Abstract
Conquering immunosuppression in tumor microenvironments is crucial for effective cancer immunotherapy. It is well known that interleukin (IL)‐6, a pleiotropic cytokine, is produced in the tumor‐bearing state. In the present study, we investigated the precise effects of IL‐6 on antitumor immunity and the subsequent tumorigenesis in tumor‐bearing hosts. CT26 cells, a murine colon cancer cell line, were intradermally injected into wild‐type and IL‐6‐deficient mice. As a result, we found that tumor growth was decreased significantly in IL‐6‐deficient mice compared with wild‐type mice and the reduction was abrogated by depletion of CD8+ T cells. We further evaluated the immune status of tumor microenvironments and confirmed that mature dendritic cells, helper T cells and cytotoxic T cells were highly accumulated in tumor sites under the IL‐6‐deficient condition. In addition, higher numbers of interferon (IFN)‐γ‐producing T cells were present in the tumor tissues of IL‐6‐deficient mice compared with wild‐type mice. Surface expression levels of programmed death‐ligand 1 (PD‐L1) and MHC class I on CT26 cells were enhanced under the IL‐6‐deficient condition in vivo and by IFN‐γ stimulation in vitro. Finally, we confirmed that in vivo injection of an anti‐PD‐L1 antibody or a Toll‐like receptor 3 ligand, polyinosinic‐polycytidylic acid, effectively inhibited tumorigenesis under the IL‐6‐deficient condition. Based on these findings, we speculate that a lack of IL‐6 produced in tumor‐bearing host augments induction of antitumor effector T cells and inhibits tumorigenesis in vivo, suggesting that IL‐6 signaling may be a promising target for the development of effective cancer immunotherapies.
Keywords: Cytotoxic T cells, dendritic cells, interferon‐γ, interleukin‐6, programmed death‐ligand 1
Recently, effective immune checkpoint therapy using anti‐programmed cell death protein 1 (PD‐1), ‐programmed death‐ligand 1 (PD‐L1), and/or ‐cytotoxic T lymphocyte‐associated protein 4 antibodies to activate effector T cells in cancer patients has been reported for various solid tumors.1, 2, 3 These results indicate that cancer antigen‐specific T cells, which eliminate cancer cells, potentially exist in tumor microenvironments. As a result, blocking negative signals against tumor‐infiltrating T cells can restore the cytotoxic function toward their target cancer cells. Thus, introduction of cancer‐specific T cells to the tumor microenvironment is required as the first step for more effective cancer immunotherapies.
Dendritic cells, which are representative antigen‐presenting cells, strongly induce antigen‐specific immune responses through activation of CD4+ T and CD8+ T cells. In cancer patients, mature dendritic cells highly expressing MHC class I, MHC class II and co‐stimulatory molecules on their cell surface are crucial to induce cancer antigen‐specific effector T cells. Therefore, proper regulation of dendritic cells in the tumor microenvironment is important for induction of antitumor immunity.4, 5, 6, 7
Interleukin (IL)‐6, a pleiotropic cytokine with a variety of effects on cells and tissues, is produced by many different cells, including immune cells, fibroblasts, endothelial cells and tumor cells. IL‐6 first binds to the IL‐6 receptor (IL‐6R), inducing dimerization of gp130, a signal transducer. Gp130 dimerization rapidly activates the JAK family and several signaling pathways, including phosphatidylinositide 3‐kinase/ERK/MAPK and signal transducer and activator of transcription 3 (STAT3). STAT3 activation induces numerous effector genes involved in cell proliferation, differentiation and survival.8, 9 Various cell types, including cancer cells, cancer‐associated fibroblasts and immune cells, are well known to produce IL‐6 in the tumor‐bearing host. A previous study has reported that serum IL‐6 levels are related to the tumor stage and size, metastasis, and survival in colon cancer patients.10 Clinical studies have demonstrated that the IL‐6 level might be a good predictive biomarker for the clinical benefit of peptide vaccines.11, 12
Previously, it was reported that IL‐6 signaling suppresses MHC class II expression on murine dendritic cells through STAT3 activation and attenuates CD4+ T cell‐mediated immune responses.13, 14 Furthermore, a previous study using tumor‐bearing mouse models indicated that administration of a mAb antagonizing IL‐6R enhances T cell responses and inhibits tumor growth in vivo.15, 16 In tumor‐bearing mice, IL‐6 suppresses CD4+ T cell‐mediated immunity through downregulation of MHC class II by enhancing the arginase activity of dendritic cells. Thus, IL‐6‐mediated STAT3 activation appears to be a critical mechanism to induce dysfunctional immune system responses in the tumor microenvironment through regulation of antigen‐presenting cells. Recent studies have revealed that IL‐6 produced in tumor environments suppresses differentiation of interferon (IFN)‐ γ‐producing helper T cells and promotes subsequent tumor formation.17, 18, 19 Furthermore, we found that IL‐6 is widely produced and STAT3 is activated in tumor microenvironments of colorectal cancer patients, and the CD11b+CD11c+ population isolated from tumor tissues shows higher IL‐6 gene expression compared with the same phenotypic population isolated from PBMC.20 Blockade of IL‐6/STAT3 signaling cascades may, therefore, be a novel approach to overcome the dysfunctional antitumor immunity of cancer patients.
In this study, we further investigated the precise effects of IL‐6 on antitumor immunity and the subsequent tumorigenesis in tumor‐bearing hosts, and revealed that a lack of IL‐6 in tumor microenvironments augments type‐1 immunity, including induction of antitumor cytotoxic T cells, and inhibits tumorigenesis in vivo. These findings suggest that blockade of IL‐6 signaling may enhance immunotherapies, such as immune checkpoint inhibition and administration of immunological adjuvants, in cancer patients.
Materials and Methods
Mice and cells
BALB/c mice were obtained from Charles River Japan (Kanagawa, Japan). IL‐6−/− mice were obtained from the Center for Animal Disease Models, Research Institute for Biomedical Sciences, Tokyo University of Science (Chiba, Japan). All mice were maintained in specific pathogen‐free conditions and used at 6–8 weeks of age. Murine colon cancer cell line CT26 was obtained from the American Type Culture Collection (CRL‐2638; VA). All mouse experiments were approved by the Animal Ethics Committee of Hokkaido University (No. 14‐0062) and conducted in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the University.
