Abstract
Inorganic arsenic is a toxic naturally occurring element in soil and water in many regions of the US including the Midwest. Prostate cancer is the second most common type of cancer in men in Iowa, surpassed only by non-melanotic skin cancer. Epidemiology studies have evaluated arsenic exposure from drinking water and prostate cancer, but most have focused on high-level exposures outside the US. As drinking water from groundwater sources is a major source of arsenic exposure, we conducted an ecologic study to evaluate prostate cancer and arsenic in drinking water from public water sources and private wells in Iowa, where exposure levels are low, but duration of exposure can be long.
Arsenic data from public water systems were obtained from the Iowa Safe Drinking Water Information System for the years 1994–2003 and for private wells from two Iowa Well Water Studies, the Iowa Community Private Well Study (ICPWS, 2002–2003) and Iowa Statewide Rural Well Water Survey Phase 2 (SWIRL2, 2006–2008) that provided data for 87 Iowa counties. Prostate cancer incidence data from 2009 to 2013 for Iowa were obtained from Surveillance, Epidemiology and End Results’ SEER*Stat software. County averages of water arsenic levels varied from 1.08 to 18.6 ppb, with three counties above the current 10 ppb limit. Based on the tertiles of arsenic levels, counties were divided into three groups: low (1.08–2.06 ppb), medium (2.07–2.98 ppb), and high (2.99–18.6 ppb).
Spatial Poisson regression modeling was conducted to estimate the risk ratios (RR) of prostate cancer by tertiles of arsenic level at a county level, adjusted for demographic and risk factors. The RR of prostate cancer were 1.23 (95% CI, 1.16–1.30) and 1.28 (95% CI, 1.21–1.35) in the medium and high groups, respectively, compared to the low group after adjusting for risk factors. The RR increased to 1.36 (95% CI, 1.28–1.45) in the high group when analyses were restricted to aggressive prostate cancers (Gleason score ≥ 7). This study shows a significant dose-dependent association between low-level arsenic exposure and prostate cancer, and if this result is replicated in future individual-level studies, may suggest that 10 ppb is not protective for human health.
Keywords: arsenic, prostate cancer, drinking water, Iowa, private well water
INTRODUCTION
Arsenic is a naturally occurring element in the earth’s crust and has anthropogenic uses, including wood treatment, pigments, and semiconductor processing (IARC, 2009). The primary source of arsenic exposure is drinking water (Chung et al., 2014; Smith et al., 1992). In the Midwest of the United States including Northern Iowa, soils, composed of glacial sediments that migrated 16,000–12,000 years ago during the late Wisconsin-aged glacial drift, contain higher concentrations of arsenic (Erickson and Barnes, 2005). Arsenic easily migrates from contaminated soil into ground water depending on geochemical conditions (Erickson and Barnes, 2005; Michael, 2013). Public water systems are regulated with a current drinking water standard for arsenic at 10 ppb under the Safe Drinking Water Act (EPA, 2009), but private wells are not regulated or monitored.
Arsenic is classified as a group 1 carcinogen by IARC as there are sufficient experimental and epidemiologic studies supporting causation of skin, bladder, and lung cancers (IARC, 2009). As summarized in the IARC monograph, potential mechanisms of arsenic carcinogenesis may be through oxidative DNA damage, gene amplification, aneuploidization (by spindle disturbance), inhibition of DNA repair enzymes, and changes in DNA methylation and histone modifications, leading to altered gene expressions and changes in cell differentiation, proliferation, and response to signaling pathways (IARC, 2009). Prostate cancer has also been reported to be positively associated with arsenic exposure (IARC, 2009). Several epidemiologic studies of populations in Taiwan, Australia, Europe, Chile or the US reported increasing mortality or incidence of prostate cancer with arsenic exposure (Bardach et al., 2015; Brown et al., 2002; Bulka et al., 2016; Chen and Wang, 1990; Chen et al., 1988; Garcia-Esquinas et al., 2013; Gunduz et al., 2015; Hinwood et al., 1999; Lewis et al., 1999; Nunez et al., 2016; Rivara et al., 1997; Wu et al., 1989; Yang et al., 2008). Some measured arsenic levels in urine (Garcia-Esquinas et al., 2013) or levels in top soil (Nunez et al., 2016), but the majority focused on drinking water as source of arsenic exposure. Of these, most evaluated the association of high-level arsenic exposure and prostate cancer and all observed a positive association. For example, prostate cancer mortality was found to increase with exposure to arsenic up to 2500 ppb in Taiwan (Yang et al., 2008). Reports on the effects of low-level arsenic exposure on prostate cancer are sparse. One study from Denmark did not observe an association of low-level arsenic (0.05–25.5 ppb, mean 1.2 ppb) and prostate cancer (Baastrup et al., 2008). One recent ecologic study reported on the effect of low-level arsenic exposure and showed a 5–10 % increased incidence of prostate cancer from arsenic exposure in a dose-response manner in Illinois counties with arsenic levels ranging from 0.33 to 16.23 ppb in public drinking water (Bulka et al., 2016). This finding and the fact that prostate cancer has the highest cancer incidence in men in the US (excluding non-melanotic skin cancer) (Brawley, 2012; IARC, 2009), justifies further analysis of the risks of low-level arsenic exposure from drinking water and prostate cancer. We therefore conducted an ecologic study to investigate the association between low-level arsenic exposure from drinking water and the incidence of prostate cancer in Iowa, accounting for both public water sources and private water wells as source of drinking water.
