Abstract
Introduction
Gestational phthalate exposures have been adversely associated with attention, externalizing, and internalizing behaviors in childhood. Early childhood temperament may be a marker of later behavioral patterns. We therefore sought to determine whether gestational phthalate exposures were associated with infant and toddler temperament.
Methods
The Mount Sinai Children’s Environmental Health Study is a prospective cohort study of children born between May 1998 and July 2001 in New York City (N=404). Phthalate metabolites were measured in spot urine samples collected from pregnant women in their third trimester. Child temperament was assessed by parental report at 12-months using the Infant Behavior Questionnaire (IBQ) (N=204) and at 24-months using the Toddler Behavior Assessment Questionnaire (TBAQ) (N=279). We used multiple linear regression to evaluate associations between urinary phthalate metabolites and eleven temperament domains.
Results
Phthalate biomarker concentrations were weakly associated with lower gross motor activity levels as well as higher duration of orienting at the 12-month assessment. Mono(3-carboxypropyl) phthalate (MCPP), monobenzyl phthalate (MBzP) and the sum of metabolites of di(2-ethylhexyl) phthalate (ΣDEHP) were associated with lower levels of smiling and laughing at 12 months. At 24-months, social fear and lower pleasure was linked to higher concentrations of MCPP and MBzP, and higher ΣDEHP was weakly associated with increased anger levels at 24-months.
Conclusions
Though we observed some weak associations between biomarkers of prenatal exposure to phthalates and temperament at 12- and 24-months, overall phthalates biomarkers were not strongly associated with alterations in temperament.
Keywords: Phthalate, Temperament, Environmental exposure, Infant, Toddler
1. Introduction
Temperament has been conceptualized as enduring individual differences in behavioral propensities (Rothbart et al. 2000). Early childhood temperament patterns have been suggested to be potential precursors or modifiers of later psychopathology (reviewed in Nigg 2006; De Pauw and Mervielde 2010), including Attention-Deficit/Hyperactivity Disorder (ADHD) (Nigg 2006; Willoughby et al. 2016) and internalizing disorders (Nigg 2006). While twin studies suggest moderate genetic heritability of temperament (20–60%) (Saudino 2005), it is also suspected that environmental factors influence its development. For example, studies have found associations between certain dimensions of temperament and socioeconomic status (Jansen et al. 2009), prenatal drug use (Locke et al. 2016; Weiss et al. 2007; Richardson et al. 2008), prenatal binge drinking (Molteno et al. 2014; Alvik et al. 2011), maternal depression (Davis et al. 2007; Stroustrup et al. 2016; Sugawara et al. 1999; Melchior et al. 2012), and anxiety during pregnancy (Blair et al. 2011; Austin et al. 2005).
While much attention has been focused on the potential influence of prenatal toxicant exposure and child cognitive and behavioral development (Grandjean and Landrigan 2014) comparatively little attention has been paid to the influence of early life toxicant exposures and the development of temperament. However, temperament may indeed mediate or modify the association between early life exposures, such as maternal stress (Zhu et al. 2014), prenatal depression (Stroustrup et al. 2016), and perhaps exposures to environmental toxicants, on later neurodevelopmental outcomes. For example, recently maternal lead exposure was found to increase risk for a difficult infant temperament (i.e. high activity, low approach, low adaptability, high intensity, negative mood, low persistence), and to enhance the already deleterious effect of maternal depression on infant temperament (Stroustrup et al. 2016). Consequently, temperament may be associated with later childhood behavioral development, and associations with toxicant exposures may highlight early developmental impacts on self-regulatory processes.
Phthalates are used as plasticizers in a variety of commercial products. Low molecular weight (LMW) phthalates (for example, diethyl phthalate (DEP)) are sometimes present in personal care products, such as perfume, shampoo, nail polish, and lotion (ATSDR 1995, 2001). High molecular weight (HMW) phthalates (for example, di(2-ethylhexyl) phthalate (DEHP)) are typically used as polyvinyl chloride plasticizers in applications such as flooring, tubing, wall covering, and medical devices (ATSDR 1997, 2002). People may be exposed to phthalates by dermal contact, ingestion of contaminated food products, and inhalation. Phthalates are quickly metabolized and excreted from the body in urine and feces. Despite rapid metabolism, studies suggest moderate stability in concentrations of some phthalate metabolites over time because of frequent, albeit episodic, exposure to some phthalate sources (e.g., cosmetics) (Adibi et al. 2008; Dewalque et al. 2015; Hauser et al. 2004; Teitelbaum et al. 2008).
A growing literature suggests potential links between prenatal phthalate exposures and neurodevelopmental outcomes in children (Miodovnik et al. 2011; Factor-Litvak et al. 2014; Y. Kim et al. 2011; Polanska et al. 2014; Whyatt et al. 2012; Engel et al. 2010; Kobrosly et al. 2014; Lien et al. 2015; Yolton et al. 2011; Swan et al. 2010), though not all studies report non-sex specific associations (Braun et al. 2014; Doherty et al. 2017; Gascon et al. 2015; Huang et al. 2015; Tellez-Rojo et al. 2013; Engel et al. 2009). A number of studies specifically reported associations between phthalate exposures and behavioral difficulties. For example, B. N. Kim et al. (2009) reported an association between metabolites of DEHP and parent-reported ADHD. Another study found associations between metabolites of LMW phthalates in maternal urine from pregnancy and aggression, conduct problems, attention difficulties, externalizing problems, and low adaptability on the Behavior Assessment System for Children- Parent Rating Scales (BASC) (Engel et al. 2010). This same study also reported links between increased prenatal LMW phthalate metabolites and lower emotional control and poorer executive function on the Behavior Rating Inventory of Executive Function (BRIEF) (Engel et al. 2010). Two studies also reported some associations between particular phthalates in prenatal urine samples and behavior problems in children as reported by mothers on the Child Behavior Checklist (CBCL) (Whyatt et al. 2012; Kobrosly et al. 2014). For example, Whyatt et al. (2012) described an association between monobutyl phthalate (MnBP; a metabolite of di-n-butyl phthalate [DnBP]) and monobenzyl phthalate (MzBP; a metabolite of butylbenzyl phthalate [BBzP]) and clinically withdrawn behaviors, and MBzP and clinically internalizing behaviors. Many of these studies also reported some differential associations between phthalates and behaviors in male compared to female children (Engel et al. 2010; Whyatt et al. 2012; Kobrosly et al. 2014).
Given the studies suggesting associations between phthalate exposures and childhood behavior and psychological literature implicating temperament patterns in development of psychopathology, we hypothesized that maternal prenatal concentrations of urinary phthalates biomarkers may also be associated with early childhood temperament. Thus, we sought to examine this hypothesis in a longitudinal study of child development with existing markers of prenatal phthalate exposure and multiple assessments of early childhood temperament. Since previous studies reported sex-phthalate interactions, we also assessed associations between phthalate and temperament among male and female children.
2. Material and methods
2.1 Study participants and data collection
The Mount Sinai Children’s Environmental Health study is a prospective cohort study of primiparous women with singleton pregnancies. Pregnant women were enrolled during prenatal visits at Mount Sinai Diagnostic and Treatment Center or two private practice clinics. All children in the study were born between May 1998 and July 2001 at Mount Sinai Hospital.
A single urine sample was collected from women between weeks 25 and 40 of pregnancy. During the third trimester, the women were administered a questionnaire about sociodemographic characteristics, medical conditions and lifestyle. The quality of the home environment was assessed using the Home Observation for Measurement in the Environment (HOME) Inventory at 12 and 24 months of age (Caldwell and Bradley 1984). Birth and delivery characteristics were collected from the medical record database at the Mount Sinai Department of Obstetrics, Gynecology and Reproductive Science. Institutional Review Boards at the Mount Sinai School of Medicine and the University of North Carolina at Chapel Hill approved the study. The analysis of blinded specimens at the Centers for Disease Control and Prevention (CDC) laboratory was determined not to constitute engagement in human subjects research.
2.2 Phthalate metabolite measurements
Concentrations of ten phthalate metabolites in the maternal urine samples were quantified at the CDC according to methods described previously (Kato et al. 2005; Silva et al. 2008). For concentrations below the limit of detection (LOD), we imputed the value of the LOD divided by the square root of two.
