Abstract
Background
Prenatal chemical exposures may adversely affect neurodevelopment, but few studies have examined the persistence of these associations. We examined whether associations between prenatal bisphenol A (BPA) or polybrominated diphenyl ether (PBDE) exposures persist or resolve as children age.
Methods
We followed 346 mother-child pairs (enrolled 2003–2006) from Cincinnati, OH from pregnancy until children were 8 years old. We measured BPA in urine collected at 16 and 26 weeks gestation and PBDE-47 in serum collected at 16 weeks gestation. We administered repeated measures of children’s behavior, mental/psychomotor development, and IQ from ages 1–8 years. We determined if associations of BPA or PBDE-47 with child neurobehavior persisted or resolved as children aged using linear mixed models and estimated neurobehavioral measure reproducibility using intraclass correlation coefficients (ICCs).
Results
Higher BPA in girls and higher PBDE-47 in both boys and girls were associated with more externalizing behaviors; these associations persisted from ages 2–8 years (exposure × age interaction p-values≥0.36). Higher PBDE-47 concentrations were associated with decreases in MDI from ages 1–3 years (PBDE-47 × age interaction p-value=0.03) and persistently lower IQ at ages 5 and 8 years (PBDE-47 × age interaction p-value=0.56). Mental/psychomotor abilities had fair reproducibility from ages 1–3 years (ICCs~0.4), cognitive abilities from ages 5 to 8 years had excellent reproducibility (ICCs=0.7–0.8), and parent-reported behaviors from ages 2–8 years had poor to good reproducibility (ICCs=0.38–0.59).
Conclusions
Prenatal BPA and PBDE-47 concentrations were persistently associated with more externalizing behaviors. PBDE-47 concentrations were inversely associated with cognitive abilities that strengthened over time.
Keywords: Children, bisphenol A, epidemiology, halogenated flame retardants, prenatal, neurodevelopment
Introduction
The developing brain is exquisitely sensitive to environmental inputs, and there are many neurotoxicant exposures that increase the risk of neurobehavioral disorders.1,2 However, studies of potential neurotoxicants have generally not considered whether these exposures influence the trajectory of children’s behavior and cognition.3 Two potential neurotoxicants of interest are bisphenol A (BPA) and polybrominated diphenyl ethers (PBDEs); exposure to both is ubiquitous among pregnant women in the United States and associated with decrements in cognitive abilities and behavior problems among children in prior cohort studies.4–11
Determining if the effects of neurotoxicant exposures change over time requires studies with repeated neurobehavioral assessments. Since some epidemiological studies only assess neurobehavior once, it is not possible to determine if the effect of these exposures persists, strengthens, or resolves as children age. Identifying persistent effects is important as toxicant-induced deficits early in life may be a prelude to more severe impairments later in life, as is the case with lead.12 Alternatively, neurotoxicant-associated deficits that manifest in infancy or early childhood may not persist if the plasticity of the developing brain allows for recovery from early life insults. In addition, neurobehavioral trajectories of typically developing children have not been well described, yet understanding variations and progression in development could help define what level of deviation is within normal limits. While some studies have explored this, they are limited by a focus on specific domains (e.g., IQ), use of outdated versions of instruments, or short follow-up intervals.13–17
To address these gaps, we used a prospective birth cohort study of 346 mother-child dyads with repeated measures of child behaviors, mental and psychomotor development, and cognition to determine if previously observed associations of prenatal BPA and PBDE exposure with child behavior and IQ in this cohort were associated with trajectories of neurobehavior between ages 1 and 8 years. We also characterized the variability and patterns of these neurobehavioral measures over the first 8 years of life.
Methods
Study Participants
The Health Outcomes and Measures of the Environment (HOME) Study is a prospective pregnancy and birth cohort that has followed mothers and their children in the greater Cincinnati, OH metropolitan area from the 2nd trimester of pregnancy (March 2003–January 2006) until their singleton children were on average age 8.1 (range: 7.5–10) years (March 2012–July 2014).18,19 We designed the study to assess the relationship between low-level environmental chemical exposures and child neurobehavior. Inclusion criteria for pregnant women at enrollment included: 1) 16±3 weeks gestation, 2) ≥18 years old, 3) living in a home built before 1978, 4) no history of HIV infection, and 5) not taking medications for seizure or thyroid disorders. We enrolled women living in homes built before 1978 in order to study the neurotoxic effects of early life lead exposure, as these homes would be more likely to have lead hazards. All women provided informed consent for themselves and their children. The institutional review boards of Cincinnati Children’s Hospital Medical Center, the cooperating delivery hospitals, and the Centers for Disease Control and Prevention (CDC) approved this study.
Prenatal BPA and PBDE Exposure Assessment
We assessed prenatal BPA exposure by measuring total (free + conjugated) BPA concentrations in urine samples collected in polypropylene specimen cups at 16 and 26 weeks gestation using previously described analytic chemistry methods.20 To account for urine dilution, we measured urinary creatinine concentrations using a kinetic assay and creatinine-standardized BPA concentrations (BPA in ng/mL divided by creatinine in mg/dL).21 If a woman had two urine samples (~95% of women), we averaged log10-transformed creatinine-standardized urinary BPA concentrations; otherwise, we used the available log10-transformed creatinine-standardized concentration. We assessed PBDE exposure by measuring concentrations of PBDE-47, the most abundant PBDE congener in our participants, in serum collected from women at 16 weeks gestation using previously described analytic chemistry methods.22 To account for inter-individual differences in blood lipid concentrations we measured serum triglycerides and total cholesterol concentrations using an enzymatic assay;23 PBDE-47 concentrations were lipid-standardized (PBDE-47 in pg/mL divided by mg/dL of lipids) and log10-transformed for analyses.
Urine and serum samples were collected following the recommended best practices for collecting and processing biospecimens that are to be used for environmental chemical exposure assessment.24 Both urine and serum samples were refrigerated after collection, stored at or below −20° C within 24 hours of collection, and shipped to the CDC laboratories on dry ice where they were analyzed using previously described methods that included quality control samples and reagent blanks.20,22 The limit of detection (LOD) for the BPA assay was 0.4 ng/ml, while the LOD for the PBDE-47 assay was defined as the highest of the lowest point on the calibration curve (0.5 pg/µL) or three times the standard deviation of the method blank concentrations. Concentrations <LOD were assigned a value of LOD/√2.25 The coefficients of variation for quality control samples were <10%.