METHODS
Arsenic exposure data
Arsenic testing data from 1994 to 2003 from 723 public water systems in 97 of the 99 Iowa counties were obtained from the Iowa Safe Drinking Water Information System. Two counties were excluded because they did not have their own public water stations and therefore county-specific arsenic data were not available. Two different studies, the Iowa Community Private Well Study (ICPWS, 2002–2003) and the Iowa Statewide Rural Well Water Survey Phase 2 (SWRL2, 2006–2008), with water samples analyzed by the Iowa State Hygienic Laboratory, were combined and provided arsenic concentration data in water samples from 709 private wells in 89 counties (CHEEC, 2009; IDNR, 2004). In the ICPWS, 103 wells were randomly selected from 50 counties, and 133 wells were selected from 15 counties with assumed higher risk of contamination. In SWRL2, 116 wells, which were included in the original SWRL (1988–1989) study and still active currently, and additional 357 randomly selected wells were sampled (https://cheec.uiowa.edu/swrl2). Although the period of the Iowa Statewide Rural Well Water Survey was outside the 1994–2003 period, it was utilized in this study because a temporal stability of arsenic levels in ground water has been reported in studies from Argentina and Nevada (Concha et al., 2006; Steinmaus et al., 2005). All datasets were obtained from the Center for Health Effects of Environmental Contamination at the University of Iowa (https://cheec.uiowa.edu/cooperative-projects). There were no active private wells and therefore no well water data in 10 counties. Thus arsenic data for both public water systems and private wells were available for 87 of the 99 counties in Iowa. County arsenic levels in the drinking water were calculated from average arsenic levels in both municipal water and private well water with weighting by the percentages of county populations who use municipal water and private well water. If the concentration was lower than the limit of detection (LOD), half of LOD was assigned as the level (Lopez-Carrillo et al., 2010). LODs were 1 ppb for private wells, and varied from 0.01 to 10 ppb for public water systems. Iowa counties were categorized into three groups by tertiles of average arsenic level in the drinking water: low exposure (1.08–2.06 ppb), medium exposure (2.07–2.98 ppb), and high exposure groups (2.99–18.6 ppb).
Prostate cancer incidence data
Data of all new prostate cancer cases in the state of Iowa between 2009 and 2013 were obtained from the Surveillance, Epidemiology, and End Results (SEER) program via SEER*Stat version 8.3. This database provided the number of observed cases and the population distribution by age-specific categories for each county (Table 1). As people of other races accounted for 5.5% of population in Iowa, they were excluded and only white males were included in this study. Aggressive prostate cancer was identified as distant stage at diagnosis and/or poorly differentiated (Gleason score ≥ 7) or undifferentiated tumor grades using this software.
Table 1.
Prostate cancer cases and average annual population for 2009–2013 for white males by age group
| Age group In years |
Number of cases1 (%) | Annual average population2 (%) |
|---|---|---|
| 30–39 | 3 (0.003) | 171,826 (20.3) |
| 40–49 | 143 (1.43) | 186,727 (22.1) |
| 50–59 | 1,966 (19.8) | 207,248 (24.5) |
| 60–69 | 4,019 (40.4) | 146,164 (17.3) |
| 70–79 | 2,624 (26.4) | 82,276 (9.7) |
| 80+ | 1,192 (12.0) | 51,523 (6.1) |
| Total | 9,947 (100.0) | 845,764 (100.0) |
All newly diagnosed prostate cancer cases in Iowa for 2009–2013 were included.
Population of white men in Iowa for 2009–2013 was obtained from SEER*Stat software and is based on U.S. Census estimates, and an average annual population was calculated.
Covariates data
Percentages of the population that obtain their drinking water from a public water system or private well by county were obtained from Water Use Data for the Nation as part of National Water Information System (NWIS) for 1995 and 2000. Poverty rate for 2009–2013 was obtained from the Small Area Income and Poverty Estimates (SAIPE) of the Census Bureau and used as a proxy measure for socioeconomic status, which is related to cancer patterns (Singh et al., 2003). Poverty rate indicates the percentage of population below the federal poverty level based on household size and income (Proctor et al., 2016). Information on general cancer risk factors, including tobacco smoking rate (current smoker), binge drinking rate (5 or more drinks an occasion during the past 30 days), and obesity rate (BMI ≥ 30) in each county for 1997–2013 was acquired from the Iowa Behavioral Risk Factor Surveillance System (BRFSS) through a research agreement with Iowa Department of Public Health (Park et al., 2006; Samanic et al., 2004; Thun et al., 1997; Yun et al., 2005). These data were also restricted to the white male population at the county level. In addition, county-level pesticide-use density was included as a proxy of pesticide exposure (Reynolds et al., 2005), calculated by annual pesticide use divided by land area. Pesticide use data and county land area were obtained from the U.S. Geologic Survey and U.S. Department of Agriculture (USDA, 2004; USGS, 2017).
Statistical analysis
The standardized incidence ratio (SIR) was calculated by dividing the number of observed cases by the number of expected cases of prostate cancer for each county. The expected number of prostate cancer cases for each county was calculated using indirect standardization, based on the age-specific prostate cancer rates in 10-year age groups (30–39, 40–49, 50–59, 60–69, 70–79, and 80+; Table 1) of the entire state of Iowa over the 2009–2013 period as a standard and population distribution by age for each county obtained via SEER*Stat from the U.S. Census Bureau.
