Abstract
The preventive effect of coffee on cancer at different sites has been reported, but the effect on all‐sites cancer incidence has not been extensively investigated. We evaluated the association between frequency of coffee consumption and risk of all‐sites cancer incidence and mortality among 39 685 men and 43 124 women (age 40–79 years, at baseline), in the Three‐Prefecture Cohort Study. The association between frequency of coffee consumption and risk of all‐sites cancer incidence and mortality was assessed by a Cox proportional hazards regression model, adjusted for potential confounders. During 411 341 person‐years among men and 472 433 person‐years among women, a total of 4244 men and 2601 women developed cancer at different sites and a total of 3021 men and 1635 women died of cancer at different sites. We showed an inverse association between frequency of coffee consumption and all‐sites cancer incidence in both men and women. Comparing participants who consumed coffee with those who never drank coffee, the adjusted hazard ratios (95% confidential interval) for all‐sites cancer incidence was 0.74 (0.62–0.88) for coffee consumption of ≥5 cups/day in men (P for trend < 0.001) and 0.76 (0.58–1.02) in women (P for trend = 0.020). Coffee consumption frequency was inversely associated with mortality from all‐sites cancer. In this population, increasing coffee consumption resulted in a decreased risk of all‐sites cancer incidence and mortality.
Keywords: Cancer incidence, cancer mortality, coffee consumption, cohort, epidemiology
Coffee is one of the most widely consumed beverages1 and almost 40% of adults drink coffee daily in Japan.2, 3 Coffee contains a number of bioactive compounds,4 and its anti‐inflammatory effect, mediated by caffeine and chlorogenic acid, is well recognized.2, 5 Research indicates that regular coffee consumption over a lifetime may have a beneficial effect for health.2
The International Agency for Research on Cancer Monograph working group has been discussing the association between coffee consumption and carcinogenicity,6 but the association of coffee consumption with all‐sites cancer mortality remains controversial. Indeed, preceding meta‐analysis or cohort studies have reported that coffee consumption was associated with both reduced3 and non‐increased1, 2, 7, 8, 9 risk of all‐sites cancer mortality. However, its association with the risk of site‐specific cancer incidence, such as breast cancer10, 11 colon cancer,12, 13 pancreas cancer,14, 15, 16 liver cancer,17, 18, 19 and prostate cancer20 is also controversial. Furthermore, the effect of coffee consumption on all‐sites cancer incidence has not been extensively investigated.
The Three‐Prefecture Cohort Study is a prospective population‐based observational study that targeted almost 100 000 inhabitants with a 15‐year follow‐up. We evaluated the association of coffee consumption with all‐sites cancer incidence and mortality. Our hypothesis was that high frequent coffee consumption was associated with a reduced risk of all‐sites cancer incidence and mortality among the Japanese population.
Materials and Methods
Study design, settings, and patients
The Three‐Prefecture Cohort Study was originally a prospective observational study to assess the long‐term effects of air pollution on mortality from lung cancer and respiratory diseases. Details of this target population and baseline survey method have been previously described.21, 22 Briefly, the study areas were urban/rural areas in Miyagi Prefecture, Aichi Prefecture, and Osaka Prefecture, Japan. The study participants were all residents aged ≥40 years. A total of 117 029 self‐administered questionnaires in sealed envelopes were distributed by hand to target individuals in cooperation with the municipal government in each area between 1983 and 1985. The total number of respondents was 104 567; of these, 100 629 were eligible subjects, excluding participants who duplicatedly answered a questionnaire or did not provide details of their name/sex/date of birth, as investigators could not follow the outcome data. We undertook this study in accordance with the Declaration of Helsinki and ethical guidelines for epidemiological research. The study was approved by the institutional review board of the National Cancer Center (Tokyo, Japan) and the Ethics Committee of Osaka University School of Medicine (Osaka, Japan). The agreement or permission for the baseline survey involving municipality residents was obtained from the municipal government with collaborators. Response to the questionnaire was deemed to represent agreement to participate in the survey.
In this study, we defined the study cohort as individuals aged 40–79 years at baseline, with a follow‐up period ≥1 day, and providing information on frequency of coffee consumption. We excluded the following: 19 persons whose date of beginning of follow‐up was unified in each area after various dates of individual response to the questionnaire, 3568 persons aged ≥80 years, and 14 233 persons who did not answer the question on coffee consumption. Finally, our study population consisted of 82 809 persons (39 685 men and 43 124 women; Fig. 1).