Infant and Child Neurobehavioral Battery
We repeatedly assessed children’s behaviors, mental and psychomotor development, and cognitive abilities from ages 1 to 8 years (Supplemental Table 1). We administered valid and reliable neurobehavioral instruments that have been used extensively in epidemiological studies and for which measured domains of these instruments have been associated with several early life neurotoxicant exposures (see Supplemental Methods for description of testing procedures).26 For the purpose of this analysis, we focused on neurobehavioral domains previously associated with prenatal exposure to BPA or PBDEs in this cohort; these included behavior measured with the Behavioral Assessment System for Children-2 (BASC-2), mental and psychomotor development measured with the Bayley Scales of Infant Development-II (BSID-II), and child cognitive abilities measured with two age-specific Wechsler instruments (Wechsler Preschool and Primary Scales of Intelligence-III [WPPSI-III] and the Wechsler Intelligence Scale for Children-IV [WISC-IV]).
We evaluated children’s behavior using the preschool and child versions of the BASC-2 at ages 2, 3, 4, 5, and 8 years. The BASC-2 is a valid and reliable parent-reported assessment of a child’s adaptive and problem behaviors in community and home settings.27 We examined three of the BASC-2 composite scales: Externalizing Problems, which reflects disruptive behavior problems; Internalizing Problems, such as depression and anxiety; and the Behavioral Symptoms Index, a measure of a child’s overall level of problem behaviors. We also examined eight clinical subscales (in secondary analysis of reproducibility described below): hyperactivity, aggression, anxiety, depression, somatization, atypicality, withdrawal, and attention.
Higher scores on the BASC-2 indicate worse behaviors, and values ≥60 (i.e., ≥1 standard deviation above the mean) are used to identify children with “at-risk” scores. At-risk scores are indicative of behaviors significant enough to warrant treatment, further evaluation, and assignment of a possible clinical diagnosis.27 Few children had clinically significant scores (≥70) on the BASC-2, so we did not evaluate this as an outcome.
We assessed children’s mental and psychomotor development using the BSID-II at ages 1, 2, and 3 years.28 The BSID-II produces two scaled scores, the mental development index (MDI) and the psychomotor development index (PDI). The MDI assesses cognitive and language abilities, while the PDI assesses gross and fine motor skills. We administered the WPPSI-III and the WISC-IV at ages 5 and 8 years, respectively, to evaluate children’s overall cognitive ability (Full-Scale IQ [FSIQ]), perceptual reasoning and organization skills (Performance IQ [PIQ]), and verbal abilities (Verbal IQ [VIQ]).29,30
Higher scores on the BSDI-II, WPPSI-III, and WISC-IV indicate better performance. Scores ≤70 on the BSID-II, WPPSI-III, and WISC-IV are typically used to identify clinically significant deficits in mental or psychomotor development or cognitive abilities. Because of the small number of children who had scores ≤70, we classified children with scores ≤85 (i.e., ≤1 standard deviation below the mean) as having “at-risk” scores on these instruments.
Statistical Analyses
BPA and PBDE Exposures and Repeated Neurobehavioral Assessments
We conducted two complementary sets of analyses to examine how prenatal exposure to BPA and PBDEs influences child neurobehavior over the first 8 years of life. For these analyses we focused on relations of: 1) prenatal BPA exposure and externalizing behaviors (BASC-2) in girls, and 2) prenatal PBDE exposure with children’s cognitive development (BSID-II MDI and WPPSI-III/WISC-IV FSIQ) and externalizing behaviors (BASC-2) in all children. We chose these relations because we previously observed and reported associations between these specific exposures and neurobehavioral domains in this cohort.4,5 For BPA, we focus on the results from models with girls only because we previously observed sexually dimorphic associations between BPA and children’s neurobehavior.5
In the first analysis we used linear mixed models with a random intercept, a random linear slope for age, a fixed exposure slope, an unstructured within-subject covariance matrix, and robust standard errors to estimate the change in mean neurobehavioral test scores across terciles of BPA or PBDE-47 concentrations and per 10-fold increase. These estimates can be regarded as summary measures of the overall association between BPA/PBDE-47 concentrations and neurobehavioral scores across assessment ages. We included mother-child pairs with complete exposure and covariate data, as well as at least one neurobehavioral measure during follow-up. We extended this analysis and examined whether the rate of neurobehavioral test scores changed across terciles of BPA or PBDE concentrations among mother-child pairs with complete data and at least two measures of the same neurobehavioral test. Our model included terms for BPA or PBDE tercile, child age, covariates, and an interaction between BPA or PBDE tercile and child age. This interaction term allowed us to estimate the rate of change in neurobehavioral test scores in each BPA or PBDE tercile and to test whether the association between BPA or PBDE concentrations and these neurobehavioral outcomes persisted, attenuated, or became greater as children aged. In addition, it let us estimate the subject-specific change in neurobehavioral test score across ages.
We identified potential confounders using directed acyclic graphs that were based on our prior work in this cohort.4,31 We adjusted our models for maternal age at delivery, race, education, IQ, marital status, serum cotinine concentrations during pregnancy, household income, baseline depressive symptoms (Beck Depression Inventory-II), and the quality and quantity of the caregiving environment at age 1 year (Home Observation for Measurement of the Environment Inventory).32,33 Between 11 and 21 children were missing covariate data in the various analyses. Given the relatively small proportion of children missing covariate data, we used complete case analyses and did not impute missing data.
Variability and Patterns of Child Neurobehavior
We conducted additional analyses to describe the magnitude and patterns of variability in longitudinal trajectories of children’s neurobehavior. First, we estimated the reproducibility of the individual test scores across time by calculating the intraclass correlation coefficient (ICC) between repeated neurobehavioral measures using linear mixed models with a compound symmetric within-subject correlation matrix and a fixed linear effect for child age. For equicorrelated measures, ICC values of 0 to <0.4, 0.4 to <0.75, and ≥ 0.75 indicate poor, fair to good, and excellent reproducibility within an individual, respectively.34
We augmented the reproducibility analysis described above by determining whether children with at-risk scores at baseline were more likely to have at-risk scores at subsequent study visits. We did this by modeling the relative risk (RR) of having an at-risk score (i.e., at-risk vs. low-risk) at subsequent study visits as a function of at-risk score status at the baseline study visit using generalized estimating equations with Poisson errors, a logarithmic link, a working unstructured correlation matrix, and robust standard errors.35 We defined at-risk scores as described above.
Finally, we estimated the age-related change in repeated neurobehavioral test scores to describe the average change and variation in neurobehavior. We modeled repeated neurobehavioral measures as a function of child age using a linear mixed model with a random intercept, a random linear slope for age, and an unstructured within-subject covariance matrix. From this model, we used subject-specific effects to estimate the rate of change in neurobehavioral test scores for individual study participants, thus, allowing us to assess the magnitude and patterns of between-subject variability in these longitudinal trajectories.