For these analyses, we focused on five phthalate metabolites: monoisobutyl phthalate (MiBP, a metabolite of diisobutyl phthalate), MnBP, monoethyl phthalate (MEP, a metabolite of DEP), mono(3-carboxypropyl) phthalate (MCPP, a major di-n-octyl phthalate metabolite, a minor DnBP metabolite and a metabolite of several high molecular weight phthalates), and MBzP. We calculated the molar sum of four metabolites of DEHP: (mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethylhexyl) phthalate (MEHP)), and refer to this as ΣDEHP.
2.3 Temperament assessment
When the children were approximately 12-months old, mothers were asked to complete the Infant Behavior Questionnaire (IBQ) – 1978 version (Rothbart 1981). This 91-item questionnaire contains Likert-type scales for which the parent is asked to indicate how often the infant engaged in certain behaviors in the past week. Item responses are averaged to create summary scores that represent six dimensions of toddler temperament: activity level (17 items) with higher scores indicating greater gross motor activity, distress to limitations (20 items) with higher scores revealing increased frustration in response to being prevented from completing certain actions, distress and latency to sudden changes or novel stimuli (16 items) with higher scores signifying greater distress, duration of orienting (11 items) with higher scores suggesting increased interaction and vocal communication with an item without an alteration in stimulation, smiling and laughing (15 items) with high scores indicating increased smiling or laughing, and soothability (8 items) with higher scores revealing increased reduction of distress when soothing attempted. The IBQ originally contained 11 items on the soothability scale, but we dropped three items (92, 93, and 94). The scoring documentation for the 1978 version of the IBQ states that reliability for the soothability scale had only thus far been established without items 92 and 93. For item 94, mothers were asked to specify other methods for soothing the child and this was missing for the majority of children in our study.
Mothers completed a 108 item Toddler Behavior Assessment Questionnaire (TBAQ) (Goldsmith 1996) when the children were approximately 24-months of age. This parent-report assessment consists of five content scales: activity level (20 items) with higher scores signifying a greater level of movement, anger (28 items) with higher scores implying greater proneness to crying or protesting when in conflict, social fear (19 items) with higher scores revealing more inhibition or withdrawal in new social situations, interest (22 items) with higher scores implying longer time persisting in independent play, and pleasure (19 items) with higher scores specifying increased smiling, laughing, and positive vocal communication.
Participants missing greater than 25% of scale items were coded as missing that summary score. Thus, the number of mother-child pairs with outcome information differed for each temperament scale.
2.4 Statistical analyses
We examined the distributions of the temperament scales and calculated the internal consistencies (Cronbach’s alpha) of each scale to assess whether or not the different items represent the same underlying construct. We also calculated correlations between scores of the different scales. We compared the characteristics of study participants in the cohort eligible for the analysis to the participants with at least one 12-month temperament content scale and with at least one 24-month temperament content scale.
To examine how temperament content scales differed across mother-child characteristics, we calculated medians and interquartile ranges (IQRs) for each temperament content scale by categories of the characteristics. Since some of the temperament scale had non-normal distributions, we tested for differences between the groups using Kruskal Wallis tests.
We used multiple linear regression to examine associations between phthalate metabolite concentrations and each of the temperament scales in SAS version 9.4 (SAS Institute Inc., Cary, NC). We examined regression diagnostics using PROC REG. Since some of the initial regression models indicated heteroskedasticity of the residuals, we natural log-transformed the temperament scales. We completed a series of analyses examining the relationship between the outcomes and each natural log-transformed phthalate metabolite with adjustment for natural log creatinine concentration in the model. In the main results, we present the percent change in each of the temperament scales for each 10% increase in phthalate concentration. In supplemental results, we also z-standardized the log-transformations in order to interpret the results on a standard deviation scale. Our models were adjusted for maternal alcohol use during pregnancy (no, yes), marital status during pregnancy (married or living with baby’s father, single/divorced/widowed/separated), maternal race (non-Hispanic white, non-Hispanic black, Hispanic/other), breastfeeding (no, yes), maternal education (high school or less, some college, college graduate), child’s sex (male, female), maternal age at delivery (continuous), the total HOME score from the time period (12-month or 24-month) of the outcome assessment (continuous) and smoking during pregnancy (no, yes). The results of the regression models presented here were fit using PROC GLM. Regression analyses were restricted to mother-child pairs with phthalate biomarkers data, a urinary creatinine concentration >10 mg/dL, and an available summary score on each individual temperament scale. Because there was minimal missing covariate data, we also restricted our analysis to mother-child pairs with complete covariate information.
Since there is evidence of heterogeneous associations between phthalate metabolites concentrations and neurodevelopmental outcomes by sex, we also fit models where we allowed for interactions between log-transformed phthalate metabolites and child’s sex. Based on the results of these models, we estimated the associations between phthalate metabolites concentrations and the temperament scales for each sex.
3. Results
3.1 Characteristics of analytic sample
The Mount Sinai Children’s Environmental Health study recruited 479 pregnant women. “Among these women, 75 were excluded because of miscarriage (n=1), fetal or infant death (n=2), medical complications (n=3), genetic anomalies (n=5), birth at less than 32 weeks completed gestation or birth weight of less than 1,500 g (n=5), not supplying a urine specimen prior to delivery (n=12), not continuing to participate in the study (n=19), or moving to a new hospital or outside of New York City (n=28) (Engel et al. 2007). Thus, 404 mother-child pairs were eligible for these analyses. Among 404 mother-child pairs in the original cohort, 204 had a score for at least one 12-month temperament scale and 279 had a score for at least one 24-month temperament scale. The mothers completing at least one 12-month temperament evaluation for their child were more likely to be white, have a college degree, or 30 years of age or older than mothers in the original cohort (Table 1). The mothers of children with at least one 24-month temperament scale were more likely to have reported breastfeeding, but were otherwise similar compared to those in the original cohort.
Table 1.
Participant characteristics for mother-child pairs in original cohort, for mother-child pairs with at least one IBQ scale, and for mother-child pairs with at least one TBAQ scale.
| Participant Characteristics | Original Cohort (N=404) | Some IBQ data (N=204) | Some TBAQ data (N=279) | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| N | % | N | % | N | % | |
| Alcohol use ab | ||||||
| No | 337 | 83.4 | 165 | 80.9 | 234 | 83.9 |
| Yes | 59 | 14.6 | 33 | 16.2 | 38 | 13.6 |
| Marital status a | ||||||
| Married/cohabiting | 215 | 53.2 | 116 | 56.9 | 144 | 51.6 |
| Other | 189 | 46.8 | 88 | 43.1 | 135 | 48.4 |
| Maternal race | ||||||
| White | 86 | 21.3 | 58 | 28.4 | 64 | 22.9 |
| Black | 112 | 27.7 | 53 | 26 | 73 | 26.2 |
| Hispanic/other | 206 | 51 | 93 | 45.6 | 142 | 50.9 |
| Breastfeeding c | ||||||
| No | 119 | 29.5 | 58 | 28.4 | 106 | 38 |
| Yes | 213 | 52.7 | 146 | 71.6 | 173 | 62 |
| Maternal education d | ||||||
| High school | 201 | 49.8 | 85 | 41.7 | 134 | 48 |
| Some college | 103 | 25.5 | 50 | 24.5 | 69 | 24.7 |
| College graduate | 98 | 24.3 | 69 | 33.8 | 76 | 27.2 |
| Child’s sex | ||||||
| Male | 220 | 54.5 | 109 | 53.4 | 151 | 54.1 |
| Female | 184 | 45.5 | 95 | 46.6 | 128 | 45.9 |
| Maternal age | ||||||
| <20 years | 142 | 35.1 | 58 | 28.4 | 94 | 33.7 |
| 20–<30 years | 176 | 43.6 | 87 | 42.6 | 120 | 43 |
| 30+ years | 86 | 21.3 | 59 | 28.9 | 65 | 23.3 |
| Smoking a | ||||||
| No | 337 | 83.4 | 170 | 83.3 | 234 | 83.9 |
| Yes | 67 | 16.6 | 34 | 16.7 | 45 | 16.1 |
Abbreviations: IBQ, Infant Behavior Questionnaire; TBAQ, Toddler Behavior Assessment Questionnaire
During pregnancy
8 missing from original cohort, 6 missing from 12-month data, 7 missing from 24-month data
72 missing from original cohort
2 missing from original cohort
For the regression analyses, 382 of the eligible 404 mother-child pairs had phthalate metabolite concentrations. We excluded six mother-child pairs with maternal urinary creatinine concentrations ≤10 mg/dL from the regression analyses. After restricting to mother-child pairs with scores on each individual scale, we excluded participants with incomplete covariate data (N=10 from the 12-month analyses and N=8 from the 24-month analyses).