Figure 1.
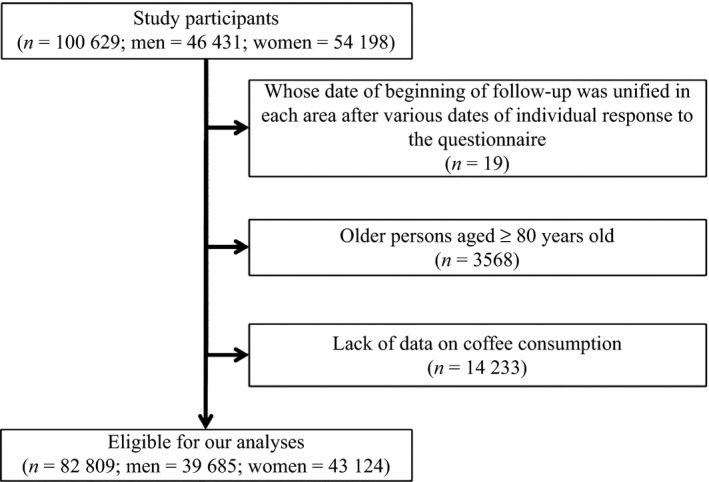
Participants in the Three‐Prefectures Cohort study included in this analysis.
Follow‐up
The follow‐up period was defined as 15 years from the baseline survey in each study area. Vital status, date of death, and date of move from the study area were confirmed by the local government using residence certificates. The cause of death was identified by a death certificate. Cancer incidence and date of diagnosis were identified from prefecture‐wide population‐based cancer registry data. The follow‐up period of cancer incidence was defined as 15 years from baseline survey in Aichi Prefecture and Osaka Prefecture, and 9 years from baseline survey in Miyagi Prefecture. The follow‐up period was defined as 15 years from baseline survey in the three prefectures.
Key group definition
The coffee consumption frequency self‐questionnaire in Aichi Prefecture and Osaka Prefecture distinguished between instant coffee and brewed coffee, whereas the coffee consumption information in Miyagi Prefecture used a single variable, with no distinction between type of coffee. Therefore, we merged two variables of coffee consumption frequency according to the combination in Table S1. Finally, we defined the categories of coffee consumption per participant, as follows: never, sometimes, 1–2 cups/day, 3–4 cups/day, and ≥5 cups/day.
Study end‐points
The main outcomes were all‐sites cancer incidence and mortality. We used the International Classification of Diseases, 9th revision (ICD‐9), for data between 1983 and 1994, and the 10th revision (ICD‐10), for data between 1995 and 2000, to classify the all‐sites cancer (ICD‐9, codes 140–208; ICD‐10, codes C00–C97).1, 2
Statistical analysis
In this study, we undertook analyses according to sex. The χ2‐test was used to compare the baseline characteristics by coffee consumption category. When cancer mortality rates were calculated, person‐years of follow‐up for mortality were counted from the date of the baseline survey to the first event occurring out of the following: date of death, date of move‐out from the study area, or the end of follow‐up. For cancer incidence rates, date of diagnosis of the first primary cancer was added to the above list. A multivariate Cox proportional hazard regression model was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between coffee consumption and all‐sites cancer incidence/mortality.