Sensitivity and Secondary Analysis
In sensitivity analyses, we re-calculated our ICCs among mother-child pairs with ≥2 measures of the same neurobehavioral test to determine if the inclusion of children with only one measure biased our results. Second, we checked the assumption of equicorrelation in calculating our ICCs by comparing the fit of model specified above with several other model parameterizations.
In secondary analyses, we examined the correlation of BSID-II MDI scores at 1, 2, and 3 years of age with WISC-IV FSIQ scores at 8 years of age because many studies of neurotoxicants use the BSID-II MDI score as a measure of early life cognitive abilities. We also described the magnitude and patterns of variability in parent-reported executive function (assessed using the Behavior Rating Inventory of Executive Function) and reciprocal social behaviors (assessed using the Social Responsiveness Scale), as these are also used in studies of neurotoxicants (Supplemental Table 1).
Results
Among participants included in at least one analysis, 65% of mothers were non-Hispanic white and 55% were college educated; 54% of children were female (Supplemental Table 2). A total of 229 (59% of original cohort) children completed follow-up at age 8 years. Children who completed follow-up at age 8 years were similar to the original cohort in terms of baseline sociodemographic characteristics.18 Median maternal urinary BPA and serum PBDE-47 concentrations were 2.0 µg/g creatinine (Range: 0.4–49) and 19 ng/g lipid (Range: 1.5–1,290), respectively. Median maternal BPA (2.0 vs. 1.8 µg/g creatinine) and PBDE-47 (20 vs. 17 ng/g lipid) concentrations during pregnancy were similar among children who did and did not complete follow-up.
BPA and PBDE Exposures and Repeated Neurobehavioral Assessments
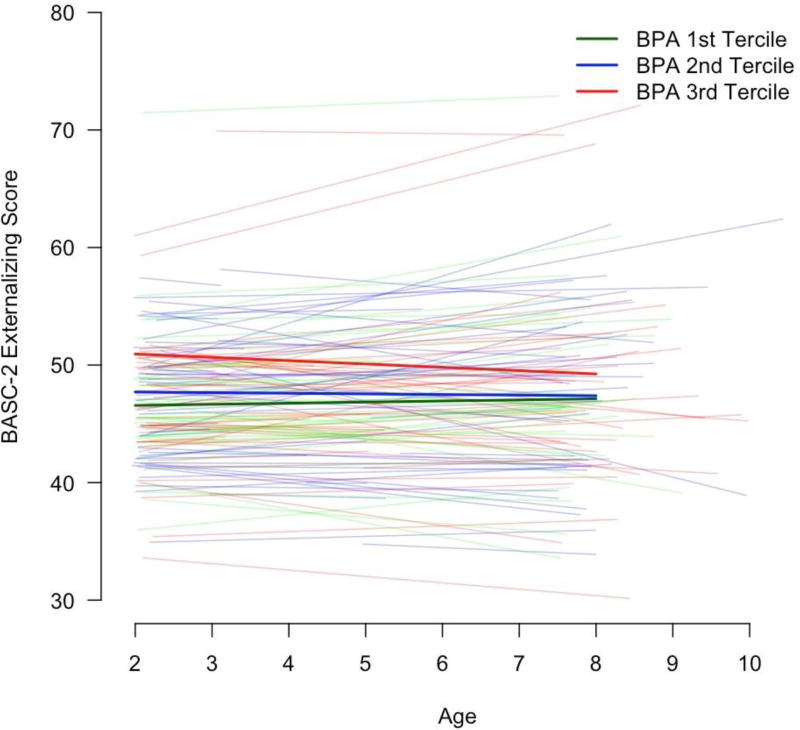
Consistent with our prior findings in this cohort, we observed that prenatal urinary BPA concentrations were associated with increased BASC-2 externalizing scores in girls between age 2 and 8 years (Table 1).5 In girls, each 10-fold increase in maternal urinary BPA concentrations was associated with a 5.9-point increase (95% CI: 1.1, 11) in BASC-2 externalizing scores and nearly 6-times the risk of having a score ≥ 60 (RR=5.8; 95% CI: 1.7, 20). The positive association between maternal urinary BPA concentrations and BASC-2 externalizing scores was stable as girls aged, neither strengthening nor attenuating (BPA tercile × age p-value=0.36). Specifically, on average, girls in the 1st, 2nd, and 3rd terciles of BPA concentrations had annual changes in BASC-2 externalizing scores of 0.1 (95% CI: −0.3, 0.5), – 0.1 (95% CI: −0.8, 0.5), and −0.3 (−0.8, 0.2), respectively (Figure 1, Supplemental Table 3). Consistent with our prior findings, BPA concentrations were not associated with externalizing behaviors in boys (Supplemental Table 4) and child sex modified the association between BPA and repeated measures of externalizing behaviors (BPA × sex interaction p-value=0.01). Prenatal BPA concentrations were not associated with child MDI, PDI, or FSIQ scores or with their trajectories over time (results not shown).
Table 1.
Adjusted difference in BASC-2 externalizing scores and relative risk of clinically significant scores (≥60) among girls between ages 2 and 8 years by prenatal urinary BPA concentrations a
| Average Difference in BASC-2 score or RR of At-Risk Scores (N=178, # repeats=603)b |
||
|---|---|---|
|
| ||
| Urinary BPA Concentration | Difference (95% CI) |
RR (95% CI) |
| Tercile 1 (0.4–1.6 µg/g Cr) | Ref | Ref |
| Tercile 2 (1.6–2.6 µg/g Cr) | 0.9 (−1.7, 3.5) | 1.1 (0.4, 3.2) |
| Tercile 3 (2.6–49 µg/g Cr) | 3.2 (0.5, 5.9) | 2.3 (1.0, 5.6) |
| Per 10-fold | 5.7 (0.8, 11) | 5.8 (1.7, 20) |
-Adjusted for maternal age at delivery, maternal race (white or non-white), maternal education (college, some college, ≤ high school), marital status (married or unmarried), serum cotinine concentrations during pregnancy, household income, maternal IQ, maternal depressive symptoms at baseline, and caregiving environment score.
-Model is estimating the average change in BASC-2 externalizing scores or RR of having an externalizing score ≥ 60 across all visits. Thus, the point estimates represent the average change in BASC-2 scores or RR with increasing prenatal urinary BPA concentrations across all visits.
Figure 1.
