Abstract
Exposure to lead (Pb) remains a significant public health concern. Lead exposure in early life impairs the normal development of numerous cognitive and neurobehavioral processes. Previous work has shown that the effects of developmental Pb exposure on gene expression patterns in the brain are modulated by various factors including the developmental timing of the exposure, level of exposure, sex, and genetic background. Using gene microarray profiling, we previously reported a significant strain-specific effect of Pb exposure on the hippocampal transcriptome, with the greatest number of differentially expressed transcripts in Long Evans (LE) rats and the fewest in Sprague Dawley (SD) rats. The present study examined the extent to which this differential effect of Pb on hippocampal gene expression might influence behavior. Animals (males and females) were tested in a trace fear conditioning paradigm to evaluate effects of Pb exposures (perinatal (PERI; gestation to postnatal day 21) or early postnatal (EPN; postnatal day 1 to day 21)) on associative learning and memory. All animals (Pb-exposed and non-Pb-exposed controls) showed normal acquisition of the conditioned stimulus (tone)-unconditioned stimulus (footshock) association. Long Evans rats showed a significant deficit in short- and long-term recall, influenced by sex and the timing of Pb exposure (PERI or EPN). In contrast, Pb exposure had no significant effect on memory consolidation or recall in any SD rats. These results further demonstrate the important influence of genetic background to the functional outcomes from developmental Pb exposure.
Keywords: developmental lead exposure, trace fear conditioning, strain differences
1. Introduction
Early life exposure to lead (Pb) impairs a variety of cognitive, behavioral and neurochemical processes resulting in a variety of potentially negative outcomes for children exposed to this potent neurotoxicant. Children with even low level Pb exposures can display cognitive/behavioral problems including deficits in learning, memory, attention, and executive function, as well as increased impulsivity and aggression (Cecil et al., 2008; Cecil and Kos, 2006; Mazumdar et al., 2011; Nigg et al., 2010; Surkan et al., 2007). Yet, developmental Pb exposure does not result in the same array or extent of deficits in all exposed individuals and consequently, no specific Pb-associated “behavioral signature” has been identified. Individuals with similar Pb exposures can express different behavioral and cognitive impairments and express different profiles of neuropsychological impairments even when subjects are assessed using the same battery of tests (Lidsky and Schneider, 2006). While a number of factors including home environment, socioeconomic status, and race can influence the susceptibility for Pb exposure (ex., (Chung et al., 2001; Gellert et al., 1993), genetic factors may also affect the toxicokinetics of Pb and the brain’s vulnerability to its neurotoxic effects (Onalaja and Claudio, 2000; Stewart et al., 2002). Additionally, genetic factors may also influence the functional outcomes from developmental Pb exposure.
While genomic variation can influence the phenotypic expression of a variety of traits, genomic variation may also influence the manner in which the brain (or other organ systems) respond to a particular toxicant, stressor, or injury. In a recent study, we used gene profiling and microarray technology to identify potential strain differences in Pb-responsive genes in the hippocampus, a brain structure well known to be adversely affected by Pb, in rats of different genetic backgrounds (i.e., Fischer 344 (F344), Long Evans (LE), and Sprague Dawley (SD)). We found significant strain-related effects of Pb on the hippocampal transcriptome that were not due to strain-related differences in brain accumulation of Pb (Schneider et al., 2014). A large number of transcripts (978) were differentially expressed in LE rats across all experimental groups (male/female, perinatal or postnatal Pb exposure), while 269 transcripts were differentially expressed in F344 rats, and only 179 transcripts were differentially expressed in SD rats (Schneider et al., 2014). The results of this study demonstrated that at least at the level of gene transcription, the response of the brain to a given Pb exposure appears to be uniquely related to the strain of the animal and suggested the possibility that differences in neurobehavioral outcomes from developmental Pb exposure in different strains of animals could occur. As there is little known about the neurobehavioral influences of strain or genetic background on cognitive outcomes following developmental Pb exposure, the present study was conducted to investigate the role that genetic variation may play in influencing cognitive outcomes from developmental Pb exposure. As we have reported deficits in associative memory in Pb-exposed LE rats (the strain that we previously found to have the largest number of hippocampal transcripts altered by Pb exposure), the current study investigated the effects of similar Pb exposures (150 ppm) on associative learning/memory in SD rats (the strain that we previously found to have the fewest number of hippocampal transcripts differentially altered by Pb exposure), using a trace fear-conditioning paradigm.