3.2 Outcome scale characteristics and demographics
Internal consistencies (Cronbach alpha coefficients) for the scales ranged from 0.72 to 0.88. Distress or and latency to novelty on the 12-month IBQ scale was moderately correlated with social fear on the 24-month TBAQ scale (Spearman correlation coefficient (ρ) = 0.41). Higher activity levels on the TBAQ were also modestly correlated with higher TBAQ anger scores (ρ = 0.51). Otherwise, correlations between the temperament scales were weak (|ρ| < 0.40) (Supplemental Table 1).
At 12-months, children of mothers with a partner were more likely than others to have lower scores on distress and latency to novel stimuli and duration or orienting scales (Table 2). Black mothers reported that their children had higher distress to novel stimuli than white, Hispanic or other race/ethnicity mothers. Duration of orienting was lower in children of white women whereas smiling and soothability scores were higher among children of Hispanic or other mothers. Breastfeeding was inversely correlated with the child’s duration of orienting. Higher maternal education was associated with the child’s lower distress to novelty and higher duration of orienting. Distress and latency to novelty were also higher among male children or children with younger mothers.
Table 2.
Medians (IQR) for each Infant Behavior Questionnaire scale by participant characteristics.
| Characteristics | Activity (N=201)
|
Distress to limitations (N=203)
|
Distress and latency to novelty (N=201)
|
Duration of orienting (N=202)
|
Smiling (N=201)
|
Soothability (N=201)
|
|---|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |
| Alcohol use ab | ||||||
| No | 4.5 (4.0 – 5.1) | 3.9 (3.3 – 4.3) | 3.2 (2.5 – 3.9) | 4.4 (3.5 – 5.1) | 5.6 (5.0 – 6.1) | 5.5 (4.8 – 6.3) |
| Yes | 4.4 (3.9 – 4.9) | 4.1 (3.3 – 4.5) | 3.4 (2.8 – 3.9) | 4.3 (3.2 – 4.8) | 5.4 (4.9 – 6.1) | 5.5 (4.5 – 6.4) |
| Marital status a | ||||||
| Married/cohabiting | 4.4 (3.9 – 5.0) | 3.9 (3.3 – 4.3) | 3.1 (2.5 – 3.8)** | 4.2 (3.4 – 4.8)** | 5.6 (5.1 – 6.0) | 5.5 (4.8 – 6.1) |
| Other | 4.5 (4.0 – 5.0) | 3.9 (3.3 – 4.4) | 3.4 (2.9 – 4.2)** | 4.5 (3.9 – 5.4)** | 5.5 (4.9 – 6.2) | 5.6 (4.9 – 6.6) |
| Maternal race | ||||||
| White | 4.5 (3.9 – 5.0) | 4.0 (3.4 – 4.5)* | 2.9 (2.5 – 3.3)** | 4.0 (3.2 – 4.8)** | 5.4 (5.0 – 5.9)** | 5.4 (4.9 – 5.9)** |
| Black | 4.6 (4.1 – 5.1) | 4.0 (3.3 – 4.4)* | 3.9 (3.1 – 4.4)** | 4.5 (3.9 – 5.1)** | 5.3 (4.9 – 5.9)** | 5.3 (4.5 – 6.0)** |
| Hispanic/other | 4.4 (3.9 – 4.9) | 3.8 (3.2 – 4.1)* | 3.1 (2.6 – 3.8)** | 4.5 (3.5 – 5.4)** | 5.8 (5.1 – 6.2)** | 5.8 (5.0 – 6.6)** |
| Breastfeeding | ||||||
| No | 4.4 (3.8 – 5.1) | 3.6 (3.1 – 4.3)* | 3.2 (2.6 – 4.2) | 4.6 (4.2 – 5.4)** | 5.5 (5.0 – 6.1) | 5.6 (5.0 – 6.3) |
| Yes | 4.4 (4.0 – 5.0) | 4.0 (3.4 – 4.3)* | 3.2 (2.7 – 3.8) | 4.3 (3.3 – 4.8)** | 5.5 (5.0 – 6.1) | 5.5 (4.8 – 6.3) |
| Maternal education | ||||||
| High school | 4.6 (4.0 – 5.1) | 3.9 (3.3 – 4.3) | 3.5 (2.8 – 4.2)** | 4.5 (3.7 – 5.4)** | 5.7 (5.0 – 6.2) | 5.8 (4.8 – 6.3) |
| Some college | 4.3 (3.9 – 4.9) | 3.8 (3.3 – 4.3) | 3.3 (3.0 – 3.9)** | 4.5 (4.0 – 4.9)** | 5.5 (5.1 – 6.1) | 5.5 (4.9 – 6.6) |
| College graduate | 4.4 (3.9 – 5.0) | 4.0 (3.4 – 4.5) | 2.8 (2.4 – 3.4)** | 3.8 (3.1 – 4.7)** | 5.4 (5.0 – 6.0) | 5.3 (4.8 – 5.9) |
| Child’s sex | ||||||
| Male | 4.5 (3.9 – 5.1) | 3.9 (3.3 – 4.3) | 3.1 (2.5 – 3.8)** | 4.3 (3.5 – 4.9) | 5.6 (5.1 – 6.1) | 5.6 (4.9 – 6.2) |
| Female | 4.4 (3.9 – 5.0) | 3.9 (3.3 – 4.5) | 3.4 (2.8 – 4.1)** | 4.4 (3.3 – 5.4) | 5.5 (4.9 – 6.1) | 5.5 (4.6 – 6.3) |
| Maternal age | ||||||
| <20 years | 4.6 (4.0 – 5.1) | 3.9 (3.3 – 4.3) | 3.7 (2.9 – 4.2)** | 4.3 (3.6 – 5.4) | 5.6 (5.1 – 6.2) | 6.0 (4.8 – 6.8) |
| 20–<30 years | 4.4 (3.9 – 4.9) | 3.9 (3.3 – 4.3) | 3.3 (2.8 – 3.9)** | 4.5 (3.6 – 4.9) | 5.5 (5.0 – 6.1) | 5.5 (4.7 – 6.3) |
| 30+ years | 4.5 (3.9 – 5.0) | 4.0 (3.3 – 4.4) | 2.8 (2.4 – 3.5)** | 4.2 (3.3 – 4.9) | 5.5 (5.0 – 6.0) | 5.4 (4.9 – 5.9) |
| Smoking a | ||||||
| No | 4.5 (3.9 – 5.1) | 3.9 (3.3 – 4.3) | 3.2 (2.6 – 3.9) | 4.4 (3.5 – 4.9) | 5.6 (5.1 – 6.1) | 5.5 (4.8 – 6.3) |
| Yes | 4.4 (4.1 – 4.9) | 3.8 (3.4 – 4.4) | 3.4 (2.7 – 4.1) | 4.5 (3.7 – 5.4) | 5.3 (4.9 – 5.8) | 5.4 (4.7 – 6.3) |
| Total HOME year 1 c | ||||||
| Tertile 1 | 4.3 (3.9 – 4.9) | 3.8 (3.2 – 4.3) | 3.3 (2.9 – 4.1) | 4.5 (3.5 – 5.4) | 5.3 (4.9 – 6.1) | 5.6 (4.9 – 6.5) |
| Tertile 2 | 4.5 (4.0 – 5.2) | 3.9 (3.2 – 4.3) | 3.1 (2.5 – 3.8) | 4.3 (3.5 – 5.1) | 5.6 (4.9 – 6.1) | 5.5 (4.5 – 6.1) |
| Tertile 3 | 4.5 (3.9 – 5.0) | 4.0 (3.4 – 4.3) | 3.2 (2.5 – 3.8) | 4.4 (3.5 – 4.9) | 5.7 (5.1 – 6.0) | 5.5 (5.0 – 6.3) |
Abbreviations: IQR, Interquartile Range; HOME, Home Observation for Measurement in the Environment
During pregnancy
6 missing alcohol use
5 missing HOME score
Kruskal Wallis test p-value < 0.10
Kruskal Wallis test p-value < 0.05
At the time of the 24-month questionnaire, activity and interest levels were higher among children born to women who did not report alcohol use during pregnancy (Table 3). Children of black or Hispanic/other mothers had higher scores on the activity level, anger, and pleasure scales than children of white mothers. Hispanic/other mothers reported higher levels of interest. Not breastfeeding was also associated with higher reported activity levels in the children. Activity, anger and pleasure scores were higher among children of younger mothers or children of mothers with lower education levels. Higher total home year score at 24-months was associated with lower activity level and anger, but higher scores on the interest scale.