Average and subject-specific BASC-2 externalizing behavior scores among HOME Study girls between ages 2 and 8 years by prenatal urinary BPA concentration tercilea,b
a-Adjusted for maternal age at delivery, maternal race (white or non-white), maternal education (college, some college, ≤ high school), marital status (married or unmarried), serum cotinine concentrations during pregnancy, household income, materna IQ, maternal depressive symptoms at baseline, and caregiving environment score
b-A linear mixed model was used to estimate the average and subject-specific change in externalizing scores from 2–8 years of age in each tercile of prenatal urinary BPA concentrations. The thicker lines show the average trajectory in each BPA tercile, while the individual lines show the subject-specific change for each of the 148 girls. The model included an age × BPA interaction term, which allows BASC-2 scores to change as a linear function of both age and BPA tercile. This interaction term was not significant (p-value=0.36), indicating that externalizing scores did not increase or decrease as children aged for a given level of BPA.
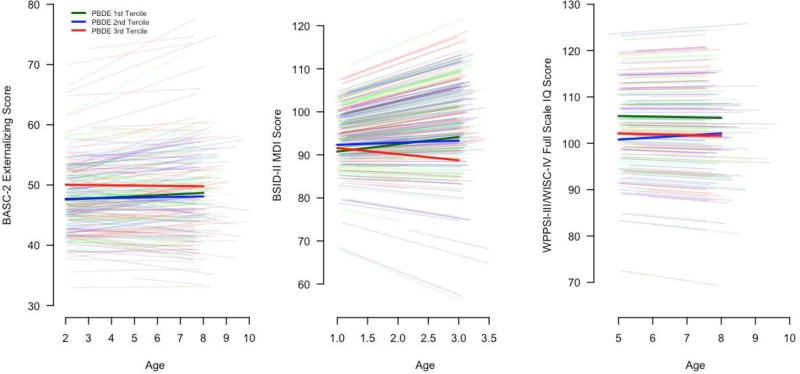
Each 10-fold increase in prenatal PBDE-47 concentration was associated with 2.0-point higher (95% CI: −0.1, 4.2) externalizing behavior scores between ages 2 and 8 years and 2-times greater risk of having a score ≥ 60 (RR: 2.0; 95% CI: 1.1, 3.6) (Table 2). The association between PBDE-47 and BASC-2 externalizing scores did not vary as children aged, suggesting that the association persisted (PBDE-47 tercile × age p-value=0.67) (Figure 2, Supplemental Table 5). Higher prenatal PBDE-47 concentrations were not associated with MDI scores at age 1 year, but PBDE-47 were inversely associated with MDI scores at age 2 and 3 years. On average, children born to women in the 3rd PBDE-47 tercile had a 1.4-point decline in MDI scores per year (95% CI: −3.3, 0.4). In contrast, MDI scores increased 1.7-point per year (95% CI: 0.2, 3.2) among children in the 1st tercile (PBDE-47 tercile × age p-value=0.04) (Figure 2, Supplemental Table 5). The inverse association between prenatal PBDE-47 and mental development at 3 years of age was consistent with the inverse association between PBDE-47 and FSIQ at 5 and 8 years of age (βper 10-fold: −4.1; 95% CI: −8.3, 0.1). No evidence emerged that the association between PBDE-47 and FSIQ changed between 5 and 8 years of age by PBDE-47 tercile (Figure 2, Supplemental Table 5) (PBDE-47 tercile×age p-value=0.56).
Table 2.
Adjusted difference in BASC-2 externalizing, BSID-II MDI, and WPPSI-III/WISC-IV FSIQ scores and relative risk of clinically significant scores between ages 2 and 8 years by prenatal serum PBDE-47 concentrations a
| Average Difference in score or RR of At-Risk Scoresb |
||
|---|---|---|
|
| ||
| Neurobehavior Scale | Difference (95% CI) |
RRc (95% CI) |
| BASC-2 Externalizing (2–8 Years of Age, n=274, repeats=1,024) | ||
| Tercile 1 (1.5–13 ng/g lipid) | Ref | Ref |
| Tercile 2 13–26 ng/g lipid) | 0.1 (−1.6, 1.8) | 1.5 (0.7, 3.3) |
| Tercile 3 (27–1,290 ng/g lipid) | 2.0 (0, 4.0) | 2.3 (1.0, 5.3) |
| Per 10-fold | 2.0 (−0.2, 4.2) | 2.0 (1.1, 3.6) |
| BSID-II MDI (1–3 Years of Age, n=291, repeats=752) | ||
| Tercile 1 (1.5–13 ng/g lipid) | Ref | Ref |
| Tercile 2 13–26 ng/g lipid) | 0.5 (−1.8, 2.9) | 0.9 (0.7, 1.3) |
| Tercile 3 (27–1,290 ng/g lipid) | −1.9 (−4.5, 0.7) | 1.3 (0.9, 1.8) |
| Per 10-fold | −1.3 (−3.7, 1.1) | 1.1 (0.8, 1.5) |
| WPPSI-III/WISC-IV FSIQ (5–8 Years of Age, n=219, repeats=373) | ||
| Tercile 1 (1.5–13 ng/g lipid) | Ref | Ref |
| Tercile 2 13–26 ng/g lipid) | −4.3 (−8.1, −0.5) | 1.0 (0.4, 2.1) |
| Tercile 3 (27–1,290 ng/g lipid) | −5.0 (−9.1, −0.9) | 0.8 (0.4, 1.8) |
| Per 10-fold | −4.1 (−8.3, 0.1) | 1.1 (0.4, 2.7) |
-Adjusted for maternal age at delivery, maternal race (white or non-white), maternal education (college, some college, ≤ high school), marital status (married or unmarried), serum cotinine concentrations during pregnancy, household income, maternal IQ, maternal depressive symptoms at baseline, and caregiving environment score.
-Model is estimating the average change in neurobehavioral test score or RR of having an at-risk score across all visits. Thus, the point estimates represent the average change in test scores or RR with increasing prenatal serum PBDE concentrations across all visits.
-Clinically significant scores for the BASC-2, MDI, and FSIQ are ≥ 60, ≤85, and ≤ 85, respectively.
Figure 2.
