing eye. BMC Dev Biol. 2008;8:7. doi: 10.1186/1471-213X-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanhart L, Kupsco J, Duronio RJ. Developmental control of growth and cell cycle progression in Drosophila. Methods Mol Biol. 2005;296:69–94. doi: 10.1385/1-59259-857-9:069. [DOI] [PubMed] [Google Scholar]
- Thomas BJ, Gunning DA, Cho J, Zipursky L. Cell cycle progression in the developing Drosophila eye: roughex encodes a novel protein required for the establishment of G1. Cell. 1994;77:1003–1014. doi: 10.1016/0092-8674(94)90440-5. [DOI] [PubMed] [Google Scholar]
- Thomas BJ, Zavitz KH, Dong X, Lane ME, Weigmann K, Finley RL, Jr, Brent R, Lehner CF, Zipursky SL. roughex down-regulates G2 cyclins in G1. Genes Dev. 1997;11:1289–1298. doi: 10.1101/gad.11.10.1289. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Carty SA, Piscopo DM, Lee JS, Wang WF, Forrester WC, Hinds PW. The retinoblastoma protein acts as a transcriptional coactivator required for osteogenic differentiation. Mol Cell. 2001;8:303–316. doi: 10.1016/s1097-2765(01)00327-6. [DOI] [PubMed] [Google Scholar]
- Tintignac LA, Leibovitch MP, Kitzmann M, Fernandez A, Ducommun B, Meijer L, Leibovitch SA. Cyclin E-cdk2 phosphorylation promotes late G1-phase degradation of MyoD in muscle cells. Exp Cell Res. 2000;259:300–307. doi: 10.1006/excr.2000.4973. [DOI] [PubMed] [Google Scholar]
- Treisman JE. Retinal differentiation in Drosophila. Wiley Interdiscip Rev Dev Biol. 2013;2:545–557. doi: 10.1002/wdev.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff T, Ready DF. The beginning of pattern formation in the Drosophila compound eye: the morphogenetic furrow and the second mitotic wave. Development. 1991;113:841–850. doi: 10.1242/dev.113.3.841. [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of Genetic Mosaics in Developing and Adult Drosophila Tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- Zielke N, Korzelius J, van Straaten M, Bender K, Schuhknecht GF, Dutta D, Xiang J, Edgar BA. Fly-FUCCI: A versatile tool for studying cell proliferation in complex tissues. Cell Rep. 2014;7:588–598. doi: 10.1016/j.celrep.2014.03.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