Table 3.
Medians (IQR) for each Toddler Behavior Assessment Questionnaire scale by participant characteristics.
| Characteristics | Activity (N=278)
|
Anger (N=268)
|
Social Fear (N=257)
|
Interest (N=261)
|
Pleasure (N=269)
|
|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |
| Alcohol use ab | |||||
| No | 4.5 (3.9 – 5.1)** | 4.0 (3.4 – 4.7) | 4.2 (3.6 – 4.9) | 4.9 (4.4 – 5.5)** | 5.8 (5.4 – 6.2)* |
| Yes | 4.2 (3.5 – 4.7)** | 4.0 (3.4 – 4.4) | 4.0 (3.6 – 4.7) | 4.7 (4.2 – 5.1)** | 5.5 (5.2 – 5.9)* |
| Marital status a | |||||
| Married/cohabiting | 4.3 (3.7 – 4.8)** | 3.8 (3.3 – 4.6)* | 4.1 (3.6 – 5.0) | 4.9 (4.4 – 5.4) | 5.7 (5.3 – 6.2) |
| Other | 4.6 (4.1 – 5.2)** | 4.1 (3.5 – 4.8)* | 4.1 (3.5 – 4.8) | 4.8 (4.3 – 5.5) | 5.8 (5.4 – 6.2) |
| Maternal race | |||||
| White | 3.9 (3.6 – 4.6)** | 3.6 (3.1 – 4.0)** | 4.0 (3.4 – 4.5) | 4.7 (4.3 – 5.2)** | 5.5 (5.2 – 5.8)** |
| Black | 4.8 (4.0 – 5.3)** | 4.4 (3.5 – 4.9)** | 4.3 (3.6 – 5.1) | 4.7 (4.2 – 5.4)** | 5.8 (5.4 – 6.3)** |
| Hispanic/other | 4.5 (4.0 – 5.1)** | 4.0 (3.4 – 4.9)** | 4.2 (3.6 – 4.9) | 5.0 (4.5 – 5.6)** | 5.8 (5.4 – 6.4)** |
| Breastfeeding | |||||
| No | 4.6 (4.1 – 5.2)** | 4.1 (3.4 – 4.9)* | 4.2 (3.6 – 5.0) | 4.8 (4.1 – 5.5) | 5.7 (5.3 – 6.0) |
| Yes | 4.4 (3.8 – 5.0)** | 3.9 (3.4 – 4.6)* | 4.1 (3.5 – 4.8) | 4.9 (4.5 – 5.4) | 5.8 (5.4 – 6.3) |
| Maternal education | |||||
| High school | 4.6 (4.2 – 5.3)** | 4.1 (3.5 – 5.0)** | 4.3 (3.7 – 5.1)* | 4.9 (4.5 – 5.5) | 5.8 (5.4 – 6.4)** |
| Some college | 4.5 (3.8 – 5.2)** | 4.0 (3.4 – 4.7)** | 4.2 (3.5 – 4.9)* | 4.8 (4.2 – 5.4) | 5.8 (5.5 – 6.1)** |
| College graduate | 4.0 (3.6 – 4.6)** | 3.6 (3.1 – 4.3)** | 4.0 (3.4 – 4.6)* | 4.8 (4.3 – 5.3) | 5.6 (5.2 – 5.9)** |
| Child’s sex | |||||
| Male | 4.4 (3.9 – 5.1) | 3.9 (3.3 – 4.6) | 4.1 (3.5 – 4.7) | 4.9 (4.3 – 5.4) | 5.8 (5.3 – 6.3) |
| Female | 4.5 (3.8 – 5.1) | 4.0 (3.5 – 4.9) | 4.3 (3.7 – 4.9) | 4.8 (4.4 – 5.3) | 5.7 (5.4 – 6.1) |
| Maternal age | |||||
| <20 years | 4.6 (4.1 – 5.3)** | 4.0 (3.4 – 4.9)** | 4.3 (3.6 – 5.0) | 4.8 (4.3 – 5.4) | 5.8 (5.4 – 6.3)** |
| 20–<30 years | 4.4 (3.8 – 5.2)** | 4.1 (3.4 – 4.8)** | 4.0 (3.6 – 5.0) | 4.9 (4.4 – 5.6) | 5.8 (5.5 – 6.2)** |
| 30+ years | 4.1 (3.7 – 4.7)** | 3.6 (3.2 – 4.3)** | 4.1 (3.6 – 4.6) | 4.8 (4.3 – 5.2) | 5.5 (5.1 – 6.1)** |
| Smoking a | |||||
| No | 4.4 (3.8 – 5.1) | 4.0 (3.4 – 4.7) | 4.2 (3.6 – 4.9) | 4.9 (4.4 – 5.4) | 5.7 (5.4 – 6.2) |
| Yes | 4.5 (3.9 – 5.1) | 4.0 (3.4 – 4.7) | 4.0 (3.3 – 4.4) | 4.7 (4.1 – 5.5) | 5.7 (5.4 – 6.2) |
| Total HOME year 2 c | |||||
| Tertile 1 | 4.7 (4.2 – 5.2)** | 4.3 (3.4 – 4.9)** | 4.1 (3.6 – 4.8) | 4.7 (4.2 – 5.2)** | 5.7 (5.4 – 6.1) |
| Tertile 2 | 4.4 (3.8 – 5.0)** | 4.0 (3.4 – 4.9)** | 4.3 (3.5 – 5.0) | 4.9 (4.5 – 5.3)** | 5.8 (5.4 – 6.2) |
| Tertile 3 | 4.1 (3.6 – 4.8)** | 3.8 (3.2 – 4.1)** | 4.1 (3.4 – 4.7) | 5.1 (4.6 – 5.5)** | 5.8 (5.3 – 6.2) |
Abbreviations: IQR, Interquartile Range; HOME, Home Observation for Measurement in the Environment
During pregnancy
7 missing alcohol use
2 missing HOME score
Kruskal Wallis test p-value < 0.10
Kruskal Wallis test p-value < 0.05
3.3 Phthalate metabolite concentrations and infant/toddler temperament
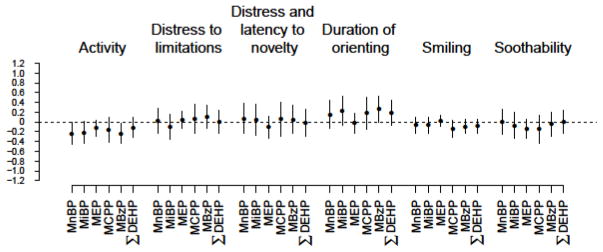
Descriptions of the phthalate metabolite concentrations in Mount Sinai Children’s Environmental Health study have been published previously (Wolff et al. 2008) and concentrations are within the ranges of the general United States population in the late 1990s – early 2000s (Silva et al. 2004). Though we observed some consistent patterns in associations between phthalate metabolite concentrations and some of the 12-month temperament scales, most associations did not meet statistical significance (Figure 1, Supplemental Table 2, Supplemental Table 3). Furthermore, the magnitude of the associations was small. Phthalate metabolites concentrations were inversely associated with reported activity levels. We found that a 10% increase in MnBP concentration was associated with a 0.2% (95% confidence interval (CI): 0.0%, 0.5%) decrease in the IBQ activity score and that a 10% increase in MBzP was associated with a 0.2% (95% CI: 0.0%, 0.4%) decrease IBQ activity score, respectively. For many of the phthalates biomarkers, higher concentrations were associated with duration of orienting scores at 12-months (% change in orienting per 10% change in MnBP=0.2 [95% CI: −0.1, 0.4], % change in orienting per 10% change in MiBP=0.2 [95% CI: −0.1, 0.5], % change in orienting per 10% change in MCPP=0.2 [95% CI: −0.1, 0.5], % change in orienting per 10% change in MBzP=0.3 [95% CI: 0.0, 0.5], % change in orienting per 10% change in ΣDEHP= 0.2 [95% CI: −0.1, 0.5]). For metabolites of high-molecular weight phthalates, increasing concentrations were associated with lower reported levels of smiling and laughing, although these associations did not meet statistical significance (% change in smiling per 10% change in MCPP=−0.1 [95% CI: −0.3, 0.0], % change in smiling per 10% change in for MBzP=−0.1 [95% CI: −0.2, 0.0], % change in smiling per 10% change in ΣDEHP=−0.1 [95% CI: −0.2, 0.1]).