Antibodies, cytokines and chemicals
Fluorescent dye‐conjugated anti‐CD45 (30‐F11), anti‐CD11c (N418), anti‐CD4 (GK1.5), anti‐CD8 (53‐6.7), anti‐H‐2Kd (AF6‐88.5), anti‐I‐Ad (AF6‐120.1), anti‐IFN‐γ (XMG1.2) and anti‐IL‐4 (11B11) mAbs were purchased from Biolegend (San Diego, CA, USA) or BD Biosciences (San Diego, CA, USA). A mAb for CD8 depletion (clone 53.6.7) and an antagonistic mAb against PD‐L1 (clone 10F.9G2) were purchased from Bio X Cell (West Lebanon, NH, USA). Recombinant murine IFN‐γ was purchased from PeproTech EC (London, UK). 7‐AAD Viability Dye was purchased from BECKMAN COULTER (Marseille Cedex, France). Phorbol 12‐myristate 13‐acetate (PMA) and A23187 calcium ionophore were purchased from Sigma‐Aldrich (St. Louis, MO, USA). Polyinosinic‐polycytidylic acid (poly I:C) was purchased from InvivoGen (San Diego, CA, USA).
Cell culture
CT26 cells were maintained in RPMI‐1640 medium (Wako Pure Chemical Industries, Osaka, Japan) supplemented with 10% FCS, 200 U/mL penicillin, 100 μg/mL streptomycin, 10 mM HEPES and 0.05 mmol/L 2‐mercaptoethanol (Sigma‐Aldrich) at 37°C in a humidified atmosphere containing 5% CO2. For flow cytometry, 2.5 × 105 CT‐26 cells were treated with 50 ng/mL IFN‐γ in 12‐well culture plates for 24 h.
Tumor‐bearing mouse model
CT26 cells (1 × 106) were injected intradermally into wild‐type and IL‐6−/− mice (day 0). The tumor size was measured by micrometer calipers from day 5. Tumor volumes were calculated using the following formula: volume (mm3) = 0.2 × (length [mm] × width [mm]) × (height [mm])2. For ex vivo analysis, CT26 cells were transfected with pMX‐IRES‐GFP, obtained from Dr T Kitamura (The University of Tokyo), by Lipofectamine 3000 (ThermoFisher Scientific, Waltham, MA, USA) and the GFP‐transduced CT26 cells were used for the subsequent flow cytometry. The anti‐CD8 mAb or control IgG (200 μg/mouse) was injected intraperitoneally into wild‐type and IL‐6−/− mice at days −1 and 5, and then every 4 days thereafter. In therapeutic experiments, the anti‐PD‐L1 mAb (200 μg/mouse), control IgG (200 μg/mouse) or poly I:C (50 μg/mouse) were injected intraperitoneally into CT26 tumor‐bearing wild‐type and IL‐6−/− mice at day 5 and then every 4 days thereafter.
Immunohistochemistry
Tumor tissues obtained from CT26 tumor‐bearing wild‐type and IL‐6−/− mice at day 14 were fixed in 4% paraformaldehyde PBS and then embedded in paraffin. After deparaffinization, antigen retrievals for CD3 and CD11c were performed with a reagent kit (pH 9.0) (415211; Nichirei Bioscience, Tokyo, Japan) at 95°C for 10 min and with proteinase K solution (S3004; Dako, Hamburg, Germany) at room temperature for 5 min, respectively. Endogenous peroxidase activity was blocked by incubation with 3% hydrogen peroxide at room temperature for 10 min. After washing with TBS, sections were treated with anti‐CD3 (ab134096; Abcam) or anti‐CD11c (GTX74940; GeneTex, Inc., Irvine, CA, USA) antibodies overnight at 4°C. Sections for CD3 and CD11c were incubated at room temperature for 30 min with Histofine Simple Stain, MAX PO (R) (424144; Nichirei Bioscience) at room temperature for 30 min or with rabbit Anti‐Hamstrer IgG (6215‐01; Southern Biotechnology Associates, Birmingham, AL, USA) at room temperature for 30 min, Histofine Simple Stain, MAX PO (R) (424144; Nichirei Bioscience) at room temperature for 30 min, TSA PLUS Biotin KIT (NEL749A001; PerkinElmer, Waltham, MA, USA) at room temperature for 5 min and VECTASTAIN Elite ABC Reagent (PK6100; Vecter Laboratories, Burlingame, CA, USA) at room temperature for 30 min. Protein expression was visualized using 3‐3′‐diaminobezidine‐4HCL at room temperature for 5 min. Finally, all sections were counterstained with Mayer's H&E.
Flow cytometry
GFP‐transduced CT26 cells (1 × 106) were intradermally injected into wild‐type and IL‐6−/− mice. Tumor tissues were obtained from CT26 tumor‐bearing wild‐type and IL‐6−/− mice at day 12. The retrieved tissues were minced using scissors and digested in a 1‐mg/mL collagenase solution (Sigma, Tokyo, Japan). Cell suspensions were prepared and stained with fluorescent dye‐conjugated mAbs after purified anti‐FcγR mAb treatment. GFP+CD45− and GFP−CD45+ populations collected from tumor tissues were determined as CT26 cancer cells and immune cells, respectively. A FACSCanto II (BD Bioscience) was used for flow cytometry. The results were analyzed with FlowJo software (Tree Star, Ashland, OR, USA). The mean fluorescence intensity was calculated in paired samples.
Intracellular cytokine staining
For detection of cytoplasmic IFN‐γ expression in T cells, single cell suspensions from tumors and regional lymph nodes (1 × 106 cells in 12‐well culture dishes) were stimulated with PMA (25 ng/mL) and A23187 calcium ionophore (1 μg/mL) for 4 h in the presence of brefeldin A. Then, the cells were harvested and stained with the anti‐CD4 mAb, anti‐CD8 mAb and 7‐AAD, and fixed with 4% paraformaldehyde. After permeabilization, the fixed cells were stained with the anti‐IFN‐γ mAb. Data were acquired by flow cytometry.
Statistical analyses
In vitro experiments were repeated at least three to five times. In vivo experiments consisting of 5–10 mice in each group were independently performed two to three times. Single representative experiments are indicated in figures. Mean values and SD were calculated for each dataset. Significant differences in the results were determined by a one‐way anova and Dunnett's post‐test. In some experiments, the two‐tailed Student's t‐test was used for evaluation of the difference between two groups. The P‐values of *P < 0.05 were indicated as statistically significant in the figures, using the two‐sided Student's t‐test. P < 0.05 was considered as significant.