2. Materials and Methods
2.1 Animals and Treatments
The use of animals was in compliance with NIH Guidelines for the Care and Use of Laboratory Animals and the study was approved by the institutional animal care and use committee at Thomas Jefferson University. Long Evans and Sprague Dawley rats and two Pb exposure paradigms (perinatal (PERI) exposure and early postnatal (EPN)) were used. The rats were acclimatized to the animal facility for at least a week before the start of the experiments. All experimental animals were generated in house to reduce outside sources of potential variability. In the PERI exposure group, 50–55 day old LE dams (Envigo Laboratories) and SD dams (Taconic Laboratories) were fed chow (RMH 1000) with or without added Pb acetate (150 ppm or 0 ppm) for 14 days prior to breeding and remained on the same diet through weaning, as previously described (Anderson et al., 2016). Thus, dams were continuously exposed to Pb for approximately 55–60 days. Litters were culled to equal numbers of pups to standardize litter size, with an aim of having eight pups per litter. Equal numbers of males and females were maintained wherever possible and were exposed to Pb from gestation through lactation (i.e., to postnatal day 21). At weaning, rats were housed four to a standard cage (940 cm2) with ad lib access to chow (no added Pb) and water until behavioral testing, beginning at postnatal day 55. For animals in the EPN group, 50–55 day old dams were fed RMH 1000 chow with no added Pb during gestation and were then fed chow with or without added Pb acetate (150 ppm or 0 ppm) beginning at parturition and pups continued to receive the same exposure to Pb through weaning at postnatal day 21. All animals were exposed to a 12 h:12 h light:dark cycle for the duration of the experiment. The number of individuals (n) tested in each group were (LE and SD, respectively): Control (no Pb): male (n = 16, 18), female (n = 16, 18); Perinatal Pb exposure: male (n = 16, 18), female (n = 16, 18); Early postnatal Pb exposure: male (n = 16, 18), female (n = 16, 18). Throughout the study, all rats were weighed every two weeks to ensure that the Pb exposure was well tolerated. No more than one male and one female from any litter were included in any behavioral group to prevent litter effects.
2.2 Blood lead analysis
Trunk blood samples were collected into EDTA-containing collection tubes at time of euthanasia from littermates of experimental animals not used for behavioral studies on postnatal day 14 and from behaviorally tested animals at postnatal day 65 and analyzed for Pb levels using an ESA LeadCare II Blood Lead Analyzer system version 1.09 and LeadCare II Blood Lead Test kit (Magellan Diagnostics, MA). Briefly, 50 μl of whole blood was mixed with 250 μl of diluted hydrochloric acid solution (0.34 M) and was applied to the sensor strip of the LeadCare II Blood Lead Analyzer system. Values were recorded in μg/dL. Additionally, blood samples were obtained from dams by retro-orbital bleed before Pb exposure and at parturition and analyzed as described above.
2.3 Trace Fear Conditioning
Trace fear conditioning was carried out using Ugo Basile Fear Conditioning systems (30cm deep × 34cm wide × 41.5cm high) and ANY-maze software (Version 4.99; Stoelting Co., Wood Dale, IL) that automatically measured the freezing response. The trace fear conditioning protocol consisted of habituation, acquisition training, and short- and long-term retrieval testing at Days 1, 2 and 10 post acquisition training as previously described (Anderson et al., 2016). Animals were habituated to the fear conditioning chamber, located within a dimly lit sound attenuating enclosure with white background noise, for 15 minutes one day prior to the acquisition trials. During acquisition trials, animals were given 180 seconds to habituate to the test chamber and then given a series of 6 pairings of tone (conditioned stimulus; CS, 3000 Hz, 80 dB for 15 seconds)-shock (unconditioned stimulus; US, 0.8mA for 1.0 second) with a 20 second trace period between CS and US and pseudorandom inter-trial-intervals of 1–3 mins. Freezing behavior, defined by absence of all but respiratory movements, was measured every second during the 20 seconds of the trace period. Retention testing occurred at 1, 2 and 10 days post acquisition. For retention testing, animals were placed back into the same chamber in which they were initially trained but with different visual and olfactory cues. On each retention testing day, animals were habituated to the chamber for 180 seconds followed by presentation of 3 tones for 15 seconds each, in the absence of foot shock, with a pseudo random ITI (1–4 mins.) between presentation of tones. Freezing was measured every second during the 20 seconds after tone presentation.