Figure 1.
Estimates of percent change in 12-month infant temperament scales (and 95% confidence interval) for a 10% increase in prenatal phthalate metabolite concentration.
Regression models are adjusted for maternal alcohol use during pregnancy, marital status during pregnancy, maternal race, breastfeeding, maternal education, child’s sex, maternal age at delivery, the total HOME score at 12-months, smoking during pregnancy, and the natural log of the creatinine concentration. Number of subjects in analyses: Activity (N=178), Distress to limitations (N=180), Distress and latency to novelty (N=178), Duration of orienting (N=179), Smiling (N=178), Soothability (N=178).
For the 24-month TBAQ questionnaire, the patterns across phthalate metabolites were not as clear. We observed evidence of a non-significant positive association between ΣDEHP and anger levels (% change in anger per 10% change in ΣDEHP=0.2 [95% CI: 0.0, 0.4]) (Figure 2, Supplemental Table 4, Supplemental Table 5). We also observed borderline associations between concentrations of MCPP and MBzP and increased social fear (% change in social fear per 10% change in MCPP=0.3 [95% CI: −0.1, 0.6], % change in social fear per 10% change in MBzP=0.3 [95% CI: 0.0, 0.5]). Finally, MCPP and MBzP concentrations were inversely associated with scores on the pleasure scale (% change in pleasure per 10% change in MCPP=−0.2 [95% CI: −0.4, −0.1], % change in pleasure per 10% change in MBzP=−0.1 [95% CI: −0.2, 0.0]).
Figure 2.
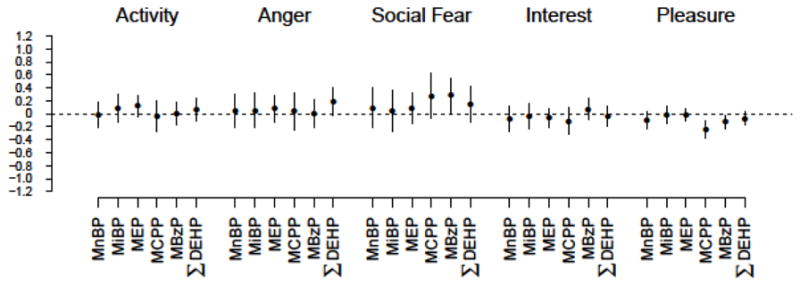
Estimates of percent change in 24-month infant temperament scales (and 95% confidence interval) for a 10% increase in prenatal phthalate metabolite concentration.
Regression models are adjusted for maternal alcohol use during pregnancy, marital status during pregnancy, maternal race, breastfeeding, maternal education, child’s sex, maternal age at delivery, the total HOME score at 24-months, smoking during pregnancy, and the natural log of the creatinine concentration. Number of subjects in analyses: Activity (N=255), Anger (N=245), Social Fear (N=234), Interest (N=239), Pleasure (N=247).
Though sex-by-phthalate interaction terms were not statistically significant at the p-value < 0.10 level, the associations between phthalate metabolite concentrations and duration of orienting scores at 12-months were stronger among girls (Supplemental Table 6 and 7). There was evidence of heterogeneity by sex on the distress and latency to novelty scale with higher concentrations of MnBP (interaction p-value=0.07), MCPP (interaction p-value=0.01), and MBzP (interaction p-value=0.03) being more strongly associated with higher distress to novelty among girls. At 24-months, we did not observe any evidence of heterogeneity by sex in the association between phthalate metabolites and temperament (Supplemental Table 8, Supplemental Table 9).
4. Discussion
4.1 Findings
In this prospective cohort study, we generally did not observe strong statistical evidence for associations between maternal urinary phthalate metabolites concentrations during pregnancy and parent-reported child temperament at 12 and 24-months of age. Based on the 12-month temperament questionnaire, there were borderline associations between phthalate metabolites concentrations and lower activity levels, particularly for MnBP and MBzP. These same phthalate metabolites were also weakly associated with higher duration of orienting. Additionally, MCPP, MBzP and ΣDEHP metabolites were weakly, but not significantly, associated with less smiling and laughing. At 24 months, higher concentrations of MCPP and MBzP were associated with higher social fear and lower pleasure. There was also some suggestion of a positive association between ΣDEHP and anger levels. However, there was no association with activity level at the 24-month visit.
4.2 Previous Literature
Early childhood temperament has been associated with maternal social, behavioral and environmental exposures experienced in the prenatal and early life period (Stroustrup et al. 2016), and with later psychopathology (Nigg 2006). The continuity of temperament from infancy through early childhood has been found to be stable, suggesting the importance of the early developmental period in the establishment of self-regulatory capabilities (Carranza et al. 2013; Komsi et al. 2006). Therefore, infant and toddler temperament might serve as a potential marker of later behavioral outcomes. For example, Willoughby et al. (2016) reported associations between higher reactivity (fear, anger, and activity) and lower regulation (inhibition and persistence of attention) in the first three years of life and parent- and teacher-reported ADHD behaviors when the children were in first grade. As such, identifying negative temperamental features may be useful for targeting early interventions to at risk children.
Previous studies have found associations between exposure to phthalates and behavioral difficulties and social impairment in older children (B. N. Kim et al. 2009; Engel et al. 2010; Whyatt et al. 2012; Kobrosly et al. 2014; Chopra et al. 2014; Miodovnik et al. 2011; Park et al. 2015). Whyatt et al. (2012) reported associations between maternal concentrations of MnBP and MBzP during the third trimester and increased withdrawal and internalizing behaviors in children at three years of age as assessed through the maternally reported Child Behavior Checklist (CBCL). Kobrosly et al. (2014) reported associations between higher maternal MiBP urinary concentrations during pregnancy and attention problems, aggressive behavior, oppositional defiance, and conduct problems in children at 6–10 years of age according to a parent-reported CBCL.
In this same cohort, we previously reported associations between maternal urinary concentrations of metabolites of low molecular weight phthalates during pregnancy and worse BASC scores for aggression, conduct problems, attention difficulties, depression, externalizing problems, and adaptability, and with the BRIEF emotional control scale and global executive composite index when the children were 4–9 years old (Engel et al. 2010). In the present paper, we extended this research to investigate the relation between gestational phthalate exposure and temperament because temperament at 12- and 24-months of age is closer in time to the initial phthalate exposure measurements, leaving less opportunity for postnatal environmental exposures and home environment conditions to potentially confound the effects of prenatal exposures.