The model was adjusted for potential confounders as follows: age group (40–49, 50–59, 60–69, and 70–79 years); area (Miyagi Prefecture rural, Miyagi Prefecture urban, Aichi Prefecture rural, Aichi Prefecture urban, Osaka Prefecture rural, and Osaka Prefecture urban); body mass index (men: 14.02–20.19, 20.20–21.79, 21.80–23.14, 23.15–24.79, and 24.80–39.76 kg/m2, or unknown; women: 14.02–19.99, 20.00–21.49, 21.50–23.06, 23.07–24.99, and 25.00–40.00 kg/m2, or unknown); history of smoking (never, past, current [0–19 pack‐years, 20–39 pack‐years, and ≥40 pack‐years], or unknown); frequency of alcohol consumption (never, past, occasional, or almost every day, and unknown); rice consumption (0–1, 2, 3, 4, 5, and 6–25 cups/day, or unknown); consumption of food items, such as bread, meat, fish, egg, milk, green and yellow vegetables, non‐green and non‐yellow vegetables, fruit, miso soup, pickled vegetables (almost never, 1–2 times/month, 1–2 times/week, 3–4 times/week, almost every day, or unknown); beverages, such as black tea or green tea (almost never, sometimes, 1–2, 3–4, and ≥5 cups/day, or unknown); job type (professional, technical, civil, managerial, clerical, sales, agricultural, forestry and fisheries, construction, transport and communications, craftsman, production process personnel, laborer, security personnel, service personnel, unemployed, or unknown); history of hypertension (current, past, never, or unknown); history of diabetes (current, past, never, or unknown); history of stroke (current, past, never, or unknown); and history of heart disease (current, past, never, or unknown). Subgroup analysis according to smoking history (current, never) was carried out using the multivariable Cox proportional hazard model for all‐sites cancer incidence or mortality, to check the effect of residual confounding and/or the effect modification by smoking. To evaluate reverse causation, we estimated the risk of mortality excluding participants who died within 3 years from baseline. The effect modification was evaluated by P‐values for the association with smoking status. In addition, we evaluated the association between all‐sites cancer and coffee consumption frequency in each prefecture as a sensitivity analysis. All analyses were undertaken with STATA version 13 MP (Stata Corp., College Station, TX, USA) and the statistical significance level was set at 0.05.
Results
Tables 1 and 2 show baseline characteristics of the study cohort by coffee consumption frequency and sex. Compared with men who did not drink coffee, those who consumed high amounts of coffee were more likely to be young, smoke cigarettes, and eat foods such as bread and meat; they were less likely to be current drinkers of alcoholic beverages, eat foods such as rice, fish, green and yellow vegetables, non‐green and non‐yellow vegetables, and miso soup, and drink beverages such as milk and green tea. Similar trends were observed among women. In addition, Table S2 shows the coffee consumption frequency by prefecture and sex. In both sexes, the proportion of participants with coffee consumption ≥5 cups/day was high in Osaka Prefecture and the proportion of participants who never consumed coffee was high in Miyagi Prefecture.
Table 1.
Baseline characteristics by coffee consumption frequency in men in three Japanese prefectures
| Total (n = 39 685) | Never (n = 5072) | Sometimes (n = 12 497) | 1–2 cups/day (n = 14 760) | 3–4 cups/day (n = 5380) | ≥5 cups/day (n = 1976) | P‐value | |
|---|---|---|---|---|---|---|---|
| Age, mean (SD) | 54.5 (10.1) | 57.5 (10.6) | 56.3 (10.3) | 53.6 (9.7) | 51.1 (8.9) | 51.9 (9.4) | <0.001 |
| History of hypertension, n (%) | 8,123 (20.5) | 1,414 (27.9) | 2979 (23.8) | 2,737 (18.5) | 741 (13.8) | 252 (12.8) | <0.001 |