2.4 Data Analyses
Data were analyzed using a two-way analysis of variance (ANOVA) with repeated measure design followed by a Tukey test for post hoc analyses using GraphPad Prism (v. 7.02). Analysis of acquisition data included all six data points (trial 1–6) averaged to yield an estimate of percent time freezing. The analysis of the retention data (Days 1, 2 and 10) included only data from the first trial each day (Anderson et al., 2016). Statistical significance was defined at p < 0.05.
3. Results
3.1 Body Weight Analysis
Before the start of the Pb exposure, there were no within group differences in weights of males or females randomized to the Pb-exposure or the control group. There were no significant differences in body weights between controls and Pb-exposed groups within sex and strain throughout the study (data not shown). Additionally, there were no grossly observable differences in food or water consumption between the control and Pb-exposed LE and SD rats.
3.2 Blood Lead Levels
Mean blood Pb levels of LE and SD dams, were not significantly different in any of the exposure groups (PERI or EPN) (P<0.05) (Table 1). Mean blood lead levels for the Pb-exposed LE and SD rats, measured at postnatal day 14, were not statistically different across strains (Table 2). The mean blood Pb levels measured at the end of study (age 65 days) were below the detectable limits (<1 μg/dl) (data not shown).
Table 1.
Blood lead levels (BLLs) measured at weaning in Long Evans and Sprague Dawley dams.
| Condition | Control | Perinatal | Early Postnatal | |||
|---|---|---|---|---|---|---|
| Strain | Long Evans | Sprague Dawley | Long Evans | Sprague Dawley | Long Evans | Sprague Dawley |
| DAM’s | <1.00 | <1.00 | 12.22 ± 0.481 | 12.46 ± 0.710 | 4.8 ± 0.300 | 4.46 ± 0.320 |
The data are represented as μg/dL mean blood lead levels ± SD. N=5–6 animals in each group.
Table 2.
Blood lead levels (BLLs) measured at postnatal day 14 in Long Evans and Sprague Dawley pups.
| Condition | Control | Perinatal | Early Postnatal | |||
|---|---|---|---|---|---|---|
| Strain | Long Evans | Sprague Dawley | Long Evans | Sprague Dawley | Long Evans | Sprague Dawley |
| Male | <1.00 | <1.00 | 5.65 ± 0.264 | 5.425 ± 0.403 | 5.95 ± 0.387 | 5.45 ± 0.341 |
| Female | <1.00 | <1.00 | 4.38 ± 0.342 | 4.521 ± 0.303 | 5.38 ± 0.13 | 5.06 ± 0.181 |
The data are represented as μg/dL mean blood lead level ± SD. N=4–5 animals in each group.
3.3 Behavioral Outcomes
During the 180 sec acclimation period before the presentation of the first CS-US there was virtually no freezing behavior observed in either strain as is expected prior to the first presentation of the CS-US pairing (Anagnostaras et al., 1999). Additionally, there were no differences between the groups in baseline locomotor activities assessed during the initial 180 sec acclimation period, regardless of gender, Pb exposure, or the developmental time window of the Pb exposure (Peri or EPN) (data not shown).
3.3.1 Fear Learning in Long Evans Females
There was no significant main effect of Pb exposure (P>0.05) nor an interaction between the acquisition trials and Pb exposure (P>0.05) on freezing behavior during the six acquisition trials in LE females. There was a significant main effect of trial alone on learning of the CS-US association in LE females (F (5, 105) = 42.39, P<0.0001). All animals, Pb exposed (PERI and EPN) or not (Control), learned the association between CS-US pairing and showed similar degrees of freezing after the first CS-US pairing as well as at the last CS-US pairing (Fig 1A).