We reported suggestive weak associations between exposure to certain phthalates and lower activity levels and higher duration of orienting at 12-months of age. The duration of orienting scale is a visual habituation task, intended to represent the construct of persistence of attention (Rothbart et al. 2000; Rothbart 1981), which has been shown to segregate with the orienting/regulatory capacity factor in several studies (Sung et al. 2015; Gartstein and Rothbart 2003), and may serve as an important predictor of later effortful control (Gartstein et al. 2013). After the first year of life, however, the infant’s emerging ability to regulate and shift attentional focus, a core component of executive functions, may result in faster disengagement with familiar objects, and thus a lower duration of orienting (Gartstein et al. 2013). Indeed, a recent longitudinal study found that shorter duration of looking was associated with significantly higher executive function scores throughout early childhood, as compared to infants with a longer duration of looking (Cuevas and Bell 2014). In this context, increases in duration of orienting associated with increasing gestational exposure to phthalates is coherent with previously reported associations with phthalate-associated deficits in executive functions in this and other studies (Engel et al. 2010; Kobrosly et al. 2014; Factor-Litvak et al. 2014), since children in the present study were largely 12 months and somewhat older at the time of their assessment. Thus, our findings lend weak support to the hypothesis that gestational phthalate exposure may negatively impact executive functions, in particular, effortful control. Of note, an earlier analysis of data from our cohort found that increasing concentrations of the molar sum of MCPP and metabolites of high molecular weight phthalates (DEHP, BzBP) was associated with decreasing scores on the Orientation domain of the Brazelton Neonatal Behavioral Assessment Scale (BNBAS) among girls (Engel et al. 2009). The BNBAS Orientation scale represents the capacity of the newborn to engage with visual and auditory stimuli as well as the quality of alertness, so it does not measure the same construct represented by duration of orienting scale on the IBQ, but does generally point to a common affected domain over time
High scores on duration of orienting have also been linked to increased internalizing behaviors at later ages (Rothbart et al. 2000). In our study sample, somewhat lower scores on the Activity and Smile domain at 12-months, and lower scores on the Pleasure domain at 24 months along with higher scores on the Social Fear domain, suggest an internalizing component. Some studies have linked gestational exposure to phthalates with increases in internalizing behaviors in later childhood (Whyatt et al. 2012; Engel et al. 2010), also lending plausibility to these observations. Though some phthalates were associated with lower activity at 12 months, there was no association with activity at 24 months. We found a weak correlation (0.32) between activity level at 12 and 24 months. This may indicate that activity level is not necessarily consistently reported over time or that the construct assessed through the two scales is not the same.
We also evaluated if associations between maternal phthalate metabolite concentrations and temperament differed by child’s sex since earlier studies have reported differential associations between phthalates and later childhood behaviors in males as compared to females (Engel et al. 2010; Whyatt et al. 2012; Kobrosly et al. 2014). We found some evidence of heterogeneity by child’s sex in the association between certain phthalate metabolites and distress and latency to novel stimuli. Though the interaction terms were not statistically significant at the 0.05 level, the association between phthalates and duration of orienting was largely observed among the girls and not the boys. We did not find any evidence of sex heterogeneity and associations between phthalate metabolites and temperament at 24-months.
4.4 Limitations and Strengths
One limitation of our study is that temperament outcome is based on parental response. Parents may differ in their response to the questionnaire independently of the child’s actual behavior. For example, responses may be influenced by the psychological well-being of the parent and by comparative knowledge of other children. In our study, we found that the distributions of some temperament scales differed by maternal race and education as previously reported (Jansen et al. 2009). We do not know if these groups are objectively different because of situational factors or if the differences are solely a result of parental reporting. Despite the limitations of parental report, an advantage of using parental report is that parents are more familiar with the overall behavior of the child than an outside observer, and thus may be more attuned to subtle features of their responses.
Another concern of our study is that the exposure measurement was based on the concentration in a spot urine sample from one time point in pregnancy. Phthalates are rapidly metabolized and exposures are likely episodic, so we cannot guarantee that the measurement is reliable across the pregnancy period. Despite the concern of the rapid metabolism of phthalates, personal care product usage is generally consistent over time. There is also evidence that concentrations of some phthalates are moderately stable over time in samples collected from pregnant women (Adibi et al. 2008; Braun et al. 2012; Cantonwine et al. 2014; Ferguson et al. 2014; Fisher et al. 2015; Valvi et al. 2015) and non-pregnant persons (Baird et al. 2010; Dewalque et al. 2015; Frederiksen et al. 2013; Fromme et al. 2007; Hauser et al. 2004; Hoppin et al. 2002; Meeker et al. 2012; Peck et al. 2010; Preau et al. 2010; Teitelbaum et al. 2008; Townsend et al. 2013; Sexton and Ryan 2012). Also, our measurements were generally from the third trimester, so it is possible that other exposure windows may differentially impact brain and behavior development. Furthermore, early childhood exposure to phthalates may impact childhood temperament and we could not account for this in the analysis.
It is somewhat challenging to contextualize the magnitude of the reported associations given the dearth of research in this area, and the inconsistency in reporting patterns among studies. We scaled our estimates to standard deviation increases to provide intuitive comparability across scales, and provide a relative benchmark to the distribution. The largest observed effect size was a −0.22 standard deviation change in log-pleasure score with a log-unit change in MCPP concentration, which suggests relatively small changes temperament outcomes over the entire distribution of biomarkers concentrations. MCPP is also a non-specific metabolite that can derive from multiple parent phthalate compounds. We also cannot exclude the potential for unmeasured or residual confounding; in particular, we did not have information on history of maternal depression, which might impact maternal reporting of child temperament, although this may well be non-differential with respect to phthalate exposure.
Our study has numerous strengths. Our study was conducted in a socioeconomically diverse study that was followed prospectively; thus we were able to ascertain and adjust multiple potential confounders of the association between phthalates and temperament. We assessed phthalate exposure during the prenatal time period using validated biomarkers. We also measured temperament at two time points: 12-months and 24-months of age. Finally, we considered the potential for heterogeneity by sex based on prior studies suggesting that phthalate effects may differ by sex.
5. Conclusion
In this prospective cohort study with a diverse study sample, we found weak evidence for associations between urinary phthalate metabolite concentrations during pregnancy and child temperament at 12- and 24- months of age. This is despite evidence linking prenatal phthalate concentrations to adverse behavioral profiles later in childhood. Further research may provide relevant data to identify environmental factors that may influence temperament.
Supplementary Material
Highlights.
Prenatal phthalates metabolites were measured in spot urine samples.
Temperament was assessed by parent report at 12- and 24-months.
Prenatal phthalates were generally not associated with temperament.
Acknowledgments
Funding: This research was supported by National Institute for Environmental Health Sciences and Environmental Protection Agency Children’s Center (grants ES09584, P30ES023515, and R827039), The New York Community Trust, Agency for Toxic Substances and Disease Registry, Centers for Disease Control and Prevention (CDC), and Association for Prevention Teaching and Research. ABS was supported by a training grant from the National Institute of Environmental Health Sciences (T32 ES007018). The funding sponsors had no role in the conduct of the research or the preparation of the article for publication.