| History of diabetes, n (%) | 2911 (7.3) | 134 (9.8) | 347 (7.3) | 1017 (6.9) | 917 (6.4) | 496 (6.8) | <0.001 |
| Body mass index, mean (SD) | 22.6 (2.8) | 22.5 (2.9) | 22.7 (2.8) | 22.5 (2.8) | 22.6 (2.8) | 22.5 (2.8) | <0.001 |
| Current smoker, n (%) | 23,143 (58.3) | 2,355 (46.4) | 6,273 (50.2) | 9,082 (61.5) | 3903 (72.5) | 1530 (77.4) | <0.001 |
| Tobacco consumption (current smoker), n (%) | |||||||
| 0–19 cigarettes/day | 6784 (29.3) | 966 (41.0) | 2387 (38.1) | 2496 (27.5) | 696 (17.8) | 239 (15.6) | <0.001 |
| 20–39 cigarettes/day | 13 114 (56.7) | 1165 (49.5) | 3364 (53.6) | 5385 (59.3) | 2337 (59.9) | 863 (56.4) | |
| ≥40 cigarettes/day | 3097 (13.4) | 199 (8.5) | 477 (7.6) | 1157 (12.7) | 846 (21.7) | 418 (27.3) | |
| Missing | 148 (0.6) | 25 (1.1) | 45 (0.7) | 44 (0.5) | 24 (0.6) | 10 (0.7) | |
| Duration of smoking (current smoker), n (%) | |||||||
| 0–19 years | 802 (3.5) | 70 (3.0) | 287 (4.6) | 249 (2.7) | 146 (3.7) | 50 (3.3) | <0.001 |
| 20–39 years | 15 965 (69.0) | 1436 (61.0) | 6416 (102.3) | 3903 (43.0) | 3038 (77.8) | 1172 (76.6) | |
| ≥40 years | 5892 (25.5) | 763 (32.4) | 2226 (35.5) | 1967 (21.7) | 653 (16.7) | 283 (18.5) | |
| Missing | 484 (2.1) | 86 (3.7) | 153 (2.4) | 154 (1.7) | 66 (1.7) | 25 (1.6) | |
| Pack‐years (current smoker), n (%) | |||||||
| 0–19 pack‐years | 3580 (15.5) | 460 (19.5) | 1219 (19.4) | 1315 (14.5) | 440 (11.3) | 146 (9.5) | <0.001 |
| 20–39 pack‐years | 10 816 (46.7) | 1092 (46.4) | 2988 (47.6) | 4374 (48.2) | 1764 (45.2) | 598 (39.1) | |
| ≥40 pack‐years | 8221 (35.5) | 709 (30.1) | 1898 (30.3) | 3228 (35.5) | 1628 (41.7) | 758 (49.5) | |
| Missing | 526 (2.3) | 94 (4.0) | 168 (2.7) | 165 (1.8) | 71 (1.8) | 28 (1.8) | |
| Current drinker of alcoholic beverages, n (%) | 19 478 (49.1) | 2560 (50.5) | 6022 (48.2) | 7539 (51.1) | 2552 (47.4) | 805 (40.7) | <0.001 |
| Rice consumption ≥3 bowls/day, n (%) | 28 015 (70.6) | 3584 (70.7) | 9078 (72.6) | 10 297 (69.8) | 3746 (69.6) | 1310 (66.3) | <0.001 |
| Bread consumption almost every day, n (%) | 11 028 (27.8) | 826 (16.3) | 2,248 (18.0) | 5049 (34.2) | 2117 (39.3) | 788 (39.9) | <0.001 |
| Meat consumption almost every day, n (%) | 6264 (15.8) | 679 (13.4) | 1752 (14.0) | 2396 (16.2) | 1006 (18.7) | 431 (21.8) | <0.001 |
| Fish consumption almost every day, n (%) | 9620 (24.2) | 1608 (31.7) | 3363 (26.9) | 3124 (21.2) | 1064 (19.8) | 461 (23.3) | <0.001 |
| Egg consumption almost every day, n (%) | 12 696 (32.0) | 1667 (32.9) | 3947 (31.6) | 4659 (31.6) | 1746 (32.5) | 677 (34.3) | 0.001 |
| Milk consumption almost every day, n (%) | 12 324 (31.1) | 1776 (35.0) | 3992 (31.9) | 4630 (31.4) | 1363 (25.3) | 563 (28.5) | <0.001 |
| Green and yellow vegetable consumption almost every day, n (%) | 15 078 (38.0) | 2,223 (43.8) | 4973 (39.8) | 5336 (36.2) | 1805 (33.6) | 741 (37.5) | <0.001 |
| Non‐green and non‐yellow vegetable consumption almost every day, n (%) | 20 700 (52.2) | 2881 (56.8) | 6823 (54.6) | 7471 (50.6) | 2529 (47.0) | 996 (50.4) | <0.001 |
| Fruit consumption almost every day, n (%) | 15 768 (39.7) | 2219 (43.8) | 5357 (42.9) | 5674 (38.4) | 1814 (33.7) | 704 (35.6) | <0.001 |
| Miso soup consumption almost every day, n (%) | 22 226 (56.0) | 3260 (64.3) | 7793 (62.4) | 7749 (52.5) | 2476 (46.0) | 948 (48.0) | <0.001 |
| Pickled vegetable consumption almost every day, n (%) | 23 645 (59.6) | 3053 (60.2) | 7763 (62.1) | 8569 (58.1) | 3082 (57.3) | 1178 (59.6) | <0.001 |
| Black tea consumption ≥1 cup/day, n (%) | 2300 (5.8) | 272 (5.4) | 673 (5.4) | 912 (6.2) | 310 (5.8) | 133 (6.7) | <0.001 |
| Green tea consumption ≥1 cup/day, n (%) | 28 079 (70.8) | 3567 (70.3) | 9362 (74.9) | 10 227 (69.3) | 3631 (67.5) | 1292 (65.4) | <0.001 |
| Employed, n (%) | 28 437 (71.7) | 3595 (70.9) | 8816 (70.5) | 10 734 (72.7) | 3964 (73.7) | 1328 (67.2) | <0.001 |
Table 2.