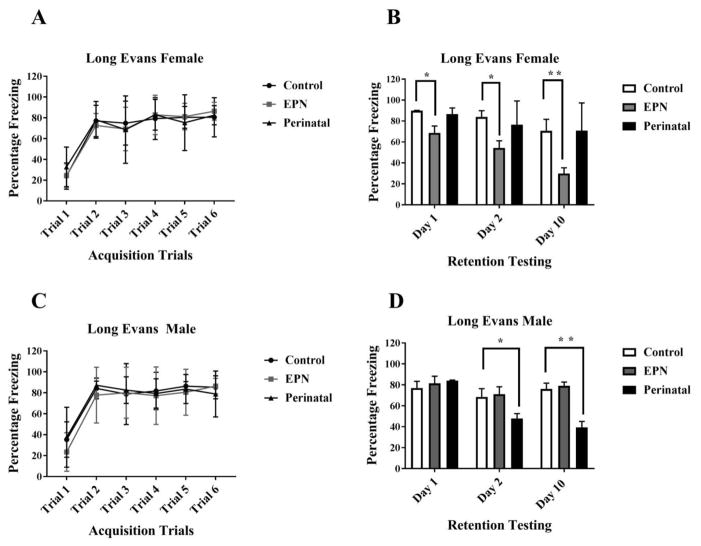
Figure 1. Effect of lead exposure on associative learning and memory during trace fear conditioning in female and male Long Evans (LE) rats.
A and C) Acquisition Trials. No significant differences were observed between groups (P>0.05). B and D) Retention Testing. A significant impairment in memory recall was observed on Day 1 (* P= 0.0450), Day 2 (* P= 0.0032) and Day 10 (** P<0.001) post acquisition in EPN females, compared to controls. A significant impairment in memory recall was observed in PERI males at Day 2 (*P= 0.0394) and Day 10 (** P>0.001), compared to controls. Data are represented as Mean ± SD. N= 16–18 animals per group.
3.3.2 Retention Testing in Long Evans Females
There were significant main effects of both Day alone (F (2, 44) = 15.32, P<0.0001) and Pb exposure alone (F (2, 22) = 17.94, P<0.0001) but no interaction between Day and Pb exposure (F (4, 44) = 1.555, P=0.2031) on post-acquisition retention of the CS-US pairing in LE female animals. Consistent with our previous data (Anderson et al., 2016), there was a significant difference in freezing between control and LE EPN females on the Day 1 of the retention testing day (P= 0.0450) but no differences in freezing responses between control and PERI Pb-exposed LE female animals (P= 0.9282). On Day 2, the freezing response in EPN Pb exposed LE female animals was further reduced compared to controls (P= 0.0032) and to PERI LE females (P= 0.0222). Deficits in memory retrieval were also observed on Day 10 of retention testing in EPN Pb-exposed LE females compared to control LE females (P <0.0001) and PERI Pb-exposed LE females (P <0.0001). No effect of Pb exposure on recall was observed in PERI Pb-exposed LE females during any post-acquisition testing (P = 0.9282, 0.6704, 0.9997 on Day 1, 2 and 10, respectively) (Fig 1B).
3.3.3 Fear Learning in Long Evans Males
There was no significant main effect of Pb exposure alone (F (2, 31) = 0.4091, P=0.6678) nor an interaction between trial and Pb exposure (F (10, 155) = 0.7875, P=0.6408) during the six acquisition trials of the CS-US association in LE males. There was a significant main effect of trial alone (F (5, 155) = 53.17, P<0.0001) on the freezing response during the acquisition trials in LE males. All groups, irrespective of Pb exposure or not, acquired the task and showed similar freezing responses after presentation of the first CS-US. At the end of the last acquisition trial, there were no differences in the freezing response between any of the groups (Fig 1C).
3.3.4 Memory Retention in Long Evans Males
Pb exposure alone (F (2, 27) = 8.156, P=0.0017) and Day alone (F (2, 54) = 12.03, P<0.0001) had significant main effects on the freezing response in LE males. Additionally, there was a significant interaction between Day and Pb exposure (F (4, 54) = 6.732, P=0.0002) on the freezing response during retention testing in LE males. There were no significant differences between control and EPN LE males during retention testing (Day 1, Day 2 or Day 10) (P= 0.8448, 0.9459, 0.9275 respectively). There was a significant difference between control and PERI LE males on retention testing on Day 2 (P= 0.0394) and on Day 10 (P <0.0001). Additionally, PERI Pb-exposed LE males had recall deficits on Day 2 and Day 10 compared to EPN Pb-exposed LE males (P= 0.0085 and <0.0001 respectively) (Fig 1D).