Results
Lack of interleukin‐6 in the tumor‐bearing host suppresses tumorigenesis and augments accumulation of immune cells in vivo
To address the relationship of IL‐6 deficiency with tumor growth in vivo, murine CT26 colorectal cancer cells were intradermally injected into wild‐type and IL6−/− mice. Tumor growth was significantly reduced in IL‐6−/− mice compared with wild‐type mice (Fig. 1a,b). Next, we evaluated the tumor‐infiltrating cells by immunohistochemistry (IHC) to evaluate the immune status of the tumor environment in CT26 tumor‐bearing wild‐type and IL‐6−/− mice. CD3+ T cells and CD11c+ dendritic cells had accumulated in tumor tissues of IL‐6−/− mice compared with wild‐type mice (Fig. 1c). We further investigated the ratios of tumor‐infiltrating immune cells by flow cytometry. As a result, CD45+ immune cells were more highly infiltrated into the tumor tissues of IL‐6−/− mice compared with wild‐type mice. In addition, CD4+ T, CD8+ T and CD11c+I‐Adhigh mature dendritic cells were more infiltrated in tumor tissues of IL‐6‐deficient mice (Fig. 1d). These data suggest that a lack of IL‐6 in the tumor‐bearing host may facilitate introduction of effector T cells and dendritic cells into the tumor microenvironment.
Figure 1.
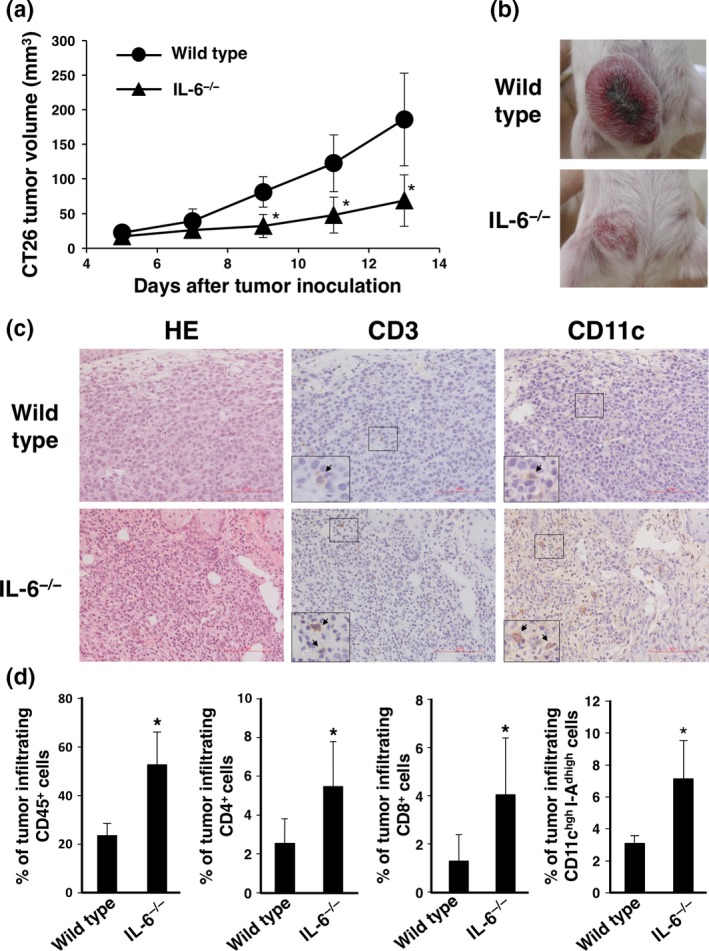
In vivo tumorigenesis and immune status under the interleukin (IL)‐6‐deficient condition. CT26 murine colon cancer cells (1 × 106) were intradermally injected into wild‐type and IL‐6−/− BALB/c mice (day 0). (a) Tumor size was monitored for 13 days after injection. Means and SD (n = 5) of the tumor volume are indicated. *P < 0.05 by Student's t‐test (b) Representative images of tumors at day 15. (c) H&E and immunohistochemistry (IHC) staining of tumor tissues collected at day 14 were performed using anti‐CD3 and anti‐CD11c antibodies. Representative images are shown. Bar represents 100 μm. (d) Tumor‐infiltrating immune cells of wild‐type and IL‐6−/− mice at day 12 were evaluated by flow cytometry using anti‐CD45, anti‐CD4, anti‐CD8, anti‐CD11c and anti‐I‐Ad mAbs. Percentages of CD45+, CD4+ T, CD8+ T and CD11c+I‐Adhigh dendritic cells were calculated. Means and SD (n = 5) are indicated. *P < 0.05 by Student's t‐test.
Interleukin‐6 produced in the tumor microenvironment may suppress antitumor effects of cytotoxic T cells in vivo
Because our previous reports demonstrated that IL‐6 attenuates T cell immune responses through the reduction of mature dendritic cells,13, 14 we investigated whether the antitumor effect of cytotoxic T cells was involved in this mouse model. As a result, depletion of CD8+ T cells enhanced tumor growth in wild‐type mice (Fig. 2a–c), indicating that CD8+ T cells killed CT26 cancer cells of this model in vivo. We further confirmed that depletion of CD8+ T cells significantly augmented tumor growth in IL‐6−/− mice (Fig. 2a–c). Therefore, these data suggest that IL‐6 produced in a tumor‐bearing host may promote in vivo tumorigenesis through suppression of antitumor effector T cells.
Figure 2.
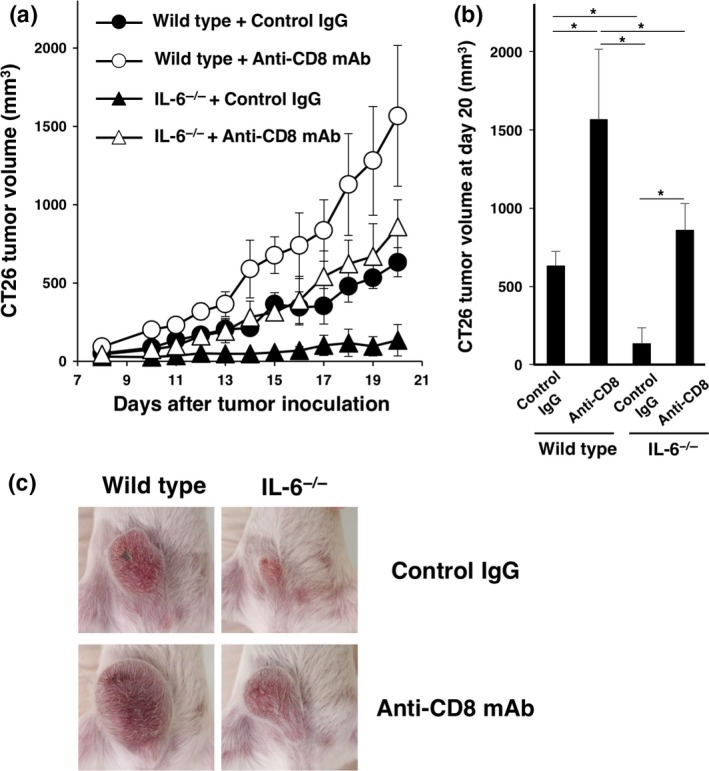
Effect of CD8+ T cells on tumorigenesis in interleukin (IL)‐6‐deficient mice. CT26 murine colon cancer cells (1 × 106) were intradermally injected into wild‐type and IL‐6−/− BALB/c mice (day 0). An anti‐CD8 mAb or control IgG (200 μg/mouse) was injected intraperitoneally into wild‐type and IL‐6−/− mice at days –1 and 5, and then every 4 days thereafter. (a) Tumor size was monitored for 13 days after injection. Means and SD (n = 5) of the tumor volume are indicated. *P < 0.05 by Dunnett's test. (b) Tumor volumes at day 20 are indicated. *P < 0.05 by Dunnett's test. (c) Representative images of tumors at day 15.