A spatial Poisson regression model analysis was used to evaluate the association between arsenic exposure and the incidence of prostate cancer (Cleophas and Zwinderman, 2016). The unit of analysis for this study was the county. Arsenic levels were trichotomized based on tertiles of arsenic concentration in drinking water. Poverty, tobacco smoking, binge drinking, and obesity rates at county level were included as covariates (Table 2). The log of the expected number of cases was used as an offset term. The Chi-square statistic was used to determine the statistical significance. Further analysis focusing on aggressive prostate cancer was performed as an indirect manner to control the potential confounding associated with prostate cancer screening. Spatial autocorrelation is reported to occur frequently in ecological data because observations from nearby locations are often more similar than would be expected on a random basis (Kissling and Carl, 2008). Spatial effect is represented by using the latitude and longitude of the central point of each county. Moran’s I statistic was calculated to test whether there was spatial autocorrelation among neighboring areas (Anselin, 1995; Kissling and Carl, 2008). Since a majority (98.5%) of the prostate cancer patients were 50 years old or older (Table 1), subjects in this age range were analyzed additionally to test for possible early onset cancers. SAS (version 9.4, Cary, NC) was used for all statistical analyses. Statistical significance was declared when the p-value was less than 0.05.
Table 2.
Summary of demographic and risk factors of adult males in county groups determined by average arsenic level
| Arsenic level in drinking water (ppb) | |||
|---|---|---|---|
|
|
|||
| 1.08–2.06 | 2.07–2.98 | 2.99–18.6 | |
| Number of counties | 29 | 29 | 29 |
| Percent private well users | 26.48 | 33.69 | 26.22 |
| Percent below poverty level | 8.92 | 8.94 | 9.40 |
| Percent current smoking | 23.79 | 22.78 | 21.86 |
| Percent binge drinking1 | 29.34 | 24.52 | 22.58 |
| Percent obesity (BMI ≥ 30) | 27.07 | 26.21 | 25.59 |
| Pesticide use density (kg/km2)2 | 154.9 | 173.4 | 159.1 |
| Percent lack of health insurance3 | 13.6 | 14.3 | 14.8 |
| Number of primary care physicians4 | 51 | 55 | 47 |
| Annual migration rate5 | 9.9 | 11.2 | 9.8 |
Data ware averaged over counties in each arsenic tertile
Five or more drinks on an occasion during the past 30 days
Annual total pesticide use (1994–2003) was divided by county land area.
Percent of people without health insurance between 18–64 years old for years 2009–2013 (USCensus, 2015).
Per 100,000 population (CHR, 2017)
Migration rates were calculated based on number of residents within a county and movers exiting that county during a 5-year period and averaged between 1995 and 2014 (USCensus, 2000; USCensus, 2012; USCensus, 2016).
Geographic mapping
Geographic data for boundaries of counties of the state of Iowa were obtained from the National Resources Geographic Information System of Iowa Department of Natural Resources. Maps were created to illustrate the arsenic level in the drinking water and SIR categorized into tertiles at the county level. All geographic mapping was conducted using ArcGIS (version 10.3, ESRI, Redlands, CA).
RESULTS
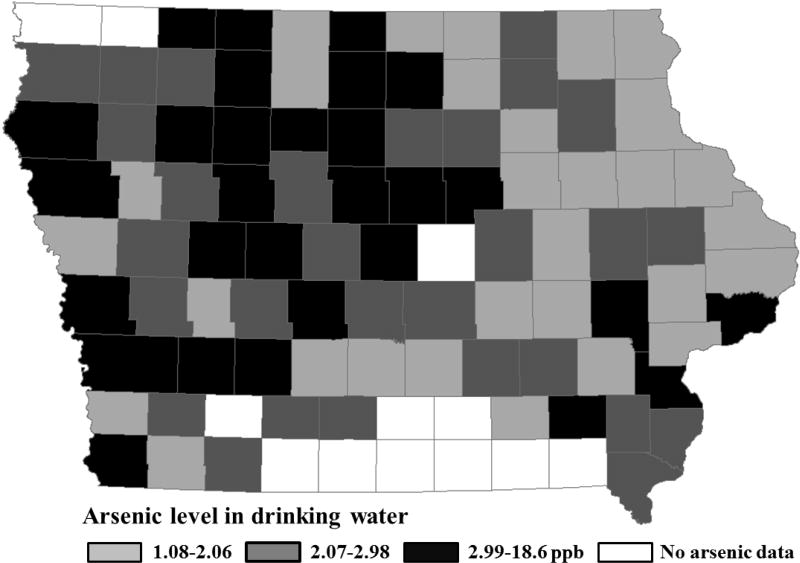
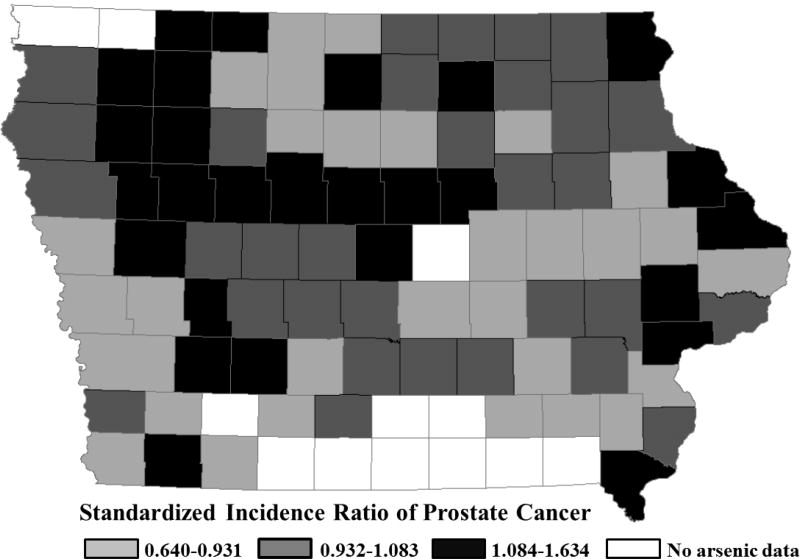
The spatial patterns of arsenic levels in drinking water and SIRs of prostate cancer in Iowa at the county level are shown in Figures 1 and 2, respectively. The SIR ranged from 0.64 in the lowest to 1.634 in the highest incidence county. Average arsenic levels in drinking water ranged from 1.08 to 18.6 ppb, although only 3 counties surpassed the current action level of 10 ppb (until January 2006, the effective EPA standard for arsenic in drinking water was 50 ppb).