Average and subject-specific BASC-2 externalizing behavior, BSID-III MDI, and WPPSI-III/WISC-IV FSIQ scores among HOME Study children between ages 1 and 8 years by prenatal serum PBDE-47 concentration tercilea,b
a-Adjusted for maternal age at delivery, maternal race (white or non-white), maternal education (college, some college, ≤ high school), marital status (married or unmarried), serum cotinine concentrations during pregnancy, household income, maternal IQ, maternal depressive symptoms at baseline, and caregiving environment score
b-A linear mixed model was used to estimate the average and subject-specific change in externalizing scores (n=251) from age 2–8 years, mental development scores from age 1–3 years (n=250), and full scale IQ from age 5–8 years (n=154) in each tercile of prenatal serum PBDE-47 concentration. The thicker lines show the average trajectory of score in each PBDE-47 tercile, while the individual lines show the subject-specific change for each child. Each model included an age × PBDE-47 interaction term, which allowed the scores to change as a linear function of both age in each PBDE-47 tercile. This interaction term was not significant for externalizing (p-value=0.67) or full scale IQ scores (p-value=0.56), but was for mental development scores (p-value=0.04).
Variability and Patterns of Child Neurobehavior
The reproducibility of repeated neurobehavioral test scores varied within and across tests (Table 3 and Supplemental Table 6). For instance, repeated BASC-2 externalizing scores had good reproducibility (ICC=0.57), whereas BASC-2 anxiety scores had poor reproducibility (ICC=0.38). BSID-II MDI (ICC=0.42) and PDI (ICC=0.39) scores had poor to fair reproducibility, respectively. FSIQ and VIQ scores had excellent reproducibility (ICCs=0.80) and PIQ had good reproducibility (ICC=0.69). Children who had clinically significant neurobehavioral test scores at baseline were at increased risk of having a clinically significant test score at a subsequent visit compared to children who had a low-risk score at baseline. In general, the magnitude of the relative risk of having a clinically significant test score at a subsequent visit followed the magnitude of the corresponding scale’s ICC (Table 1). For instance, children with a BASC-2 externalizing score ≥60 at baseline had 4.6-times the risk (95% CI: 2.8, 7.5) of an at-risk score at a subsequent visit compared to children with scores < 60 at baseline.
Table 3.
Correlation and patterns of BASC-2, BSID-II, and WPPSI-III/WISC-IV scores among HOME Study children
| Neurobehavioral Measure (Age, Years) Administered |
N (Repeats) |
Between- Child Variance |
Within- Child Variance |
ICCa | Mean Change per Year (Range) |
25th, 75th Percentile of Change per Year |
N (%) with Clinically Significant Score at Baseline |
RR of Clinically Significant Score at Follow-up Visits (95% CI)b |
|---|---|---|---|---|---|---|---|---|
| BASC-2 (2, 3, 4, 5, and 8) | 323 (1,150) | |||||||
| BSI | 42.6 | 29.4 | 0.59 | −0.3 (−1.5, 1.5) | −0.6, −0.2 | 23 (8) | 5.8 (3.5, 9.6) | |
| Externalizing | 44.9 | 34.4 | 0.57 | 0.1 (−1.6, 2.3) | −0.2, 0.3 | 23 (8) | 4.6 (2.8, 7.5) | |
| Internalizing | 42.4 | 39.3 | 0.52 | 0.1 (−0.6, 0.8) | 0, 0.2 | 11 (4) | 5.7 (3.2, 10) | |
| BSID-II (1, 2, and 3) | 328 (839) | |||||||
| MDI | 67.5 | 96.8 | 0.41 | −0.0 (−9.8, 6.8) | −1.8, 2.0 | 67 (24) | 1.7 (1.3, 2.3) | |
| PDI | 74.9 | 122.5 | 0.38 | 3.1 (−6.6, 9.5) | 1.7, 4.1 | 97 (35) | 2.2 (1.6, 3.1) | |
| WPPSI-III/WISC-IV (5 and 8) | 251 (418) | |||||||
| FSIQ | 194.2 | 48.8 | 0.80 | 0.1 (−0.8, 0.5) | −0.2, 0.1 | 24 (14) | 17 (7.4, 39) | |
| VIQ | 187.1 | 49.6 | 0.79 | −0.5 (−0.8, −0.3) | −0.6, −0.5 | 24 (14) | 7.4 (3.9, 14) | |
| PIQ | 193.7 | 77.6 | 0.71 | 1.3 (−3.7, 5.7) | 0.7, 1.5 | 24 (14) | 5.3 (2.4, 12) |
-Intraclass correlation coefficient is the ratio of the between-person variation to the sum of the between- and within-person variation.
-Relative risk of having at-risk test scores at any subsequent visit compared to children with low-risk test scores at baseline. At risk scores were ≥ 60 for the BASC-2, ≤ 85 for the BSID-II, and ≤ 85 for the WPPSI-III and WISC-IV.
On average, most repeated neurobehavioral test scores changed <0.5 points per year and declined between 2 and 8 years. Notably, BASC-2 anxiety scores increased an average of 0.6 points per year (Range: 0, 2.0) and atypicality scores decreased an average of 0.7 points per year (Range: −1.9, 1.6). BSID-II PDI scores increased an average of 3.1 (Range: −6.4, 9.6) points per year between 1 and 3 years of age, while FSIQ scores remained stable between age 5 and 8 years.
Sensitivity and Secondary Analyses
ICCs were similar when we restricted our analyses to children who had at least two measures of the same neurobehavioral test. The equicorrelation assumption we made when calculating the ICC was not satisfied for most BASC-2 scales, some executive function scales, the MDI, and the measure of reciprocal social behavior.
The correlations between FSIQ at age 8 years and BSID-II MDI scores was stronger at 2 and 3 years of age (Pearson r=0.61, r=0.67, respectively) compared to 1 year of age (Pearson r=0.22). The reproducibility of parent-reported executive function was poor to fair and parent-reported reciprocal social behaviors had good reproducibility (Supplemental Table 6).
Discussion
Using a prospective pregnancy and birth cohort study, we investigated the persistence of associations between prenatal BPA or PBDE exposures with repeated measures of children’s neurobehavior. We also quantified the reproducibility and patterns of longitudinal measurements of children’s cognition and behavior from 1 to 8 years of age. Three new findings emerge from this work. First, higher prenatal urinary BPA and serum PBDE-47 concentrations were associated with persistent increases in externalizing behaviors and risk of at-risk externalizing behavior scores from age 2–8 years among girls and all children, respectively. Second, higher prenatal serum PBDE-47 concentrations were associated with declining mental development from age 1–3 years and persistent decrements in cognitive abilities from age 5–8 years. Third, the stability of repeated neurobehavioral measures depended on the specific domain. Mental and psychomotor development varied substantially from age 1–3 years, but cognitive abilities were stable from age 5–8 years. Most parent-reported behaviors showed fair to good correlations from age 2–8 years.