3.3.5 Fear Learning in Sprague-Dawley Females
There was no significant main effect of Pb exposure alone (F (2, 21) = 0.2701, P=0.7659) nor an interaction between trial and Pb exposure (PERI and EPN) (F (10, 105) = 1.1, P=0.3694) during the six acquisition trials. There was a significant main effect of trial alone on the freezing behavior of female SD rats (F (5, 105) = 42.48, P<0.0001). All groups, irrespective of the Pb exposure or not learned the task and displayed similar freezing behavior across the six acquisition trials (Fig 2A).
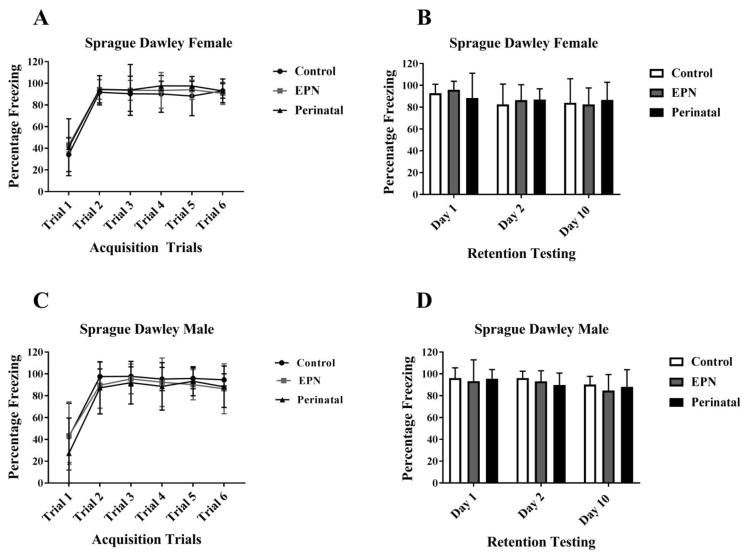
Figure 2. Effect of lead exposure on associative learning and memory during trace fear conditioning in female and male Sprague Dawley (SD) rats.
A and C) Acquisition Trials. No significant group differences were observed (P>0.05). B and D) Retention Testing. Female and Male SD rats showed no memory deficits during recall testing (P>0.05). Data are represented as Mean ± SD. N= 16–18 animals per group.
3.3.6 Memory Retention in Sprague-Dawley Females
There was no main effect of either Pb exposure alone (P>0.05) nor an interaction between Days and Pb exposure (P>0.05) on post-acquisition retention testing in SD females. There were no significant differences between Control and EPN SD females (Day 1, Day 2 and Day 10, P>0.05) nor between Control and PERI SD females (Day 1, Day 2 and Day 10, P>0.05) during retention testing. No SD females, regardless of Pb exposure, showed any retention/recall deficits (Fig 2B).
3.3.7 Fear Learning in Sprague-Dawley Males
There was no significant main effects of Pb exposure alone (P>0.05) nor an interaction between Pb exposure and trial (P>0.05) during the six acquisition trials. There was a significant main effect of trial alone on the freezing behavior in SD males (F (5, 270) = 73.5, P<0.0001), with PERI males performing significantly different from EPN males on the first trial of acquisition training (P = 0.0333). There were no significant differences in freezing responses between Controls and Pb-exposed SD males at the end of the acquisition period (Fig 2C).
3.3.8 Memory Retention Test in Sprague-Dawley Males
There was no significant main effect of Pb exposure alone (P>0.05) nor an interaction between Days and Pb exposure (P>0.05) on post-acquisition retention testing of the CS-US association in SD males. There were no significant differences in freezing responses between Control and EPN Pb-exposed SD males (Day 1, Day 2 or Day 10, P>0.05) nor between control and PERI Pb-exposed SD males during retention testing (Day 1, Day 2 or Day 10, P>0.05). There were no significant differences in freezing responses between the two Pb-exposed groups on any day of the retention/recall testing (P>0.05) (Fig 2D).