Figure 1.
Average arsenic level in drinking water and its spatial pattern by county group (29 counties/arsenic group) in the state of Iowa for the years of 1994–2003 and 2002–2008 for public and private drinking water sources, respectively.
Figure 2.
Standardized incidence ratio of prostate cancer and its spatial pattern by county group (29 counties/arsenic group) in the state of Iowa for the years 2009–2013.
There were 9,947 newly diagnosed prostate cancer cases in Iowa during 2009–2013 (Table 1). While there were no prostate cancers among white males aged under 30, as expected, over 98% of prostate cancers were diagnosed at age 50 and older.
In our crude model, the risk of prostate cancer significantly increased by 9% and 18% in the county groups with the medium and high range of arsenic levels in drinking water, respectively, compared to the county group with low range of arsenic levels (Table 3). After adjusting for poverty, smoking, drinking and obesity, the risk of prostate cancer significantly increased by 23% and 28% in the medium and high groups, respectively, compared to the low group.
Table 3.
Risk ratio (RR) and 95% confident interval of prostate cancer using spatial Poisson regression models
| Model | Average Arsenic level (ppb) |
RR (95% CI) | p-value | Trend |
|---|---|---|---|---|
| Crude | 1.08 – 2.06 | 1.00 (reference) | <0.001 | |
| 2.07 – 2.98 | 1.09 (1.03–1.14) | 0.002 | ||
| 2.99 – 18.6 | 1.18 (1.12–1.24) | <0.001 | ||
| Adjusted1 | 1.08 – 2.06 | 1.00 (reference) | < 0.001 | |
| 2.07 – 2.98 | 1.23 (1.16–1.30) | < 0.001 | ||
| 2.99 – 18.6 | 1.28 (1.21–1.35) | < 0.001 |
Adjusted for rates of poverty, smoking, drinking, obesity, and pesticide use density
Further analysis only focusing on aggressive prostate cancers also showed increased incidence of prostate cancer with arsenic exposure from drinking water by 11% and 32% in medium and high arsenic counties, and by 20% and 36%, respectively, after adjusting for covariates (Table 4). Once again in all models, the incidence of prostate cancer increased between county groups with increasing arsenic levels and demonstrated a dose-dependent relationship.
Table 4.
Risk ratio (RR) and 95% confident interval of aggressive prostate cancer by county arsenic group using spatial Poisson regression models
| Model | Average Arsenic level (ppb) |
RR (95% CI) | p-value | Trend |
|---|---|---|---|---|
| Crude | 1.08 – 2.06 | 1.00 (reference) | <0.001 | |
| 2.07 – 2.98 | 1.11 (1.04–1.19) | 0.002 | ||
| 2.99 – 18.6 | 1.32 (1.23–1.47) | <0.001 | ||
| Adjusted1 | 1.18 – 2.06 | 1.00 (reference) | < 0.001 | |
| 2.07 – 2.98 | 1.20 (1.13–1.29) | < 0.001 | ||
| 2.99 – 18.6 | 1.36 (1.28–1.45) | < 0.001 |
Adjusted for rates of poverty, smoking, drinking, obesity, and pesticide use density
An analysis limited to patients aged 50 and older at diagnosis (results not shown) were not appreciably different from risk ratios for all ages shown in Tables 3 and 4.
DISCUSSION
In this ecologic study, a positive association between arsenic exposure from drinking water and prostate cancer was found using spatial Poisson regression modeling. Prostate cancer incidences were significantly higher in counties with higher levels of arsenic in public and private drinking water in a dose-dependent manner. A stronger association was observed after adjusting for demographic and risk factor such as poverty, smoking, drinking, and obesity for each county. These results are consistent with previous studies that showed positive associations of arsenic exposure and prostate cancer (Bulka et al., 2016; Hinwood et al., 1999; Hsu et al., 2013).
In this study, arsenic levels for both public and private drinking water were included in the analysis. As private wells are not regulated, water analysis data were limited or not available in a previous study (Bulka et al., 2016). However, two sources of arsenic testing data were available for Iowa private wells. Most private wells were sampled only once or twice during the study periods. Since no temporal change of arsenic level in groundwater was observed in the prior studies and urine arsenic levels were relatively constant over a 10-year period in a population exposed to similar arsenic levels as in Iowa, the use of these data seems appropriate (Concha et al., 2006; Navas-Acien et al., 2009; Steinmaus et al., 2005). Water sample data from 709 private wells representing 89 of the 99 counties in Iowa were available. Most of these wells were randomly selected. Although this represents only a small fraction of the total number of private wells used for drinking water, these data provide a first, state-wide analysis of arsenic levels in private well water in Iowa. After excluding two additional counties that had no arsenic data for public water since they used water supplied from neighboring counties, a total of 87 counties are included in this study, providing a nearly complete coverage of the state.