Interferon‐γ‐producing CD4+ T and CD8+ T cells accumulate in tumor tissues of interleukin‐6‐deficient mice
We further evaluated the cytokine production abilities of tumor‐infiltrating CD4+ T cells and CD8+ T cells in wild‐type and IL‐6−/− mice. As a result, higher numbers of IFN‐γ‐producing CD4+ T cells were present in the tumor tissues of IL‐6−/− mice compared with wild‐type mice (Fig. 3a,b). IL‐10‐ and IL‐17‐producing CD4+ T cells were not altered between IL‐6−/− and wild‐type mice (Fig. 3a,b). Furthermore, we confirmed that IFN‐γ‐producing CD8+ T cells were present in the tumor tissues of IL‐6−/− mice compared with wild‐type mice (Fig. 3a,b). IFN‐γ, but not IL‐10‐producing or IL‐17‐producing CD4+ T cells had increased in the draining lymph nodes of IL‐6−/− mice compared with wild‐type mice (Fig. S1). These data indicate that IL‐6 produced in the tumor‐bearing host attenuates both Th1 and Tc1 types of immunity not only in the tumor sites but also in total body, suggesting that IL‐6 might be a promising target to introduce antitumor effector T cells into tumor microenvironments.
Figure 3.

Cytokine‐producing ability of tumor infiltrating CD4+ T and CD8+ T cells under the interleukin (IL)‐6‐deficient condition. CT26 murine colon cancer cells (1 × 106) were intradermally injected into wild‐type and IL‐6−/− BALB/c mice (day 0). Cytokine production by CD4+ T and CD8+ T cells in tumor tissues of wild‐type and IL‐6−/− mice at day 12 were evaluated by flow cytometry using anti‐IFN‐γ, anti‐IL‐10, anti‐IL‐17, anti‐CD4 and anti‐CD8 mAbs. (a) Representative intracellular staining profiles of CD4+ T cells. Percentages of IFN‐γ‐ producing, IL‐10‐producing or IL‐17‐producing CD4+ T cells were calculated. Means and SD (n = 5) are indicated. *P < 0.05 by Student's t‐test. N.S. indicates not statistically significant. (b) Representative intracellular staining profiles of CD8+ T cells. Percentages of IFN‐γ‐producing CD8+ T cells were calculated. Means and SD (n = 5) are indicated. *P < 0.05 by Student's t‐test.
In this model, we found significant differences between the anti‐CD8 mAb‐treated wild‐type and IL‐6−/−, suggesting the existence of a CD8+ T‐independent beneficial effect of IL‐6 deficiency (Fig. 2b). To address the involvement of CD4+ T cells in the tumorigenesis, we performed depletion of CD4+T cells by using anti‐CD4 mAb in this model. In the early phase, such as at day 9 after inoculation of CT26 cells, the tumor growth was enhanced by in vivo injection of anti‐CD4 mAb; however, we found that depletion of CD4+ T cells significantly suppressed CT26 tumor growth at a later phase under IL‐6‐deficient condition (Fig. S2a–c). These data not only indicate that IL‐6 will suppress anti‐tumor function of CD4+ T cells at induction phase but also suggest that suppression of anti‐tumor immunity by Tregs may be involved in this model.
We further investigated the effect of both CD4+ T and CD8+ T depletions and compared with CD8+ T single depletion in IL‐6‐deficient condition. As the result, we found that there was no significant difference between the both depletions and CD8+ T single depletion (Fig. S2a–c). These data suggest that IL‐6 will suppress CD8+ T cells and other immune cells, but not CD4+ T cells, or may also directly stimulate cancer cells to augment the tumor growth in this model mice.
Type‐1 condition enhances expression levels of MHC class I and PD‐L1 on CT26 cells in vitro and in vivo
We examined the characteristic features of CT26 cancer cells under the type‐1 immune condition. IFN‐γ stimulation enhanced surface expression levels of H‐2Kd, MHC class I and PD‐L1 on CT26 cells in vitro (Fig. 4a). In addition, we found that both MHC class I and PD‐L1 expression levels on CT26 cells were significantly augmented in tumor‐bearing IL‐6−/− mice compared with wild‐type mice (Fig. 4b).
Figure 4.