We acknowledge the technical assistance of Ella Samandar and Jim Preau (CDC, Atlanta, GA) in measuring the urinary concentrations of phthalate biomarkers
Footnotes
Disclaimer: The use of trade names is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Services or the Centers for Disease Control and Prevention (CDC). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adibi JJ, Whyatt RM, Williams PL, Calafat AM, Camann D, Herrick R, et al. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environmental Health Perspectives. 2008;116(4):467–473. doi: 10.1289/ehp.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvik A, Torgersen AM, Aalen OO, Lindemann R. Binge alcohol exposure once a week in early pregnancy predicts temperament and sleeping problems in the infant. Early Human Development. 2011;87(12):827–833. doi: 10.1016/j.earlhumdev.2011.06.009. [DOI] [PubMed] [Google Scholar]
- ATSDR. Toxicological Profile for Diethyl Phthalate. Atlanta, GA: Agency for Toxic Substances and Disease Registry; 1995. [PubMed] [Google Scholar]
- ATSDR. Toxicological Profie for Di-n-ocytylphthalate. Atlanta, GA: Agency for Toxic Substances and Disease Registry; 1997. [Google Scholar]
- ATSDR. Toxicological Profile for Di-n-butyl Phthalate. Atlanta, GA: Agency for Toxic Substances and Disease Registry; 2001. [PubMed] [Google Scholar]
- ATSDR. Toxicological Profile for Di(2-ethylhexyl) Phthalate. Atlanta, GA: Agency for Toxic Substances and Disease Registry; 2002. [PubMed] [Google Scholar]
- Austin MP, Hadzi-Pavlovic D, Leader L, Saint K, Parker G. Maternal trait anxiety, depression and life event stress in pregnancy: relationships with infant temperament. Early Human Development. 2005;81(2):183–190. doi: 10.1016/j.earlhumdev.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Baird DD, Saldana TM, Nepomnaschy PA, Hoppin JA, Longnecker MP, Weinberg CR, et al. Within-person variability in urinary phthalate metabolite concentrations: measurements from specimens after long-term frozen storage. Journal of Exposure Science & Environmental Epidemiology. 2010;20(2):169–175. doi: 10.1038/jes.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair MM, Glynn LM, Sandman CA, Davis EP. Prenatal maternal anxiety and early childhood temperament. Stress. 2011;14(6):644–651. doi: 10.3109/10253890.2011.594121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Just AC, Yolton K, Calafat AM, Sjodin A, et al. Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children: the HOME study. Environmental Health Perspectives. 2014;122(5):513–520. doi: 10.1289/ehp.1307261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, et al. Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environmental Health Perspectives. 2012;120(5):739–745. doi: 10.1289/ehp.1104139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell BM, Bradley RH. Administration Manual: Home Observation for Measurement of the Environment (Revised edition) Little Rock, AR: University of Arkansas at Little Rock; 1984. [Google Scholar]
- Cantonwine DE, Cordero JF, Rivera-Gonzalez LO, Anzalota Del Toro LV, Ferguson KK, Mukherjee B, et al. Urinary phthalate metabolite concentrations among pregnant women in Northern Puerto Rico: distribution, temporal variability, and predictors. Environment International. 2014;62:1–11. doi: 10.1016/j.envint.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carranza JA, Gonzalez-Salinas C, Ato E. A longitudinal study of temperament continuity through IBQ, TBAQ and CBQ. Infant Behavior & Development. 2013;36(4):749–761. doi: 10.1016/j.infbeh.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Chopra V, Harley K, Lahiff M, Eskenazi B. Association between phthalates and attention deficit disorder and learning disability in U.S. children, 6–15 years. Environmental Research. 2014;128:64–69. doi: 10.1016/j.envres.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas K, Bell MA. Infant attention and early childhood executive function. Child Development. 2014;85(2):397–404. doi: 10.1111/cdev.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA. Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(6):737–746. doi: 10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- De Pauw SS, Mervielde I. Temperament, personality and developmental psychopathology: a review based on the conceptual dimensions underlying childhood traits. Child Psychiatry and Human Development. 2010;41(3):313–329. doi: 10.1007/s10578-009-0171-8. [DOI] [PubMed] [Google Scholar]
- Dewalque L, Pirard C, Vandepaer S, Charlier C. Temporal variability of urinary concentrations of phthalate metabolites, parabens and benzophenone-3 in a Belgian adult population. Environmental Research. 2015;142:414–423. doi: 10.1016/j.envres.2015.07.015. [DOI] [PubMed] [Google Scholar]
- Doherty BT, Engel SM, Buckley JP, Silva MJ, Calafat AM, Wolff MS. Prenatal phthalate biomarker concentrations and performance on the Bayley Scales of Infant Development-II in a population of young urban children. Environmental Research. 2017;152:51–58. doi: 10.1016/j.envres.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Berkowitz GS, Barr DB, Teitelbaum SL, Siskind J, Meisel SJ, et al. Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioral Assessment Scale in a multiethnic pregnancy cohort. American Journal of Epidemiology. 2007;165(12):1397–1404. doi: 10.1093/aje/kwm029. [DOI] [PubMed] [Google Scholar]
- Engel SM, Miodovnik A, Canfield RL, Zhu C, Silva MJ, Calafat AM, et al. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environmental Health Perspectives. 2010;118(4):565–571. doi: 10.1289/ehp.0901470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Zhu C, Berkowitz GS, Calafat AM, Silva MJ, Miodovnik A, et al. Prenatal phthalate exposure and performance on the Neonatal Behavioral Assessment Scale in a multiethnic birth cohort. Neurotoxicology. 2009;30(4):522–528. doi: 10.1016/j.neuro.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Factor-Litvak P, Insel B, Calafat AM, Liu X, Perera F, Rauh VA, et al. Persistent Associations between Maternal Prenatal Exposure to Phthalates on Child IQ at Age 7 Years. PloS One. 2014;9(12):e114003. doi: 10.1371/journal.pone.0114003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Ko YA, Mukherjee B, Meeker JD. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environment International. 2014;70:118–124. doi: 10.1016/j.envint.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Arbuckle TE, Mallick R, LeBlanc A, Hauser R, Feeley M, et al. Bisphenol A and phthalate metabolite urinary concentrations: Daily and across pregnancy variability. Journal of Exposure Science & Environmental Epidemiology. 2015;25(3):231–239. doi: 10.1038/jes.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen H, Kranich SK, Jorgensen N, Taboureau O, Petersen JH, Andersson AM. Temporal variability in urinary phthalate metabolite excretion based on spot, morning, and 24-h urine samples: considerations for epidemiological studies. Environmental Science & Technology. 2013;47(2):958–967. doi: 10.1021/es303640b. [DOI] [PubMed] [Google Scholar]
- Fromme H, Bolte G, Koch HM, Angerer J, Boehmer S, Drexler H, et al. Occurrence and daily variation of phthalate metabolites in the urine of an adult population. International Journal of Hygiene and Environmental Health. 2007;210(1):21–33. doi: 10.1016/j.ijheh.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Gartstein MA, Bridgett DJ, Young BN, Panksepp J, Power T. Origins of Effortful Control: Infant and Parent Contributions. Infancy. 2013;18(2):149–183. doi: 10.1111/j.1532-7078.2012.00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein MA, Rothbart MK. Studying infant temperament via the revised infant behavior questionnaire. Infant Behavior and Development. 2003;26(1):64–86. [Google Scholar]
- Gascon M, Valvi D, Forns J, Casas M, Martinez D, Julvez J, et al. Prenatal exposure to phthalates and neuropsychological development during childhood. International Journal of Hygiene and Environmental Health. 2015;218(6):550–558. doi: 10.1016/j.ijheh.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH. Studying temperament via construction of the Toddler Behavior Assessment Questionnaire. Child Development. 1996;67(1):218–235. [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurology. 2014;13(3):330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environmental Health Perspectives. 2004;112(17):1734–1740. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppin JA, Brock JW, Davis BJ, Baird DD. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environmental Health Perspectives. 2002;110(5):515–518. doi: 10.1289/ehp.02110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HB, Chen HY, Su PH, Huang PC, Sun CW, Wang CJ, et al. Fetal and Childhood Exposure to Phthalate Diesters and Cognitive Function in Children Up to 12 Years of Age: Taiwanese Maternal and Infant Cohort Study. PloS One. 2015;10(6):e0131910. doi: 10.1371/journal.pone.0131910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen PW, Raat H, Mackenbach JP, Jaddoe VW, Hofman A, Verhulst FC, et al. Socioeconomic inequalities in infant temperament: the generation R study. Social Psychiatry and Psychiatric Epidemiology. 2009;44(2):87–95. doi: 10.1007/s00127-008-0416-z. [DOI] [PubMed] [Google Scholar]
- Kato K, Silva MJ, Needham LL, Calafat AM. Determination of 16 phthalate metabolites in urine using automated sample preparation and on-line preconcentration/high-performance liquid chromatography/tandem mass spectrometry. Analytical Chemistry. 2005;77(9):2985–2991. doi: 10.1021/ac0481248. [DOI] [PubMed] [Google Scholar]
- Kim BN, Cho SC, Kim Y, Shin MS, Yoo HJ, Kim JW, et al. Phthalates exposure and attention-deficit/hyperactivity disorder in school-age children. Biological Psychiatry. 2009;66(10):958–963. doi: 10.1016/j.biopsych.2009.07.034. [DOI] [PubMed] [Google Scholar]