Most studies on the health effects of arsenic exposure from water have been conducted in countries with high arsenic levels of up to 2500 ppb, such as Taiwan, Bangladesh, and Chile (Argos et al., 2014; Marshall et al., 2007; Yang et al., 2008). There are a limited number of studies conducted in locations with low-level arsenic exposure. In a Utah study, the risk of death from prostate cancer was elevated by 45% from exposure to drinking water arsenic ranging from 14 to 166 ppb (Lewis et al., 1999). A prospective cohort study observed a 3.3 times increased mortality for prostate cancer by comparing the groups of the 80th and 20th percentile of urine arsenic level that ranged from <10 to 61 ppb in American Indians from Arizona, Oklahoma, North Dakota, and South Dakota (Garcia-Esquinas et al., 2013). Recently, a study conducted in Illinois, a Midwest state with low arsenic levels ranging from 0.33 to 16.23 ppb, showed a relationship between arsenic exposure from public drinking water with prostate cancer (Bulka et al., 2016). As the range of average county arsenic levels in drinking water from public sources and private wells in Iowa was 1.08–18.6 ppb, our arsenic levels were similar to those in the Illinois study and we found a similar association with prostate cancer.
The mechanism of arsenic carcinogenesis may be related to changes in the expression of various genes (IARC, 2009). The biological plausibility of the increase in risk of prostate cancer by arsenic exposure is provided by experimental studies. Based on these studies several mechanisms of arsenic-induction of prostate cancer have been proposed including overexpression of matrix metalloproteinase-9, heme oxygenase-1, and ras, the decreased expression of thrombospondin, and the induction of acquired androgen independence (Benbrahim-Tallaa and Waalkes, 2008; Benbrahim-Tallaa et al., 2005; Benbrahim-Tallaa et al., 2007; Witz, 2008). In a recent study, the alteration of stromal TGFβ signaling by arsenic exposure was shown to be related to progression of prostate cancer (Shearer et al., 2016).
We considered a latency period between exposure to arsenic and the incidence of prostate cancer as we included an exposure period that was between 1994 and 2003, while incident prostate cancers diagnosed between 2009 and 2013. The latency period for peak occurrence of increased cancer mortality after arsenic exposure can be very long, on average 20–25 years, but even longer periods of over 50 years as well as increases of childhood cancers due to arsenic exposure, suggesting short latency times, have been summarized in a recent review (Naujokas et al., 2013). A minimal latency period of 5 years, as reported and used for other exposures and cancer types (Farioli et al., 2013; Ferreccio et al., 2013; Melak et al., 2014; Steinmaus et al., 2013) seemed therefore justified. It should be pointed out, however, that according to those studies the increased risk for adverse health effects due to arsenic exposure may continue for a lifetime, further highlighting the urgent need for more in-depth, data-driven arsenic risk assessment.
Risk factors and potential confounding factors were considered and adjusted for in the analyses. As the risk of prostate cancer increases significantly in older ages, the effects of age were excluded by using indirect standardization. Racial disparities were also excluded by focusing only on white males. Smoking, binge drinking, and obesity were considered, although these factors showed an inverse relationship to arsenic levels. The percentage of population below the federal poverty level was included as a proxy of socioeconomic status (SES) as high SES is linked to a lower rate of uninsured and better access to health care including PSA screening (Adler and Newman, 2002; Ross et al., 2006; Rundle et al., 2013). The percentage of population without health insurance was correlated with poverty (Pearson’s correlation coefficient=0.61) and therefore not included. In addition, county-level pesticide density was included as a proxy of pesticide exposure, as pesticide exposure is known to increase the risk for prostate cancer (Alavanja et al., 2003; Band et al., 2011; Cockburn et al., 2011; Koutros et al., 2013). The adjustment for these risk factors resulted in higher risk ratios of 10–15% compared to the crude rate. Performing the analyzes using all ages or only those above 50 did not show significant differences, suggesting that arsenic exposure may not cause an earlier onset of prostate cancer. Potential confounding associated with prostate cancer screening was evaluated by performing restricted analyses of only aggressive prostate cancer cases. The effect was negligible for the medium arsenic group, but increased the RR by 14% and 8% for the crude and adjusted model, respectively, for the high arsenic group. This interesting finding may imply that arsenic in drinking water could possibly act in accelerating the progression of prostate cancer. This possibility needs to be further investigated using prostate cancer mortality data.
There are some limitations in this study. First, there could be differential misclassification of arsenic exposure in this study (Smith et al., 2016). individual differences in the consumption of water and the consumption of other sources of water such as nonresidential water, bottled water, and other beverages and the use of arsenic-specific filtration system were not considered as such information was not available. Given that use of bottled and filtered water could reduce the arsenic exposure, analysis of individual levels of arsenic and methylated arsenic in urine would provide better data about current exposure (Concha et al., 2006), but could misclassify exposure levels from earlier years. High LODs with a high percentage of samples below the LOD could also lead to exposure misclassification. For example, any sample with a LOD of 10 ppb was assigned a LOD of 10/2 or 5 ppb, automatically placing it in the high exposure group. However, all private water wells had LODs of 1 ppb and the number of public water samples below a LOD of 10 ppb was small (12%) and further reduced when multiple measurements of the same water source on the same day, month or year were averaged. In addition, an analysis of data after excluding all public water samples with LODs over 1 ppb still showed the significant dose-response relationship between arsenic exposure and prostate cancer, with the risk decreased by 6–10% compared to the current results. This underscores the robustness of the findings. Second, other risk factors for prostate cancer including family history of prostate cancer, hormonal, and other genetic factors could not be considered because those data were not available (Alberti, 2010; Hebert et al., 1998; Rowlands et al., 2009). Since Iowans have a high degree of German and Scandinavian ancestry, future studies could investigate the influence of inherited susceptibility by comparing Iowa with other states. Third, African-Americans have a higher risk of prostate cancer, but because of the small number of African-Americans in Iowa, the study was restricted to whites. Last, this study is an ecological study conducted with county-level data because individual-level data were not available. Individual-level analysis would be required for a stronger conclusion with better exposure assessment and consideration of risk factors (Greenland, 2001).