Previous studies, including ones from this cohort, have reported that prenatal BPA and PBDE exposures are associated with neurobehavioral decrements in children.4–8,10,36–39 The presented work extends our prior findings from this cohort and shows that associations between prenatal urinary BPA concentrations and externalizing behaviors in girls endure to school-age.5 Prior reports from the HOME Study and other United States cohorts reported that prenatal serum PBDE concentrations were associated with cognitive decrements, increased externalizing behaviors, more attention problems, and impairments in executive function; many of these associations were observed in school-aged children.4,6–10 The presented findings show that our previously observed associations between prenatal serum PBDE-47 concentrations and child neurobehavior persist to school age.
This study is the first to formally test whether associations between prenatal BPA or PBDE exposures persist as children age, but we were unable to examine the potential mechanisms of this observation. This remains an important area of research as the developing brain may be able to adapt to neurotoxicant insults because of its plasticity and associations between prenatal toxicant exposures and child neurobehavior may resolve as children age.40 On the other hand, the developing brain may be exquisitely sensitive to neurotoxicant exposures and disruption of neurulation, synaptogenesis, or neuronal differentiation, proliferation, or migration during critical periods of development could increase the risk of behavioral disorders or cognitive deficits.1 The need to formally evaluate trajectories of neurobehavior is highlighted by the observed decline in children’s MDI scores as they aged with increasing prenatal serum PBDE-47 concentrations. This association would have been missed if we had only one measure of cognitive development during infancy.
There are a variety of mechanisms by which prenatal BPA or PBDE exposures may adversely affect children’s neurodevelopment. In animal and in vitro studies, BPA and PBDEs can affect the synthesis, action, transport, or metabolism of neurotransmitters, thyroid hormones, or gonadal hormones.41,42 Thyroid hormones are critical for proper neurodevelopment and may be a mechanism underlying these associations given evidence from this cohort suggesting that BPA and PBDEs can affect thyroid hormone homeostasis during gestation and in the neonate.43–45 The ability of BPA to act on gonadal hormone systems may explain the sexually dimorphic associations we and others have observed since gonadal hormones play an important role in sexually-dimorphic neurodevelopment.46 We are not aware of specific mechanisms that would explain why prenatal PBDE-47 concentrations were associated with more rapid declines in children’s MDI scores, but we speculate that PBDE exposure could set off a cascade of events that do not manifest until later in childhood or affect postnatal neurodevelopmental events such as synaptic pruning or myelination. Alternatively, the reduced misclassification of children’s cognitive abilities as they age may enhance our ability to detect associations of prenatal neurotoxicant exposures with cognitive measures at later ages. This hypothesis is supported by the greater reproducibility of cognitive measures at ages 5 and 8 years compared to ages 1 to 3 years and the stronger correlation between cognitive measures at ages 3 and 8 years compared to ages 1 and 8 years.
In these data, prenatal BPA and PBDE exposures were persistently associated with poorer neurobehavioral outcomes. However, additional studies estimating the impact of prenatal BPA and PBDE exposures on adolescent or adult outcomes are warranted. Prior studies suggest that associations between childhood lead exposure and cognitive decrements precede more profound neurobehavioral sequelae including criminal behavior in adulthood and structural changes in the brain of adults.12,47,48 The results of the present study and prior studies of BPA, PBDEs, and lead suggest that early life exposure to some neurotoxicants may place children on a trajectory of development from which they cannot recover. Because prior studies show that neurobehavioral trajectories during childhood and adolescence are important predictors of adult health outcomes, it is necessary to consider both the relative change in children’s neurobehavior as they age, as well as the absolute differences in neurobehavior at any age.49
It will be important to determine if the potential effects of prenatal exposures continue to influence neurobehavior after adolescence, a period of dramatic changes in the brain.50 Moreover, neurotoxic or endocrine disrupting exposures during adolescence might adversely affect adolescent neurodevelopment. This is particularly relevant for chemicals like BPA and PBDEs that may affect gonadal hormones, which in turn play an important role in adolescent brain development.51,52 Consistent with the presented findings, prior studies show that repeated measures of IQ and behavior have good to excellent correlation over intervals <1 year.13–17 Previously observed correlations between repeated IQ measures over periods >1 year are also consistent with our results; two studies from the United States and New Zealand reported that repeated IQ measures were highly correlated over a period of ~3 to 6 years (R>0.72) and that most children had similar full scale IQ scores at multiple visits.14,15 A prior study of 4–16 year old Dutch children found a high degree of correlation between repeated measures of parent-reported behavior (Child Behavior Checklist) over a period of up to 8 years,16,17 which is consistent with our findings. When it is not feasible or of interest to obtain repeated neurobehavioral measures, our results show that a single measurement of IQ would be sufficient to achieve reproducibility at a level of 0.8 (0.8 = 194.2/(194.2 + 48.8/1) [reproducibility calculated from variance components in Table 3, n of 1 used for number of repeated measures to achieve reproducibility at a level of 0.8). However, for parent-reported measures of behavior, the Spearman-Brown “prophecy” formula suggests that two or more repeated measures may be necessary to achieve reproducibility at a level of 0.8.53 It should be noted that the equicorrelation assumption was not satisfied for all neurobehavioral measures in this study, thus, the presented ICCs should be interpreted cautiously.
In this cohort of typically developing children, the average annual rate of change in most neurobehavioral domains was small (<0.5 points per year). However, our subject-specific analyses indicated that there were some children who had annual rates of change ≥ 1 point per year, particularly on the BASC-2 and BSID-II scales. Some of the within-person variation in these neurobehavioral domains likely reflects the dynamic nature of development in the first few years of life, but the role of environmental factors in this variation is not well understood. Thus, future studies should consider how a broad suite of early life factors influences this variation in neurobehavioral trajectories, as well as its consequences in later life.
The strengths of this study include the prospective assessment of prenatal BPA and PBDE exposure, detailed and longitudinal assessment of children’s neurobehavior from infancy to school-age, and use of linear mixed models to evaluate changes in children’s neurobehavioral scores as a function of their prenatal BPA and PBDE exposures. There are some limitations that should be acknowledged. First, there are other methods to examine trajectories of neurobehavioral scores, like latent class mixed models; these can identify distinct classes of neurobehavioral scores that vary over the life course.49 We initially applied these methods, but resulting classes had insufficient numbers of children to support subsequent analyses. Thus, this is a direction for future studies with larger sample sizes. Second, while we adjusted for many potential confounders, adjusting for other factors associated with higher BPA or PBDE exposure and poorer child neurobehavioral scores may attenuate the observed associations towards the null. Finally, we did not evaluate whether there are periods of heightened vulnerability to BPA or PBDE exposures or if postnatal exposures contributed to neurobehavioral trajectories, but there are statistical methods for identifying discrete windows of vulnerability.54 In the case of postnatal BPA exposures, which varies substantially within a person, it would be possible to use time-varying BPA exposures to estimate both the cross-sectional and longitudinal effects of BPA exposure on neurobehavior using the mixed model framework.19,55
Conclusion
We used repeated measures of neurobehavior in a cohort of typically developing children to show how two prevalent neurotoxicant exposures may impact both the absolute level and trajectory of child behavior and cognition. Prenatal BPA exposure was associated with persistent increases in externalizing behaviors among girls from ages 2–8 years. Prenatal PBDE exposure was associated with persistent increases in externalizing behaviors from ages 2–8 years, declines in mental development from ages 1–3 years, and persistent decrements in IQ from ages 5–8 years. Given that higher prenatal PBDE-47 concentrations were associated with declines in children’s mental development scores from ages 1–3 years and persistent decrements in IQ, future studies should consider whether PBDE exposures have lingering effects that do not manifest until later in childhood.