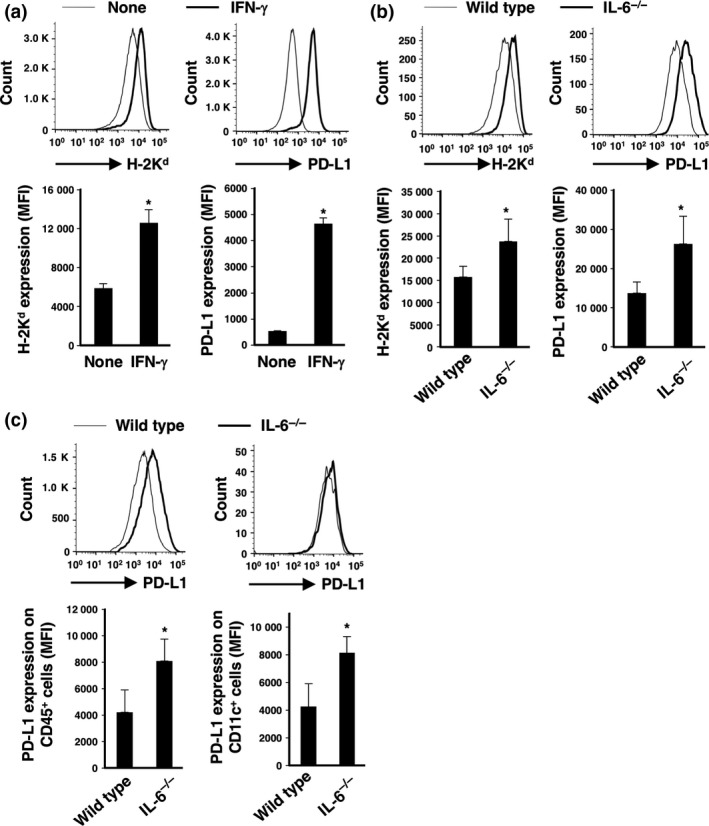
Expression levels of MHC class I and PD‐L1 on CT26 cells in vitro and in vivo. (a) CT26 cells were stimulated with IFN‐γ (50 ng/mL) for 24 h in vitro. Surface expression levels of MHC class I and PD‐L1 on CT26 cells were evaluated by flow cytometry using anti‐H‐2Kd and anti‐PD‐L1 mAbs. Representative profiles are shown. Mean fluorescence intensities (MFI) for the expression levels of H‐2Kd and PD‐L1 were calculated. Means and SD (n = 3) are indicated. *P < 0.05 by Student's t‐test. (b) GFP‐transduced CT26 murine colon cancer cells (1 × 106) were intradermally injected into wild‐type and IL‐6−/− BALB/c mice (day 0). Surface expression levels of MHC class I and PD‐L1 on GFP + CD45− CT26 cells at day 27 were evaluated by flow cytometry using anti‐H‐2Kd and anti‐PD‐L1 mAbs. Representative profiles are shown. MFI for the expression levels of H‐2Kd and PD‐L1 were calculated. Means and SD (n = 3) are indicated. *P < 0.05 by Student's t‐test. (c) Surface expression levels of MHC class I and PD‐L1 on GFP − CD45+ cells and GFP − CD45+ CD11c+ cells at day 27 were evaluated by flow cytometry using anti‐H‐2Kd and anti‐PD‐L1 mAbs. Representative profiles are shown. MFI for the expression levels of H‐2Kd and PD‐L1 were calculated. Means and SD (n = 3) are indicated. *P < 0.05 by Student's t‐test.
We further investigated the PD‐L1 expression levels on CD45+ immune cells and CD11c+ dendritic cells in tumor tissues of wild‐type and IL‐6−/− mice. As a result, we confirmed that PD‐L1 expression levels on both populations in IL‐6−/− mice were significantly enhanced compared with those in wild‐type mice (Fig. 4c).
These data suggest that CT26 cells and immune cells including dendritic cells attenuated antitumor responses by effector T cells through the PD‐L1 expressions under the IL‐6‐deficient condition in vivo, whereas MHC class I‐restricted recognition by cytotoxic CD8+ T cells was increased.
Interleukin‐6 deficiency facilitates antitumor effects of immune checkpoint therapy using the anti‐PD‐L1 mAb
To investigate the effect of immune checkpoint inhibition under the IL‐6‐deficient condition, we injected the anti‐PD‐L1 mAb into CT26 tumor‐bearing wild‐type and IL‐6−/− mice. We found that in vivo injection of the anti‐PD‐L1 mAb into IL‐6‐deficient mice significantly reduced tumorigenesis compared with wild‐type mice (Fig. 5a,b).
Figure 5.
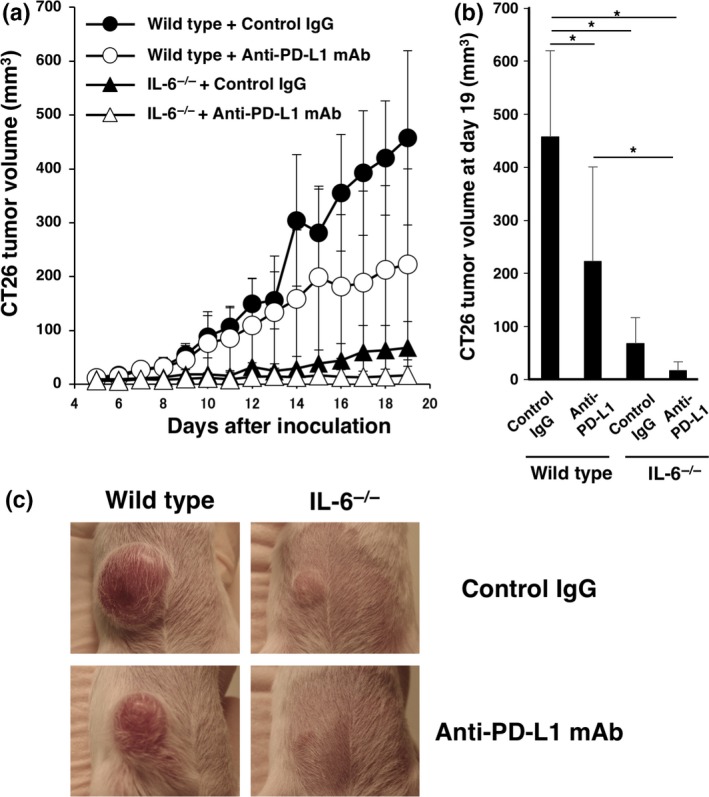
Effect of PD‐L1 blockade on tumorigenesis in interleukin (IL)‐6‐deficient mice. CT26 murine colon cancer cells (1 × 106) were intradermally injected into wild‐type and IL‐6−/− BALB/c mice (day 0). Anti‐PD‐L1 mAb or control IgG (200 μg/mouse) was injected intraperitoneally into wild type and IL‐6−/− mice at day 5 and then every 4 days thereafter. (a) Tumor size was monitored for 19 days after injection. Means and SD (n = 5) of the tumor volume are indicated. (b) Tumor volumes at day 19 are indicated. *P < 0.05 by Dunnett's test. (c) Representative images of tumors at day 15.
Moreover, we performed PD‐L1 combination therapy on IL‐6−/− mice using 4T1, murine breast cancer cells. As a result, we confirmed that tumor growth of the 4T1 model mice was significantly reduced under IL‐6‐deficient condition. The therapy using anti‐PD‐L1 mAb under IL‐6‐deficient condition was very effective as well as CT26, murine colon cancer cells (Fig. S3a–c). In this study, we further evaluated the effect of in vivo injection anti‐IL‐6R mAb into CT26 tumor‐bearing wild‐type mice. As a result, we confirmed that the administration of anti‐IL‐6R mAb significantly suppressed CT26 tumor growth in this therapy model (Fig. S4a–c).
These data suggest that the lack of IL‐6, which enhanced type‐1 immunity in the tumor‐bearing state, enhances the antitumor effect of immune checkpoint therapy using an anti‐PD‐L1 mAb.