- Kim Y, Ha EH, Kim EJ, Park H, Ha M, Kim JH, et al. Prenatal exposure to phthalates and infant development at 6 months: prospective Mothers and Children’s Environmental Health (MOCEH) study. Environmental Health Perspectives. 2011;119(10):1495–1500. doi: 10.1289/ehp.1003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobrosly RW, Evans S, Miodovnik A, Barrett ES, Thurston SW, Calafat AM, et al. Prenatal phthalate exposures and neurobehavioral development scores in boys and girls at 6–10 years of age. Environmental Health Perspectives. 2014;122(5):521–528. doi: 10.1289/ehp.1307063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komsi N, Raikkonen K, Pesonen AK, Heinonen K, Keskivaara P, Jarvenpaa AL, et al. Continuity of temperament from infancy to middle childhood. Infant Behavior & Development. 2006;29(4):494–508. doi: 10.1016/j.infbeh.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Lien YJ, Ku HY, Su PH, Chen SJ, Chen HY, Liao PC, et al. Prenatal exposure to phthalate esters and behavioral syndromes in children at 8 years of age: Taiwan Maternal and Infant Cohort Study. Environmental Health Perspectives. 2015;123(1):95–100. doi: 10.1289/ehp.1307154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke RL, Lagasse LL, Seifer R, Lester BM, Shankaran S, Bada HS, et al. Effects of prenatal substance exposure on infant temperament vary by context. Development and Psychopathology. 2016;28(2):309–326. doi: 10.1017/S0954579415000504. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, Hauser R. Urinary phthalate metabolites and their biotransformation products: predictors and temporal variability among men and women. Journal of Exposure Science & Environmental Epidemiology. 2012;22(4):376–385. doi: 10.1038/jes.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior M, Chastang JF, de Lauzon B, Galera C, Saurel-Cubizolles MJ, Larroque B, et al. Maternal depression, socioeconomic position, and temperament in early childhood: the EDEN Mother-Child Cohort. Journal of Affective Disorders. 2012;137(1–3):165–169. doi: 10.1016/j.jad.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Miodovnik A, Engel SM, Zhu C, Ye X, Soorya LV, Silva MJ, et al. Endocrine disruptors and childhood social impairment. Neurotoxicology. 2011;32(2):261–267. doi: 10.1016/j.neuro.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteno CD, Jacobson JL, Carter RC, Dodge NC, Jacobson SW. Infant emotional withdrawal: a precursor of affective and cognitive disturbance in fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research. 2014;38(2):479–488. doi: 10.1111/acer.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT. Temperament and developmental psychopathology. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2006;47(3–4):395–422. doi: 10.1111/j.1469-7610.2006.01612.x. [DOI] [PubMed] [Google Scholar]
- Park S, Lee JM, Kim JW, Cheong JH, Yun HJ, Hong YC, et al. Association between phthalates and externalizing behaviors and cortical thickness in children with attention deficit hyperactivity disorder. Psychological Medicine. 2015;45(8):1601–1612. doi: 10.1017/S0033291714002694. [DOI] [PubMed] [Google Scholar]
- Peck JD, Sweeney AM, Symanski E, Gardiner J, Silva MJ, Calafat AM, et al. Intra- and inter-individual variability of urinary phthalate metabolite concentrations in Hmong women of reproductive age. Journal of Exposure Science & Environmental Epidemiology. 2010;20(1):90–100. doi: 10.1038/jes.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanska K, Ligocka D, Sobala W, Hanke W. Phthalate exposure and child development: the Polish Mother and Child Cohort Study. Early Human Development. 2014;90(9):477–485. doi: 10.1016/j.earlhumdev.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Preau JL, Jr, Wong LY, Silva MJ, Needham LL, Calafat AM. Variability over 1 week in the urinary concentrations of metabolites of diethyl phthalate and di(2-ethylhexyl) phthalate among eight adults: an observational study. Environmental Health Perspectives. 2010;118(12):1748–1754. doi: 10.1289/ehp.1002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Goldschmidt L, Willford J. The effects of prenatal cocaine use on infant development. Neurotoxicology and Teratology. 2008;30(2):96–106. doi: 10.1016/j.ntt.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart MK. Measurement of temperament in infancy. Child Development. 1981;52:569–578. [Google Scholar]
- Rothbart MK, Ahadi SA, Evans DE. Temperament and personality: origins and outcomes. Journal of Personality and Social Psychology. 2000;78(1):122–135. doi: 10.1037//0022-3514.78.1.122. [DOI] [PubMed] [Google Scholar]
- Saudino KJ. Behavioral genetics and child temperament. Journal of Developmental and Behavioral Pediatrics. 2005;26(3):214–223. doi: 10.1097/00004703-200506000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton K, Ryan AD. Using exposure biomarkers in children to compare between-child and within-child variance and calculate correlations among siblings for multiple environmental chemicals. Journal of Exposure Science & Environmental Epidemiology. 2012;22(1):16–23. doi: 10.1038/jes.2011.30. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, et al. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environmental Health Perspectives. 2004;112(3):331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Preau JL, Jr, Needham LL, Calafat AM. Cross validation and ruggedness testing of analytical methods used for the quantification of urinary phthalate metabolites. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences. 2008;873(2):180–186. doi: 10.1016/j.jchromb.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Stroustrup A, Hsu HH, Svensson K, Schnaas L, Cantoral A, Solano Gonzalez M, et al. Toddler temperament and prenatal exposure to lead and maternal depression. Environmental Health: A Global Access Science Source. 2016;15(1):71. doi: 10.1186/s12940-016-0147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara M, Kitamura T, Toda MA, Shima S. Longitudinal relationship between maternal depression and infant temperament in a Japanese population. Journal of Clinical Psychology. 1999;55(7):869–880. doi: 10.1002/(sici)1097-4679(199907)55:7<869::aid-jclp8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Sung J, Beijers R, Gartstein MA, de Weerth C, Putnam SP. Exploring temperamental differences in infants from the United States of America (US) and the Netherlands. European Journal of Developmental Psychology. 2015;12(1):15–28. doi: 10.1080/17405629.2014.937700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Liu F, Hines M, Kruse RL, Wang C, Redmon JB, et al. Prenatal phthalate exposure and reduced masculine play in boys. International Journal of Andrology. 2010;33(2):259–269. doi: 10.1111/j.1365-2605.2009.01019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, et al. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environmental Research. 2008;106(2):257–269. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Tellez-Rojo MM, Cantoral A, Cantonwine DE, Schnaas L, Peterson K, Hu H, et al. Prenatal urinary phthalate metabolites levels and neurodevelopment in children at two and three years of age. Science of the Total Environment. 2013;461–462:386–390. doi: 10.1016/j.scitotenv.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend MK, Franke AA, Li X, Hu FB, Eliassen AH. Within-person reproducibility of urinary bisphenol A and phthalate metabolites over a 1 to 3 year period among women in the Nurses’ Health Studies: a prospective cohort study. Environmental Health: A Global Access Science Source. 2013;12(1):80. doi: 10.1186/1476-069X-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvi D, Monfort N, Ventura R, Casas M, Casas L, Sunyer J, et al. Variability and predictors of urinary phthalate metabolites in Spanish pregnant women. International Journal of Hygiene and Environmental Health. 2015;218(2):220–231. doi: 10.1016/j.ijheh.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Weiss SJ, St Jonn-Seed M, Harris-Muchell C. The contribution of fetal drug exposure to temperament: potential teratogenic effects on neuropsychiatric risk. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2007;48(8):773–784. doi: 10.1111/j.1469-7610.2007.01745.x. [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Liu X, Rauh VA, Calafat AM, Just AC, Hoepner L, et al. Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environmental Health Perspectives. 2012;120(2):290–295. doi: 10.1289/ehp.1103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby MT, Gottfredson NC, Stifter CA Family Life Project I. Observed temperament from ages 6 to 36 months predicts parent- and teacher-reported attention-deficit/hyperactivity disorder symptoms in first grade. Development and Psychopathology. 2016:1–14. doi: 10.1017/S0954579415001236. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, et al. Prenatal phenol and phthalate exposures and birth outcomes. Environmental Health Perspectives. 2008;116(8):1092–1097. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolton K, Xu Y, Strauss D, Altaye M, Calafat AM, Khoury J. Prenatal exposure to bisphenol A and phthalates and infant neurobehavior. Neurotoxicology and Teratology. 2011;33(5):558–566. doi: 10.1016/j.ntt.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Sun MS, Hao JH, Chen YJ, Jiang XM, Tao RX, et al. Does prenatal maternal stress impair cognitive development and alter temperament characteristics in toddlers with healthy birth outcomes? Developmental Medicine and Child Neurology. 2014;56(3):283–289. doi: 10.1111/dmcn.12378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.