Supplementary Material
Highlights.
Prenatal BPA and PBDE exposures were associated with more externalizing behaviors.
Prenatal PBDE exposure was associated with poorer cognitive abilities.
The association between PBDE and cognitive abilities strengthened as children aged.
Reproducibility of neurobehavioral measures from age 1–8 years varied by domain.
Acknowledgments
We thank Drs. Antonia Calafat and Andreas Sjodin and their laboratory staff at the Centers for Disease Control and Prevention for analyzing maternal urinary bisphenol A and serum polybrominated diphenyl ether concentrations.
Sources of Financial Support: This work was supported by NIEHS grants R00 ES020346, R01 ES024381, P01 ES11261, RO1 ES01475, and R01 ES020349.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests: The authors have no competing financial interests to declare.
Obtaining Data: The HOME Study principal investigators have actively engaged in collaborative data-sharing. Investigators should contact Drs. Joseph M. Braun [joseph_braun_1@brown.edu] or Kimberly Yolton (kimberly.yolton@cchmc.org) to obtain data or code from this paper. Requests for data require that investigators submit a project proposal which is evaluated by the HOME Study Data Sharing Committee.
References
- 1.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet neurology. 2014;13(3):330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellinger DC, Matthews-Bellinger JA, Kordas K. A developmental perspective on early-life exposure to neurotoxicants. Environment international. 2016;94:103–112. doi: 10.1016/j.envint.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Chen A, Yolton K, Rauch SA, et al. Prenatal polybrominated diphenyl ether exposures and neurodevelopment in U.S. children through 5 years of age: the HOME study. Environmental health perspectives. 2014;122(8):856–862. doi: 10.1289/ehp.1307562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun JM, Kalkbrenner AE, Calafat AM, et al. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. 2011;128(5):873–882. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowell WJ, Lederman SA, Sjodin A, et al. Prenatal exposure to polybrominated diphenyl ethers and child attention problems at 3–7 years. Neurotoxicology and teratology. 2015;52(Pt B):143–150. doi: 10.1016/j.ntt.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eskenazi B, Chevrier J, Rauch SA, et al. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environ Health Perspect. 2013;121(2):257–262. doi: 10.1289/ehp.1205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herbstman JB, Mall JK. Developmental Exposure to Polybrominated Diphenyl Ethers and Neurodevelopment. Curr Environ Health Rep. 2014;1(2):101–112. doi: 10.1007/s40572-014-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sagiv SK, Kogut K, Gaspar FW, et al. Prenatal and childhood polybrominated diphenyl ether (PBDE) exposure and attention and executive function at 9–12years of age. Neurotoxicology and teratology. 2015 doi: 10.1016/j.ntt.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vuong AM, Yolton K, Webster GM, et al. Prenatal polybrominated diphenyl ether and perfluoroalkyl substance exposures and executive function in school-age children. Environmental research. 2016;147:556–564. doi: 10.1016/j.envres.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect. 2011;119(6):878–885. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright JP, Dietrich KN, Ris MD, et al. Association of prenatal and childhood blood lead concentrations with criminal arrests in early adulthood. PLoS medicine. 2008;5(5):e101. doi: 10.1371/journal.pmed.0050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iverson GL. Pediatric Forensic Neuropsychology. Oxford: Oxford University Press; 2012. [Google Scholar]
- 14.Moffitt TE, Caspi A, Harkness AR, Silva PA. The natural history of change in intellectual performance: who changes? How much? Is it meaningful? Journal of child psychology and psychiatry, and allied disciplines. 1993;34(4):455–506. doi: 10.1111/j.1469-7610.1993.tb01031.x. [DOI] [PubMed] [Google Scholar]
- 15.Watkins MW, Smith LG. Long-term stability of the Wechsler Intelligence Scale for Children--Fourth Edition. Psychol Assess. 2013;25(2):477–483. doi: 10.1037/a0031653. [DOI] [PubMed] [Google Scholar]
- 16.Verhulst FC, van der Ende J. Six-year stability of parent-reported problem behavior in an epidemiological sample. Journal of abnormal child psychology. 1992;20(6):595–610. doi: 10.1007/BF00911243. [DOI] [PubMed] [Google Scholar]
- 17.Verhulst FC, Van der Ende J. The eight-year stability of problem behavior in an epidemiologic sample. Pediatric research. 1995;38(4):612–617. doi: 10.1203/00006450-199510000-00023. [DOI] [PubMed] [Google Scholar]
- 18.Braun JM, Kalloo G, Chen A, et al. Cohort Profile: The Health Outcomes and Measures of the Environment (HOME) study. International journal of epidemiology. 2016 doi: 10.1093/ije/dyw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stacy SL, Eliot M, Calafat AM, et al. Patterns, Variability, and Predictors of Urinary Bisphenol A Concentrations during Childhood. Environmental science & technology. 2016;50(11):5981–5990. doi: 10.1021/acs.est.6b00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye X, Bishop AM, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for measuring parabens, triclosan, and other environmental phenols in human milk. Anal Chim Acta. 2008;622(1–2):150–156. doi: 10.1016/j.aca.2008.05.068. [DOI] [PubMed] [Google Scholar]
- 21.Larsen K. Creatinine assay in the presence of protein with LKB 8600 Reaction Rate Analyser. Clinica chimica acta; international journal of clinical chemistry. 1972;38(2):475–476. doi: 10.1016/0009-8981(72)90146-5. [DOI] [PubMed] [Google Scholar]
- 22.Jones R, Anderson S, Zhang Y, Edenfield E, Sjodin A. Semi-automated extraction and cleanup method for the measurement of organohalogen compounds and halogenated phenols in human serum. Paper presented at: Proceedings of Dioxin; 2010; San Antonio, TX. [Google Scholar]
- 23.Phillips DL, Pirkle JL, Burse VW, Bernert JT, Jr, Henderson LO, Needham LL. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Archives of environmental contamination and toxicology. 1989;18(4):495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- 24.Calafat AM. Contemporary Issues in Exposure Assessment Using Biomonitoring. Current epidemiology reports. 2016;3(2):145–153. doi: 10.1007/s40471-016-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hornung RW, Reed LD. Estimation of Average Concentration in the Presence of Nondetectable Values. Applied occupational and environmental hygiene. 1990;5(1):46–51. [Google Scholar]