In vivo injection of the anti‐PD‐L1 mAb into interleukin‐6‐deficient mice promotes accumulation of T cells and mature dendritic cells in the tumor microenvironment
We evaluated immune cells in the tumor environment of CT26 tumor‐bearing wild‐type and IL‐6−/− mice injected with the anti‐PD‐L1 mAb by IHC. CD3+ T cells and CD11c+ dendritic cells were highly accumulated in the tumor tissues of anti‐PD‐L1 mAb‐injected IL‐6−/− mice (Fig. 6a). We further investigated the ratios of tumor‐infiltrating immune cells by flow cytometry. As a result, cytotoxic CD8+ T cells and CD11c+I‐Adhigh mature dendritic cells were more infiltrated in the tumor tissues of IL‐6‐deficient mice injected with the anti‐PD‐L1 mAb compared with wild‐type mice (Fig. 6b). These data suggest that a lack of IL‐6 in the tumor‐bearing host may facilitate the introduction of antitumor effector T cells and mature dendritic cells into the tumor microenvironment by anti‐PD‐L1 mAb treatment.
Figure 6.

Immune status of anti‐PD‐L1 mAb‐treated tumor‐bearing mice under the interleukin (IL)‐6‐deficient condition. CT26 murine colon cancer cells (1 × 106) were intradermally injected into wild‐type and IL‐6−/− BALB/c mice (day 0). (a) Immunohistochemistry (IHC) staining of tumor tissues collected at day 27 was performed using anti‐CD3 and anti‐CD11c mAbs. Representative images are shown. Bar represents 100 μm. (b) Tumor‐infiltrating immune cells of wild‐type and IL‐6−/− mice at day 27 were evaluated by flow cytometry using anti‐CD8, anti‐CD11c and anti‐I‐Ad mAbs. Percentages of CD8+ T cells and CD11c+I‐Adhigh dendritic cells were calculated. Means and SD (n = 3) are indicated. *P < 0.05 by Dunnett's test.
Poly I:C administration under the interleukin‐6‐deficient condition shows a strong antitumor effect in vivo
Administration of immunological adjuvants occasionally induces IL‐6 production in vivo.21 As a therapeutic experiment, we injected poly I:C, a TLR3 ligand, into tumor‐bearing wild‐type and IL‐6−/− mice. The poly I:C injection significantly reduced tumorigenesis in both IL‐6‐deficient mice and wild‐type mice (Fig. 7a,b).
Figure 7.
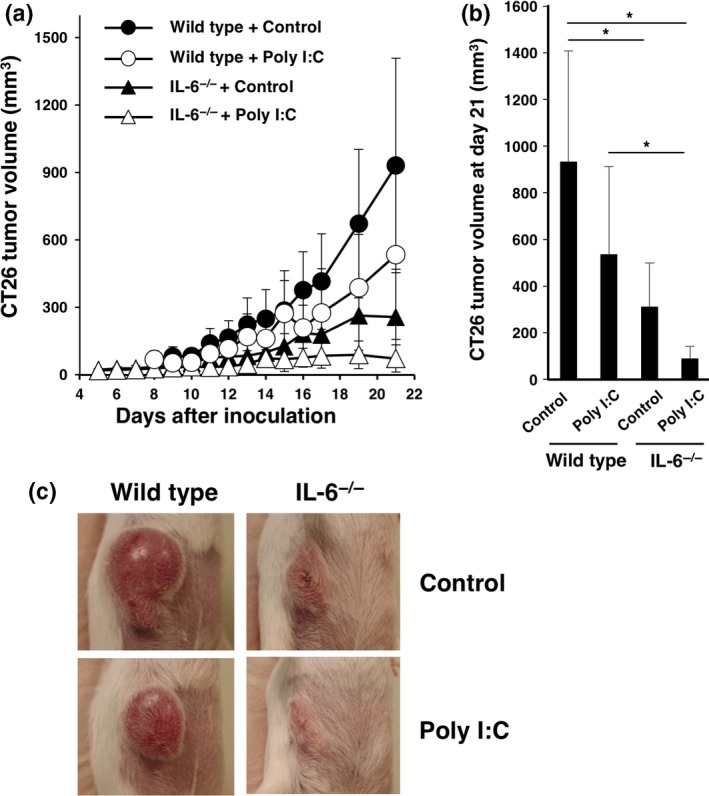
Effect of poly I:C treatment on tumorigenesis in interleukin (IL)‐6‐deficient mice. CT26 murine colon cancer cells (1 × 106) were intradermally injected into wild‐type and IL‐6−/− BALB/c mice (day 0). Poly I:C (50 μg/mouse) or control PBS was injected intraperitoneally into wild‐type and IL‐6−/− mice at day 5 and then every 4 days thereafter. (a) Tumor size was monitored for 21 days after injection. Means and SD (n = 5) of the tumor volume are indicated. (b) Tumor volumes at day 21 are indicated. *P < 0.05 by Dunnett's test. (c) Representative images of tumors at day 15.
In this study, we further investigated IL‐12 production by CD11c+ cells from tumor tissues of polyI:C‐ or control PBS‐treated wild‐type or IL‐6−/− mice. As a result, we confirmed that the IL‐12p35 gene expression levels of control‐stimulated and poly I:C‐stimulated CD11c+ cells of wild‐type mice were significantly low compared with those of IL‐6−/− mice, respectively. Furthermore, we found that the poly I:C stimulation in CD11c+ cells induce IL‐12 production in both IL‐6‐free condition and in IL‐6‐sufficient condition (Fig. S5). These data suggest that poly I:C‐induced IL‐6 production suppresses antitumor immunity and may promote in vivo tumorigenesis, whereas the polyI:C injection induces IL‐12 production by dendritic cells in tumor‐bearing host.