4. Discussion
The results of this study demonstrate that both genetic background and sex may play important roles in determining neurobehavioral outcomes from Pb exposure. In addition to the developmental timing of Pb exposure, sex, rearing environment, and the amount of Pb exposure being effect modifiers of outcomes from developmental Pb exposure (Anderson et al., 2016; Anderson et al., 2013; Schneider et al., 2012b; Schneider et al., 2014), genetic background also plays an important role in determining the extent and nature of the brain’s response to Pb. The findings from the current behavioral study extend previous findings from this lab (Schneider et al., 2014) in which we identified strain-related differences in hippocampal gene expression profiles in response to Pb exposure in LE, F344, and SD rats, and help to put the results of the prior transcriptomic study into a functional context. In that study, significant strain-specific effects of Pb on the hippocampal transcriptome were described, with 978 transcripts differentially expressed in LE rats across all experimental groups, and only 179 transcripts differentially expressed in SD rats (Schneider et al., 2014). Interestingly, the differential expression of some of the key genes involved in learning and memory including immediate-early genes such as Arc (Activity Regulated Cytoskeleton Associated Protein), Npas4 (Neuronal PAS domain protein 4) and Egr (Early growth response protein) were significantly affected by Pb exposure in LE rats but not in SD rats, suggesting a potential molecular link to the behavioral differences currently observed. Significant associative memory impairments in LE rats and no such impairments in SD rats, despite the same Pb-exposures, together with the previous gene expression data, suggest the relative vulnerability and resiliency, respectively, of these two strains to developmental Pb-exposure may differ, which is important for neurobehavioral work related to Pb toxicity using these two strains. While short and long-term memory impairments have been reported in Pb-exposed SD rats using the Morris water maze, this occurred in animals with very high BLLs (56.8 ± 4.4 μg/dL) and sex effects were not examined (Xu et al., 2009), making these data difficult to compare with those from the present study. Perinatal Pb exposure (250ppm) has also been reported to impair dendritic spine formation on hippocampal neurons, although BLLs were not reported, sex effects were not examined, and the functional consequences of the reported effects on spine formation in naïve animals are unknown (Hu et al., 2014). Thus, developmental Pb exposure may have detrimental effects on the brain and behavior in SD rats, however, using a relatively low Pb exposure, effects were not observed in the particular behavioral paradigm we used, suggesting potential strain, dosage, and task-dependent effects of Pb-induced neurotoxicity.
The neurobiological bases for the sex-specific differences in response to Pb exposure are not completely known but may at least in part arise as a result of differential effects of Pb on sex-specific genes (McCarthy and Arnold, 2011; Schneider et al., 2011). Likewise, the mechanisms underlying the relative vulnerability and resiliency of these two strains, LE and SD, are also not currently clear. Further study of these two strains could provide important information regarding molecular mechanisms that might underlie resiliency or vulnerability to developing behavioral/cognitive dysfunction as a result of Pb exposure and may help design strategies to mitigate Pb-induced neurotoxicity.
During trace fear-associated learning, there is an important interplay between the medial prefrontal cortex (mPFC) and hippocampus for the organization, consolidation, and retrieval of memories (Xu and Sudhof, 2013). Pre-training hippocampal lesions impair trace fear conditioning (McEchron et al., 1998) while mPFC has been suggested as a site for trace fear memory storage (Corcoran and Quirk, 2007; Song et al., 2015). Damage to the mPFC has a negative impact on memory retrieval during trace conditioning (McLaughlin et al., 2002; Powell et al., 2001). We (Schneider et al., 2012; Schneider et al., 2012b) and others (Wang et al., 2013; Xu et al., 2009) have shown the detrimental effects of developmental Pb exposure on hippocampal and PFC gene expression and functioning. Thus, our results suggest the possibility that exposure to Pb may disrupt the interplay between the mPFC and hippocampus and the necessary plasticity required for the forming and recalling the associative memory and that this effect is modified by sex and developmental timing of Pb exposure in LE rats and that such effects are absent in SD rats.
The use of more than one strain to investigate potential neurotoxicant effects increases the power of the experiment by preventing the use of a strain that might be resistant to the neurotoxicant or to the effects on the particular outcome being measured (Festing, 1993). The advantage of using outbred strains of rats is that they more closely mimic the genetic diversity of the population and are relevant preclinical models for assessing neurotoxicity in contrast to inbred strains that show minimal genetic variation and less likely to represent the inter-individual variability (i.e., genetic variation) present in the heterogeneous human population (Festing, 2010, 2010a). Yet, our data suggest that even when using outbred strains (both LE and SD are outbred strains) the response to a particular neurotoxicant may vary between strains based on their particular genetic backgrounds and suggest that a multi-strain approach and multi-behavior approach may provide a more comprehensive understanding of differences in genetic-based vulnerability and resilience to developmental neurotoxicants.