- 26.Dietrich KN, Eskenazi B, Schantz SL. Principles and practices of neurodevelopmental assessment in children: lessons learned from the Centers for Children's Environmental Health and Disease Prevention Research. Environ Health Perspect. 2005;113(10):1437–1446. doi: 10.1289/ehp.7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynolds CR, Kamphaus RW. Behavior Assessment System for Children. Bloomington, MN: Pearson; 2002. [Google Scholar]
- 28.Bayley N. Bayley Scales of Infant Devleopment. 2. San Antonio TX: The Psychological Corporation; 1993. [Google Scholar]
- 29.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. Third. San Antonio, TX: The Psychological Corporation; 2002. [Google Scholar]
- 30.Wechsler D. Wechsler Intelligence Scale for Children-IV. 3. San Antonio: The Psychological Corporation; 2003. [Google Scholar]
- 31.Braun JM, Kalkbrenner AE, Calafat AM, et al. Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environ Health Perspect. 2011;119(1):131–137. doi: 10.1289/ehp.1002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beck AT, Steer RA, Brown GK. Beck Depression Inventory - 2nd Edition (BDI-II) San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- 33.Caldwell B, Bradley R. HOME Inventory Administration Manual. Little Rock, AK: University of Arkansas at Little Rock; 2003. [Google Scholar]
- 34.Rosner B. Fundamentals of Biostatistics. 4. Pacific Grove, CA: Duxbury; 2000. [Google Scholar]
- 35.Zou G. A modified poisson regression approach to prospective studies with binary data. American journal of epidemiology. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 36.Roen EL, Wang Y, Calafat AM, et al. Bisphenol A exposure and behavioral problems among inner city children at 7–9 years of age. Environ Res. 2015 doi: 10.1016/j.envres.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harley KG, Gunier RB, Kogut K, et al. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environmental research. 2013 doi: 10.1016/j.envres.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evans SF, Kobrosly RW, Barrett ES, et al. Prenatal Bisphenol A Exposure and maternally reported behavior in boys and girls. Neurotoxicology. 2014 doi: 10.1016/j.neuro.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casas M, Forns J, Martinez D, et al. Exposure to bisphenol A during pregnancy and child neuropsychological development in the INMA-Sabadell cohort. Environmental research. 2015;142:671–679. doi: 10.1016/j.envres.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 40.Johnston MV. Plasticity in the developing brain: implications for rehabilitation. Developmental disabilities research reviews. 2009;15(2):94–101. doi: 10.1002/ddrr.64. [DOI] [PubMed] [Google Scholar]
- 41.Dingemans MM, van den Berg M, Westerink RH. Neurotoxicity of brominated flame retardants: (in)direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system. Environmental health perspectives. 2011;119(7):900–907. doi: 10.1289/ehp.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mustieles V, Perez-Lobato R, Olea N, Fernandez MF. Bisphenol A: Human exposure and neurobehavior. Neurotoxicology. 2015;49:174–184. doi: 10.1016/j.neuro.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Romano ME, Webster GM, Vuong AM, et al. Gestational urinary bisphenol A and maternal and newborn thyroid hormone concentrations: The HOME Study. Environmental research. 2015;138:453–460. doi: 10.1016/j.envres.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vuong AM, Webster GM, Romano ME, et al. Maternal Polybrominated Diphenyl Ether (PBDE) Exposure and Thyroid Hormones in Maternal and Cord Sera: The HOME Study, Cincinnati, USA. Environmental health perspectives. 2015;123(10):1079–1085. doi: 10.1289/ehp.1408996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghassabian A, Henrichs J, Tiemeier H. Impact of mild thyroid hormone deficiency in pregnancy on cognitive function in children: lessons from the Generation R Study. Best practice & research Clinical endocrinology & metabolism. 2014;28(2):221–232. doi: 10.1016/j.beem.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Cohen-Bendahan CC, van de Beek C, Berenbaum SA. Prenatal sex hormone effects on child and adult sex-typed behavior: methods and findings. Neuroscience and biobehavioral reviews. 2005;29(2):353–384. doi: 10.1016/j.neubiorev.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Mazumdar M, Bellinger DC, Gregas M, Abanilla K, Bacic J, Needleman HL. Low-level environmental lead exposure in childhood and adult intellectual function: a follow-up study. Environ Health. 2011;10(1):24. doi: 10.1186/1476-069X-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cecil KM, Brubaker CJ, Adler CM, et al. Decreased brain volume in adults with childhood lead exposure. PLoS medicine. 2008;5(5):e112. doi: 10.1371/journal.pmed.0050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Odgers CL, Moffitt TE, Broadbent JM, et al. Female and male antisocial trajectories: from childhood origins to adult outcomes. Development and psychopathology. 2008;20(2):673–716. doi: 10.1017/S0954579408000333. [DOI] [PubMed] [Google Scholar]
- 50.Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28(1):62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peper JS, Hulshoff Pol HE, Crone EA, van Honk J. Sex steroids and brain structure in pubertal boys and girls: a mini-review of neuroimaging studies. Neuroscience. 2011;191:28–37. doi: 10.1016/j.neuroscience.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 52.Vigil P, Orellana RF, Cortes ME, Molina CT, Switzer BE, Klaus H. Endocrine modulation of the adolescent brain: a review. J Pediatr Adolesc Gynecol. 2011;24(6):330–337. doi: 10.1016/j.jpag.2011.01.061. [DOI] [PubMed] [Google Scholar]
- 53.Cappelleri JC, Zou KH, Bushmakin AG, Alvir JMJ, Alemayehu D, Symonds T. Patient-Reported Outcomes: Measurement, Implementation and Interpretation. Boca Raton, Florida: Chapman & Hall/CRC Press; 2014. [Google Scholar]
- 54.Sanchez BN, Hu H, Litman HJ, Tellez-Rojo MM. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environmental health perspectives. 2011;119(3):409–415. doi: 10.1289/ehp.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Data Analysis. 2. Hoboken: Wiley; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.