Discussion
Persisting induction and maintenance of tumor‐specific cytotoxic T cells in the tumor microenvironment are crucial to overcome cancer. The present study showed that IL‐6 produced by the tumor‐bearing host augmented tumorigenesis by decreasing introduction of antitumor effector cells, such as cytotoxic T cells and mature dendritic cells, into the tumor microenvironment. Recently, we demonstrated that IL‐6 treatment attenuates the surface expression level of MHC class II and IL‐12 production of human dendritic cells, and, in fact, impairs IFN‐γ production by CD4+ T cells in vitro.20 IL‐12 is an important cytokine for induction of Th1 immunity, because IL‐12 activates STAT4 in CD4+ T cells, inducing subsequent IFN‐γ secretion.22, 23, 24 The helper function of antigen‐specific Th1 cells is essential to induce fully activated cytotoxic T cells in tumor‐bearing hosts.25 In this study, CT26 tumor growth was enhanced by in vivo injection of anti‐CD4 mAb at an early phase; however, we found that depletion of CD4+ T cells significantly suppressed CT26 tumor growth at the later phase under IL‐6‐deficient condition. These data suggest that involvement of Tregs exists in our experiment using anti‐CD4 mAb. Furthermore, we speculate that there is a possibility that anti‐tumor effects of CD4+ T cells that can be rescued by IL‐6 deficiency may be exerted through CD8+ T cells at the later phase in our tumor‐bearing model. Generally, certain responses of CD8+ T cells, such as long‐lasting proliferation/survival or recruitment into draining LN and tumor site, are known to be helped by CD4+ T cells, which may also be mediated through a mutual interaction with dendritic cells. Therefore, we speculated that IL‐6 produced in the tumor‐bearing host suppresses type‐1 antitumor immunity involving the activation of helper and cytotoxic T cells by causing dysfunction of dendritic cells.
We isolated CD11c+ dendritic cells from tumor tissues and performed transcriptome analysis by next generation sequencing. We found 2121 genes changed between wild‐type and IL‐6−/− dendritic cells (q < 0.2) and confirmed that STAT1 gene expression (FPKM) in wild‐type and IL‐6−/− dendritic cells were 66882.5 and 84507.7, respectively, suggesting that the IL‐6‐deficient condition in the tumor‐bearing state augments the STAT1‐mediated signaling pathway in CD11c+ dendritic cells. This result is consistent with the augmented IFN‐γ production by Th1 and Tc1 cells in the IL‐6‐deficient tumor‐bearing host.
Previous studies have reported that PD‐L1 expression is induced by IFN‐γ production from lymphocytes and STAT‐1 activation based on type‐1 immunity.26, 27, 28 In general, PD‐L1 expressed on cancer cells reduces antitumor immune responses by PD‐1‐expressing effector cytotoxic T cells. In this study, we revealed that the IL‐6‐deficient condition increased Th1 and Tc1 immunity that caused augmentation of the PD‐L1 expression level on CT26 cancer cells and immune cells including dendritic cells in vivo, suggesting negative feedback regulation. Based on these findings, we blocked PD‐1/PD‐L1 signaling under the IL‐6‐deficient condition by administration of the anti‐PD‐L1 mAb in vivo. As expected, injection of the anti‐PD‐L1 mAb into CT26 tumor‐bearing IL‐6−/− mice strongly inhibited tumorigenesis in vivo. These data suggest that the mechanism of IL‐6‐dependent immunosuppression might be different from the PD1/PD‐L1‐mediated dysfunction of antitumor immunity.
A TLR3 ligand, poly I:C, among immunological adjuvants is a powerful agent to induce antitumor immunity by increasing IL‐12 production from dendritic cells in tumor‐bearing hosts.29 However, the TLR3/TRIF/MyD88‐NF‐kb signaling pathway in dendritic cells induces both IL‐6 and IL‐12 production.21 We confirmed that the IL‐6‐deficient condition promoted IL‐12 induction and the antitumor effect of immunoadjuvant therapy using poly I:C.
Generally, cancer cells as well as immune cells produced IL‐6 in tumor‐bearing hosts.16 Therefore, we have to block the immunosuppressive effects of IL‐6 from both tumor cells and the other host cells to develop more effective cancer immunotherapy. We confirmed that blockade of IL‐6 signaling by in vivo injection of anti‐IL‐6R mAb significantly suppressed tumor growth in the present study and other models as previously reported.15, 16 A recent clinical trial using an anti‐IL‐6R mAb, tocilizumab, with carboplatin/doxorubicin and IFN‐α2b has been conducted for patients with recurrent epithelial ovarian cancer.30 This clinical study reported that myeloid cells in the IL‐6R mAb‐treated patients had produced more IL‐12, while T cells were more activated and secreted higher amounts of effector cytokines including IFN‐γ. Therefore, antibodies for IL‐6R may be a good tool to block IL‐6‐signaling cascade caused by IL‐6 produced in tumor‐bearing hosts.
In this study, we found that the lack of IL‐6 in a tumor‐bearing host suppresses tumorigenesis and augments the accumulation of immune cells in vivo. Furthermore, we confirmed that the IL‐6‐deficient condition significantly promotes the antitumor effects of in vivo injection of an anti‐PD‐L1 mAb or poly I:C, an immunological adjuvant. Based on the present data, we speculate that blockade of the IL‐6 signaling pathway may promote introduction of antitumor immunity into the tumor‐bearing host. Such an approach may be a promising strategy for the development of more effective immune checkpoint blockade therapies using anti‐PD‐1/PD‐L1 mAbs and immunological adjuvants such as poly I:C for cancer patients.
Disclosure Statement
The authors have no conflict of interest to declare.
Supporting information
Fig. S1. Cytokine producing ability of CD4+ cells in draining lymph nodes under interleukin (IL)‐6‐condition.
Fig. S2. Effect of CD4+ T and CD8+ T cells on CT26 tumorigenesis in interleukin (IL)‐6‐deficient mice.
Fig. S3. Effect of PD‐L1 blockade on tumorigenesis of 4T1 murine breast cancer cells in interleukin (IL)‐6‐deficient mice.
Fig. S4. Effect of IL‐6R and PD‐L1 blockade on CT26 tumorigenesis in wild‐type mice.
Fig. S5. Effect of poly I:C treatment on IL‐12p35 gene induction in tumor‐infiltrating CD11c+ dendritic cells.
Acknowledgments
We thank Ms A. Nishiuchi for her excellent technical and secretarial assistance and Dr T. Nishimura for thoughtful advice.
Cancer Sci 108 (2017) 1959–1966
Funding Information
This work was partially supported by Grants‐in‐Aid for Scientific Research (C) (25460584 and 15K08416 to H. K. and 16K10526 to N. T.) from the Japan Society for the Promotion of Science (JSPS), Grants‐in‐Aid for JSPS Research Fellows (14J06854 to S. T. and 13J01464 to S. K.), the Platform Project for Supporting Drug Discovery and Life Science Research (Platform for Drug Discovery, Informatics, and Structural Life Science) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (H. K.), the Japan Agency for Medical Research and Development (AMED) (H. K. and A. T.), and the Joint Research Program of the Institute for Genetic Medicine, Hokkaido University (S. H. and A. T.).