5. Conclusions
The result from the current study furthers our understanding of the complex interplay between sex, genetic variation and developmental window of exposure in influencing the neurobehavioral response to developmental Pb exposure. Additional work is necessary to elucidate the molecular mechanisms underlying the influences of sex and genetic diversity as effect modifiers of developmental Pb exposure.
Highlights.
The effects of Pb acetate exposure on learning and memory were differentially associated with the timing of Pb exposure (PERI or EPN), sex, and the strain (LE or SD) of the exposed animal.
Perinatally (PERI) Pb acetate exposed male LE rats showed deficits in memory consolidation and long term recall.
Female LE rats showed deficits in memory consolidation and long term recall when exposed to Pb acetate during the early postnatal period (EPN).
No effect of Pb acetate exposure on learning and memory was observed in SD rats regardless of the type of Pb-exposure (PERI or EPN).
Acknowledgments
This project was supported by NIH grant RO1-ES015295
Footnotes
Author’s Contribution: Conceived and designed the experiments: JS. Performed the behavioral experiments: MV. Analyzed the data: MV and JS. Wrote the manuscript: MV and JS.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anagnostaras SG, Maren S, Sage JR, Goodrich S, Fanselow MS. Scopolamine and Pavlovian fear conditioning in rats: dose-effect analysis. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 1999;21(6):731–744. doi: 10.1016/S0893-133X(99)00083-4. [DOI] [PubMed] [Google Scholar]
- Anderson DW, Mettil W, Schneider JS. Effects of low level lead exposure on associative learning and memory in the rat: Influences of sex and developmental timing of exposure. Toxicology letters. 2016;246:57–64. doi: 10.1016/j.toxlet.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DW, Mettil WA, Schneider JS. Rearing environment, sex and developmental lead exposure modify gene expression in the hippocampus of behaviorally naive animals. Neurochemistry international. 2013;62(4):510–520. doi: 10.1016/j.neuint.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecil KM, Brubaker CJ, Adler CM, Dietrich KN, Altaye M, Egelhoff JC, Wessel S, Elangovan I, Hornung R, Jarvis K, Lanphear BP. Decreased brain volume in adults with childhood lead exposure. PLoS medicine. 2008;5(5):e112. doi: 10.1371/journal.pmed.0050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecil KM, Kos RS. Magnetic resonance spectroscopy and metabolic imaging in white matter diseases and pediatric disorders. Topics in magnetic resonance imaging: TMRI. 2006;17(4):275–293. doi: 10.1097/RMR.0b013e318033787e. [DOI] [PubMed] [Google Scholar]
- Chung EK, Webb D, Clampet-Lundquist S, Campbell C. A comparison of elevated blood lead levels among children living in foster care, their siblings, and the general population. Pediatrics. 2001;107(5):E81. doi: 10.1542/peds.107.5.e81. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27(4):840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festing MF. Genetic variation in outbred rats and mice and its implications for toxicological screening. Journal of experimental animal science. 1993;35(5–6):210–220. [PubMed] [Google Scholar]
- Festing MF. Inbred strains should replace outbred stocks in toxicology, safety testing, and drug development. Toxicologic pathology. 2010;38(5):681–690. doi: 10.1177/0192623310373776. [DOI] [PubMed] [Google Scholar]
- Festing MF. Improving toxicity screening and drug development by using genetically defined strains. Methods in molecular biology. 2010a;602:1–21. doi: 10.1007/978-1-60761-058-8_1. [DOI] [PubMed] [Google Scholar]
- Gellert GA, Wagner GA, Maxwell RM, Moore D, Foster L. Lead poisoning among low-income children in Orange County, California. A need for regionally differentiated policy. Jama. 1993;270(1):69–71. [PubMed] [Google Scholar]