Despite these limitations, we observed a significantly increased risk of prostate cancer in a dose-response manner as arsenic concentration in the drinking water increased even at low levels. Importantly, this study allows the identification of “hot spot” counties with higher levels of arsenic contamination and higher incidences of prostate cancer for further study and for community-level intervention strategies. As our low-level arsenic was even lower than the current regulatory level of 10 ppb and showed an increased risk of prostate cancer, arsenic awareness initiatives like for example the one in New Jersey (http://blogs.ei.columbia.edu/2016/06/16/get-the-facts-arsenic-in-new-jersey-well-water/) should be implemented in states with private wells that may contain arsenic to inform the public. In addition, stricter regulations should be discussed if this result is replicated in future studies including those with individual-level analysis.
Highlights.
Arsenic data for Iowa public water sources and private well water were analyzed
Counties grouped in tertiles: 1.08–2.06 ppb, 2.07–2.98 ppb, 2.99–18.59 ppb arsenic
Risk ratios (RR) for prostate cancer were 1.23 (median), 1.28 (high arsenic group)
For aggressive cancers the high arsenic group RR increased to 1.36 (CI 1.28–1.45)
If findings are confirmed, stricter arsenic limits in drinking water may be needed
Acknowledgments
Financial support was provided by the Training Core of the Iowa Superfund Research Program P42 ES013661 from NIEHS and the Center for Health Effects of Environmental Contaminants (CHEEC). We also appreciate the help from the Iowa Department of Public Health for access to data from the Iowa Behavioral Risk Factor Surveillance System (BRFSS) and the Iowa Department of Natural Resources for access to public water data. Cancer data were provided with contract support (N01-PC-35143 and HHSN261201300020I) from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no potential conflicts of interest.
References
- Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Affairs. 2002;21:60–76. doi: 10.1377/hlthaff.21.2.60. [DOI] [PubMed] [Google Scholar]
- Alavanja MC, et al. Use of agricultural pesticides and prostate cancer risk in the Agricultural Health Study cohort. Am. J. Epidemiol. 2003;157:800–14. doi: 10.1093/aje/kwg040. [DOI] [PubMed] [Google Scholar]
- Alberti C. Hereditary/familial versus sporadic prostate cancer: few indisputable genetic differences and many similar clinicopathological features. Eur. Rev. Med. Pharmacol. Sci. 2010;14:31–41. [PubMed] [Google Scholar]
- Anselin L. Local Indicators of Spatial Association—LISA. Geographical Analysis. 1995;27:93–115. [Google Scholar]
- Argos M, et al. Arsenic and lung disease mortality in Bangladeshi adults. Epidemiology. 2014;25:536–43. doi: 10.1097/EDE.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baastrup R, et al. Arsenic in drinking-water and risk for cancer in Denmark. Environ. Health Perspect. 2008;116:231–7. doi: 10.1289/ehp.10623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band PR, et al. Prostate cancer risk and exposure to pesticides in British Columbia Farmers. The Prostate. 2011;71:168–183. doi: 10.1002/pros.21232. [DOI] [PubMed] [Google Scholar]
- Bardach AE, et al. Epidemiology of chronic disease related to arsenic in Argentina: A systematic review. Sci. Total Environ. 2015;538:802–16. doi: 10.1016/j.scitotenv.2015.08.070. [DOI] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, Waalkes MP. Inorganic arsenic and human prostate cancer. Environ. Health Perspect. 2008;116:158–164. doi: 10.1289/ehp.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, et al. Acquisition of androgen independence by human prostate epithelial cells during arsenic-induced malignant transformation. Environ. Health Perspect. 2005;113:1134–9. doi: 10.1289/ehp.7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, et al. Mechanisms of acquired androgen independence during arsenic-induced malignant transformation of human prostate epithelial cells. Environ. Health Perspect. 2007;115:243–7. doi: 10.1289/ehp.9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawley OW. Trends in prostate cancer in the United States. J. Natl. Cancer Inst. 2012;2012:152–6. doi: 10.1093/jncimonographs/lgs035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KG, et al. Arsenic, drinking water, and health: a position paper of the American Council on Science and Health. Regul. Toxicol. Pharmacol. 2002;36:162–74. doi: 10.1006/rtph.2002.1573. [DOI] [PubMed] [Google Scholar]
- Bulka CM, et al. Arsenic in drinking water and prostate cancer in Illinois counties: An ecologic study. Environ. Res. 2016;148:450–6. doi: 10.1016/j.envres.2016.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEEC. Center for Health Effects of Environmental Contamination. University of Iowa; Iowa City, IA: 2009. Iowa Statewide Rural Well Water Survey Phase 2 (SWRL2) Results and Analysis. [Google Scholar]
- Chen C-J, Wang C-J. Ecological correlation between arsenic level in well water and age-adjusted mortality from malignant neoplasms. Cancer research. 1990;50:5470–5474. [PubMed] [Google Scholar]