References
- 1. Hodi FS, O'Day SJ, McDermott DF et al Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wolchok JD, Kluger H, Callahan MK et al Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369: 122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brahmer JR, Tykodi SS, Chow LQM et al Safety and activity of anti‐PD‐L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366: 2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012; 12: 265–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Palucka K, Banchereau J. Dendritic‐cell‐based therapeutic cancer vaccines. Immunity 2013; 39: 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature 2007; 449: 419–26. [DOI] [PubMed] [Google Scholar]
- 7. Steinman RM, Mellman I. Immunotherapy: bewitched, bothered, and bewildered no more. Science 2004; 305: 197–200. [DOI] [PubMed] [Google Scholar]
- 8. Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL‐6 family of cytokine receptors. Oncogene 2000; 19: 2548–56. [DOI] [PubMed] [Google Scholar]
- 9. Hirano T, Yasukawa K, Harada H et al Complementary DNA for a novel human interleukin (BSF‐2) that induces B lymphocytes to produce immunoglobulin. Nature 1986; 324: 73–6. [DOI] [PubMed] [Google Scholar]
- 10. Knpfer H, Preiss R. Serum interleukin‐6 levels in colorectal cancer patient—A summary of published results. Int J Colorectal Dis 2010; 25: 135–40. [DOI] [PubMed] [Google Scholar]
- 11. Makuuchi Y, Honda K, Osaka Y et al Soluble interleukin‐6 receptor is a serum biomarker for the response of esophageal carcinoma to neoadjuvant chemoradiotherapy. Cancer Sci 2013; 104: 1045–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hazama S, Takenouchi H, Tsunedomi R et al Predictive biomarkers for the outcome of vaccination of five therapeutic epitope peptides for colorectal cancer. Anticancer Res 2014; 34: 4201–5. [PubMed] [Google Scholar]
- 13. Kitamura H, Kamon H, Sawa SI et al IL‐6‐STAT3 controls intracellular MHC class II αβ dimer level through cathepsin S activity in dendritic cells. Immunity 2005; 23: 491–502. [DOI] [PubMed] [Google Scholar]
- 14. Park SJ, Nakagawa T, Kitamura H et al IL‐6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol 2004; 173: 3844–54. [DOI] [PubMed] [Google Scholar]
- 15. Sumida K, Wakita D, Narita Y et al Anti‐IL‐6 receptor mAb eliminates myeloid‐derived suppressor cells and inhibits tumor growth by enhancing T‐cell responses. Eur J Immunol 2012; 42: 2060–72. [DOI] [PubMed] [Google Scholar]
- 16. Narita Y, Kitamura H, Wakita D et al The key role of IL‐6‐arginase cascade for inducing dendritic cell‐dependent CD4(+) T cell dysfunction in tumor‐bearing mice. J Immunol 2013; 190: 812–20. [DOI] [PubMed] [Google Scholar]
- 17. Tsukamoto H, Nishikata R, Senju S et al Myeloid‐derived suppressor cells attenuate TH1 development through IL‐6 production to promote tumor progression. Cancer Immunol Res 2013; 1: 64–76. [DOI] [PubMed] [Google Scholar]
- 18. Tsukamoto H, Senju S, Matsumura K et al IL‐6‐mediated environmental conditioning of defective Th1 differentiation dampens antitumour immune responses in old age. Nat Commun 2015; 6: 6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tsukamoto H, Fujieda K, Hirayama M et al Soluble IL‐6R expressed by myeloid cells reduces tumor‐specific Th1 differentiation and drives tumor progression. Cancer Res 2017; 77: 2279–91. [DOI] [PubMed] [Google Scholar]
- 20. Ohno Y, Kitamura H, Takahashi N et al IL‐6 down‐regulates HLA class II expression and IL‐12 production of human dendritic cells to impair activation of antigen‐specific CD4(+) T cells. Cancer Immunol Immunother 2016; 65: 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu Y, Cong X, Chen L et al Synergy of TLR3 and 7 ligands significantly enhances function of DCs to present inactivated PRRSV antigen through TRIF/MyD88‐NF‐κB signaling pathway. Sci Rep 2016; 6: 23977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morinobu A, Gadina M, Strober W et al STAT4 serine phosphorylation is critical for IL‐12‐induced IFN‐gamma production but not for cell proliferation. Proc Natl Acad Sci USA 2002; 99: 12281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hsich CS, Macatonia SE, Tripp CS et al Development of TH1 CD4+ T cells through IL‐12 produced by listeria‐induced macrophage. Science 1993; 260: 547–9. [DOI] [PubMed] [Google Scholar]
- 24. Kalinski P, Hilkens CM, Snijders A et al IL‐12‐deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol 1997; 159: 28–35. [PubMed] [Google Scholar]
- 25. Nishimura T, Iwakabe K, Sekimoto M et al Distinct role of antigen‐specific T helper type 1 (Th1) and Th2 cells in tumor eradication in vivo. J Exp Med 1999; 190: 617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bellucci R, Martin A, Bommarito D et al Interferon‐g‐induced activation of JAK1 and JAK2 suppresses tumor cell susceptibility to NK cells through upregulation of PD‐L1 expression. Oncoimmunology 2015; 4: e1008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kharma B, Baba T, Matsumura N et al STAT1 drives tumor progression in serous papillary endometrial cancer. Cancer Res 2014; 74: 6519–30. [DOI] [PubMed] [Google Scholar]
- 28. Abiko K, Matsumura N, Hamanishi J et al IFN‐γ from lymphocytes induces PD‐L1 expression and promotes progression of ovarian cancer. Br J Cancer 2015; 112: 1501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Azuma M, Takeda Y, Nakajima H et al Biphasic function of TLR3 adjuvant on tumor and spleen dendritic cells promotes tumor T cell infiltration and regression in a vaccine therapy. Oncoimmunology 2016; 5: e1188244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dijkgraaf EM, Santegoets SJ, Reyners AK et al A phase I trial combining carboplatin/doxorubicin with tocilizumab, an anti‐IL‐6R monoclonal antibody, and interferon‐α2b in patients with recurrent epithelial ovarian cancer. Ann Oncol 2015; 26: 2141–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Cytokine producing ability of CD4+ cells in draining lymph nodes under interleukin (IL)‐6‐condition.
Fig. S2. Effect of CD4+ T and CD8+ T cells on CT26 tumorigenesis in interleukin (IL)‐6‐deficient mice.
Fig. S3. Effect of PD‐L1 blockade on tumorigenesis of 4T1 murine breast cancer cells in interleukin (IL)‐6‐deficient mice.
Fig. S4. Effect of IL‐6R and PD‐L1 blockade on CT26 tumorigenesis in wild‐type mice.
Fig. S5. Effect of poly I:C treatment on IL‐12p35 gene induction in tumor‐infiltrating CD11c+ dendritic cells.