- Hu F, Xu L, Liu ZH, Ge MM, Ruan DY, Wang HL. Developmental lead exposure alters synaptogenesis through inhibiting canonical Wnt pathway in vivo and in vitro. PloS one. 2014;9(7):e101894. doi: 10.1371/journal.pone.0101894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidsky TI, Schneider JS. Adverse effects of childhood lead poisoning: the clinical neuropsychological perspective. Environmental research. 2006;100(2):284–293. doi: 10.1016/j.envres.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Mazumdar M, Bellinger DC, Gregas M, Abanilla K, Bacic J, Needleman HL. Low-level environmental lead exposure in childhood and adult intellectual function: a follow-up study. Environ Health. 2011;10:24. doi: 10.1186/1476-069X-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nature neuroscience. 2011;14(6):677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8(6):638–646. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, Skaggs H, Churchwell J, Powell DA. Medial prefrontal cortex and pavlovian conditioning: trace versus delay conditioning. Behavioral neuroscience. 2002;116(1):37–47. [PubMed] [Google Scholar]
- Nigg JT, Nikolas M, Mark Knottnerus G, Cavanagh K, Friderici K. Confirmation and extension of association of blood lead with attention-deficit/hyperactivity disorder (ADHD) and ADHD symptom domains at population-typical exposure levels. Journal of child psychology and psychiatry, and allied disciplines. 2010;51(1):58–65. doi: 10.1111/j.1469-7610.2009.02135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onalaja AO, Claudio L. Genetic susceptibility to lead poisoning. Environmental health perspectives. 2000;108(Suppl 1):23–28. doi: 10.1289/ehp.00108s123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DA, Skaggs H, Churchwell J, McLaughlin J. Posttraining lesions of the medial prefrontal cortex impair performance of Pavlovian eyeblink conditioning but have no effect on concomitant heart rate changes in rabbits (Oryctolagus cuniculus) Behavioral neuroscience. 2001;115(5):1029–1038. doi: 10.1037//0735-7044.115.5.1029. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Anderson DW, Sonnenahalli H, Vadigepalli R. Sex-based differences in gene expression in hippocampus following postnatal lead exposure. Toxicology and applied pharmacology. 2011;256(2):179–190. doi: 10.1016/j.taap.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Anderson DW, Talsania K, Mettil W, Vadigepalli R. Effects of developmental lead exposure on the hippocampal transcriptome: influences of sex, developmental period, and lead exposure level. Toxicological sciences: an official journal of the Society of Toxicology. 2012;129(1):108–125. doi: 10.1093/toxsci/kfs189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Mettil W, Anderson DW. Differential effect of postnatal lead exposure on gene expression in the hippocampus and frontal cortex. Journal of molecular neuroscience: MN. 2012b;47(1):76–88. doi: 10.1007/s12031-011-9686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Talsania K, Mettil W, Anderson DW. Genetic diversity influences the response of the brain to developmental lead exposure. Toxicological sciences: an official journal of the Society of Toxicology. 2014;141(1):29–43. doi: 10.1093/toxsci/kfu101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Ehlers VL, Moyer JR., Jr Trace Fear Conditioning Differentially Modulates Intrinsic Excitability of Medial Prefrontal Cortex-Basolateral Complex of Amygdala Projection Neurons in Infralimbic and Prelimbic Cortices. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35(39):13511–13524. doi: 10.1523/JNEUROSCI.2329-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart WF, Schwartz BS, Simon D, Kelsey K, Todd AC. ApoE genotype, past adult lead exposure, and neurobehavioral function. Environmental health perspectives. 2002;110(5):501–505. doi: 10.1289/ehp.02110501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surkan PJ, Zhang A, Trachtenberg F, Daniel DB, McKinlay S, Bellinger DC. Neuropsychological function in children with blood lead levels <10 microg/dL. Neurotoxicology. 2007;28(6):1170–1177. doi: 10.1016/j.neuro.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XM, Liu WJ, Zhang R, Zhou YK. Effects of exposure to low-level lead on spatial learning and memory and the expression of mGluR1, NMDA receptor in different developmental stages of rats. Toxicology and industrial health. 2013;29(8):686–696. doi: 10.1177/0748233712436641. [DOI] [PubMed] [Google Scholar]
- Xu J, Yan HC, Yang B, Tong LS, Zou YX, Tian Y. Effects of lead exposure on hippocampal metabotropic glutamate receptor subtype 3 and 7 in developmental rats. Journal of negative results in biomedicine. 2009;8:5. doi: 10.1186/1477-5751-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Sudhof TC. A neural circuit for memory specificity and generalization. Science. 2013;339(6125):1290–1295. doi: 10.1126/science.1229534. [DOI] [PMC free article] [PubMed] [Google Scholar]