- Chen CJ, et al. Arsenic and cancers. Lancet. 1988;1:414–5. doi: 10.1016/s0140-6736(88)91207-x. [DOI] [PubMed] [Google Scholar]
- CHR. County Health Rankings and Roadmaps (CHR) University of Wisconsin Population Health Institute; 2017. [Google Scholar]
- Chung JY, et al. Environmental source of arsenic exposure. J. Prev. Med. Public Health. 2014;47:253–257. doi: 10.3961/jpmph.14.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleophas TJ, Zwinderman AH. SPSS for Starters and 2nd Levelers. Springer International Publishing; Cham: 2016. Poisson Regression for Outcome Rates (50 Patients) pp. 121–125. [Google Scholar]
- Cockburn M, et al. Prostate Cancer and Ambient Pesticide Exposure in Agriculturally Intensive Areas in California. Am. J. Epidemiol. 2011;173:1280–1288. doi: 10.1093/aje/kwr003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha G, et al. Spatial and temporal variations in arsenic exposure via drinking-water in northern Argentina. J. Health Popul. Nutr. 2006;24:317–26. [PMC free article] [PubMed] [Google Scholar]
- EPA. National Primary Drinking Water Regulations. U.S. Environmental Protection Agency; Washington, DC: 2009. [Google Scholar]
- Erickson ML, Barnes RJ. Glacial Sediment Causing Regional-Scale Elevated Arsenic in Drinking Water. Ground water. 2005;43:796–805. doi: 10.1111/j.1745-6584.2005.00053.x. [DOI] [PubMed] [Google Scholar]
- Farioli A, et al. Risk of mesothelioma following external beam radiotherapy for prostate cancer: a cohort analysis of SEER database. Cancer Causes & Control. 2013;24:1535–1545. doi: 10.1007/s10552-013-0230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreccio C, et al. Case-control study of arsenic in drinking water and kidney cancer in uniquely exposed northern Chile. Am. J. Epidemiol. 2013;178:813–8. doi: 10.1093/aje/kwt059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Esquinas E, et al. Arsenic exposure and cancer mortality in a US-based prospective cohort: the strong heart study. Cancer Epidemiol. Biomarkers Prev. 2013;22:1944–53. doi: 10.1158/1055-9965.EPI-13-0234-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S. Ecologic versus individual-level sources of bias in ecologic estimates of contextual health effects. Int. J. Epidemiol. 2001;30:1343–1350. doi: 10.1093/ije/30.6.1343. [DOI] [PubMed] [Google Scholar]
- Gunduz O, et al. Statistical analysis of causes of death (2005–2010) in villages of Simav Plain, Turkey, with high arsenic levels in drinking water supplies. Arch. Environ. Occup. Health. 2015;70:35–46. doi: 10.1080/19338244.2013.872076. [DOI] [PubMed] [Google Scholar]
- Hebert JR, et al. Nutritional and socioeconomic factors in relation to prostate cancer mortality: a cross-national study. J. Natl. Cancer Inst. 1998;90:1637–1647. doi: 10.1093/jnci/90.21.1637. [DOI] [PubMed] [Google Scholar]
- Hinwood AL, et al. Cancer incidence and high environmental arsenic concentrations in rural populations: results of an ecological study. Int. J. Environ. Res. Public Health. 1999;9:131–141. [Google Scholar]
- Hsu LI, et al. Use of arsenic-induced palmoplantar hyperkeratosis and skin cancers to predict risk of subsequent internal malignancy. Am. J. Epidemiol. 2013;177:202–212. doi: 10.1093/aje/kws369. [DOI] [PubMed] [Google Scholar]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volum 100C-6. International Agency of Research on Cancer; Lyon, France: 2009. Arsenic and arsenic compounds. [Google Scholar]
- IDNR. Iowa Community Private Well Study. Iowa Department of Natural Resources; Iowa City, IA: 2004. [Google Scholar]
- Kissling WD, Carl G. Spatial autocorrelation and the selection of simultaneous autoregressive models. Glob. Ecol. Biogeogr. 2008;17:59–71. [Google Scholar]
- Koutros S, et al. Risk of total and aggressive prostate cancer and pesticide use in the Agricultural Health Study. Am. J. Epidemiol. 2013;177:59–74. doi: 10.1093/aje/kws225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DR, et al. Drinking water arsenic in Utah: A cohort mortality study. Environ. Health Perspect. 1999;107:359–65. doi: 10.1289/ehp.99107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Carrillo L, et al. Exposure to phthalates and breast cancer risk in northern Mexico. Environ. Health Perspect. 2010;118:539–44. doi: 10.1289/ehp.0901091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall G, et al. Fifty-year study of lung and bladder cancer mortality in Chile related to arsenic in drinking water. J. Natl. Cancer Inst. 2007;99:920–8. doi: 10.1093/jnci/djm004. [DOI] [PubMed] [Google Scholar]
- Melak D, et al. Arsenic methylation and lung and bladder cancer in a case-control study in northern Chile. Toxicol. Appl. Pharmacol. 2014;274:225–31. doi: 10.1016/j.taap.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael HA. An arsenic forecast for China. Science. 2013;341:852–853. doi: 10.1126/science.1242212. [DOI] [PubMed] [Google Scholar]
- Naujokas MF, et al. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect. 2013;121:295–302. doi: 10.1289/ehp.1205875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, et al. Urine arsenic concentrations and species excretion patterns in American Indian communities over a 10-year period: the Strong Heart Study. Environ. Health Perspect. 2009;117:1428–33. doi: 10.1289/ehp.0800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez O, et al. Arsenic and chromium topsoil levels and cancer mortality in Spain. Environ. Sci. Pollut. Res. Int. 2016;23:17664–75. doi: 10.1007/s11356-016-6806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SM, et al. Impact of prediagnosis smoking, alcohol, obesity, and insulin resistance on survival in male cancer patients: National Health Insurance Corporation Study. J. Clin. Oncol. 2006;24:5017–5024. doi: 10.1200/JCO.2006.07.0243. [DOI] [PubMed] [Google Scholar]
- Proctor BD, et al. Income and Poverty in the United States: 2015. U.S. Census Bureau; Washington, DC: 2016. [Google Scholar]
- Reynolds P, et al. Residential proximity to agricultural pesticide use and incidence of breast cancer in California, 1988–1997. Environ. Health Perspect. 2005;113:993–1000. doi: 10.1289/ehp.7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivara MI, et al. Cancer risk in an arsenic-contaminated area of Chile. Toxicol. Ind. Health. 1997;13:321–338. doi: 10.1177/074823379701300217. [DOI] [PubMed] [Google Scholar]
- Ross LE, et al. The effect of physician-patient discussions on the likelihood of prostate-specific antigen testing. Journal of the National Medical Association. 2006;98:1823–1829. [PMC free article] [PubMed] [Google Scholar]
- Rowlands MA, et al. Circulating insulin-like growth factor peptides and prostate cancer risk: a systematic review and meta-analysis. Int J Cancer. 2009;124:2416–29. doi: 10.1002/ijc.24202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundle A, et al. A prospective study of socioeconomic status, prostate cancer screening and incidence among men at high risk for prostate cancer. Cancer Causes & Control. 2013;24:297–303. doi: 10.1007/s10552-012-0108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanic C, et al. Obesity and cancer risk among white and black United States veterans. Cancer Causes & Control. 2004;15:35–44. doi: 10.1023/B:CACO.0000016573.79453.ba. [DOI] [PubMed] [Google Scholar]
- Shearer JJ, et al. Inorganic arsenic-related changes in the stromal tumor microenvironment in a prostate cancer cell-conditioned media model. Environ. Health Perspect. 2016;124:1009–15. doi: 10.1289/ehp.1510090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh GK, et al. NCI Cancer Surveillance Monograph Series, Number 4. National Cancer Institute; Bethesda, MD: 2003. Area Socioeconomic Variations in U.S. Cancer Incidence, Mortality, Stage, Treatment, and Survival, 1975–1999. [Google Scholar]
- Smith AE, et al. Assessing arsenic exposure in households using bottled water or point-of-use treatment systems to mitigate well water contamination. Sci. Total Environ. 2016;544:701–10. doi: 10.1016/j.scitotenv.2015.11.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, et al. Cancer risks from arsenic in drinking water. Environ. Health Perspect. 1992;97:259–267. doi: 10.1289/ehp.9297259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus CM, et al. Drinking water arsenic in northern Chile: high cancer risks 40 years after exposure cessation. Cancer Epidemiol. Biomarkers Prev. 2013;22:623–30. doi: 10.1158/1055-9965.EPI-12-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus CM, et al. The temporal stability of arsenic concentrations in well water in western Nevada. Environonmental Research. 2005;99:164–8. doi: 10.1016/j.envres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Thun MJ, et al. Alcohol consumption and mortality among middle-aged and elderly US adults. N. Engl. J. Med. 1997;337:1705–1714. doi: 10.1056/NEJM199712113372401. [DOI] [PubMed] [Google Scholar]
- USCensus. Gross and net migration tabulations and county-to-county migration flow data (1995 to 2000) 2000 [Google Scholar]
- USCensus. 2005–2009 American Community Survey county-to-county migration files. U.S. Census Bureau; 2012. [Google Scholar]
- USCensus. Small Area Health Insurance Estimates (SAHIE) U.S. Census Bureau; 2015. [Google Scholar]
- USCensus. 2010–2014 American Community Survey county-to-county migration flows. U.S. Census Bureau; 2016. [Google Scholar]
- USDA. 2002 Census of Agriculture: Iowa. Volume 1, Geographic Area Series Part 15. U.S. Department of Agriculture; 2004. [Google Scholar]
- USGS. Estimated annual agricultural pesticide use. U.S. Geological Survey; 2017. [Google Scholar]
- Witz IP. Yin-yang activities and vicious cycles in the tumor microenvironment. Cancer Res. 2008;68:9–13. doi: 10.1158/0008-5472.CAN-07-2917. [DOI] [PubMed] [Google Scholar]
- Wu M-M, et al. Dose-response relation between arsenic concentration in well water and mortality from cancers and vascular diseases. Am. J. Epidemiol. 1989;130:1123–1132. doi: 10.1093/oxfordjournals.aje.a115439. [DOI] [PubMed] [Google Scholar]
- Yang CY, et al. Does arsenic exposure increase the risk for prostate cancer? J. Toxicol. Environ. Health A. 2008;71:1559–63. doi: 10.1080/15287390802392065. [DOI] [PubMed] [Google Scholar]
- Yun YH, et al. Cigarette smoking and cancer incidence risk in adult men: National Health Insurance Corporation Study. Cancer Detect. Prev. 2005;29:15–24. doi: 10.1016/j.cdp.2004.08.006. [DOI] [PubMed] [Google Scholar]