Baseline characteristics by consumption frequency in women in three Japanese prefectures
| Total (n = 43 124) | Never (n = 7772) | Sometimes (n = 16 148) | 1–2 cups/day (n = 14 608) | 3–4 cups/day (n = 3351) | ≥5 cups/day (n = 1245) | P‐value | |
|---|---|---|---|---|---|---|---|
| Age, years, mean (SD) | 55.0 (10.2) | 60.3 (10.6) | 55.9 (10.0) | 52.5 (9.2) | 50.0 (8.4) | 51.7 (9.2) | <0.001 |
| History of hypertension, n (%) | 9124 (21.2) | 2252 (29.0) | 3738 (23.1) | 2553 (17.5) | 408 (12.2) | 173 (13.9) | <0.001 |
| History of diabetes, n (%) | 1503 (3.5) | 31 (6.2) | 70 (3.5) | 362 (2.5) | 559 (2.1) | 481 (2.5) | <0.001 |
| Body mass index, mean (SD) | 22.5 (3.1) | 22.4 (3.4) | 22.7 (3.2) | 22.4 (3.0) | 22.3 (2.8) | 22.4 (3.1) | <0.001 |
| Current smoker, n (%) | 4744 (11.0) | 637 (8.2) | 1189 (7.4) | 1905 (13.0) | 693 (20.7) | 320 (25.7) | <0.001 |
| Tobacco consumption (current smoker), n (%) | |||||||
| 0–19 cigarettes/day | 3069 (64.7) | 448 (70.3) | 829 (69.7) | 1247 (65.5) | 392 (56.6) | 153 (47.8) | <0.001 |
| 20–39 cigarettes/day | 1484 (31.3) | 168 (26.4) | 308 (25.9) | 600 (31.5) | 267 (38.5) | 141 (44.1) | |
| ≥40 cigarettes/day | 121 (2.6) | 8 (1.3) | 24 (2.0) | 39 (2.0) | 26 (3.8) | 24 (7.5) | |
| Missing | 70 (1.5) | 13 (2.0) | 28 (2.4) | 19 (1.0) | 8 (1.2) | 2 (0.6) | |
| Duration of smoking (current smoker), n (%) | |||||||
| 0–19 years | 1532 (32.3) | 154 (24.2) | 353 (29.7) | 665 (34.9) | 247 (35.6) | 113 (35.3) | <0.001 |
| 20–39 years | 2489 (52.5) | 289 (45.4) | 602 (50.6) | 1022 (53.6) | 394 (56.9) | 182 (56.9) | |
| ≥40 years | 580 (12.2) | 161 (25.3) | 189 (15.9) | 176 (9.2) | 33 (4.8) | 21 (6.6) | |
| Missing | 143 (3.0) | 33 (5.2) | 45 (3.8) | 42 (2.2) | 19 (2.7) | 4 (1.3) | |
| Pack‐years (current smoker), n (%) | |||||||
| 0–19 pack‐years | 2878 (60.7) | 357 (56.0) | 730 (61.4) | 1226 (64.4) | 399 (57.6) | 166 (51.9) | <0.001 |
| 20–39 pack‐years | 1351 (28.5) | 188 (29.5) | 321 (27.0) | 512 (26.9) | 220 (31.7) | 110 (34.4) | |
| ≥40 pack‐years | 359 (7.6) | 58 (9.1) | 89 (7.5) | 122 (6.4) | 51 (7.4) | 39 (12.2) | |
| Missing | 156 (3.3) | 34 (5.3) | 49 (4.1) | 45 (2.4) | 23 (3.3) | 5 (1.6) | |
| Current drinker of alcoholic beverages, n (%) | 2552 (5.9) | 365 (4.7) | 731 (4.5) | 1029 (7.0) | 312 (9.3) | 115 (9.2) | <0.001 |
| Rice consumption ≥3 bowls/day, n (%) | 25 722 (59.6) | 4957 (63.8) | 10 189 (63.1) | 8,157 (55.8) | 1,785 (53.3) | 634 (50.9) | <0.001 |
| Bread consumption almost every day, n (%) | 14 528 (33.7) | 1576 (20.3) | 3742 (23.2) | 6966 (47.7) | 1595 (47.6) | 649 (52.1) | <0.001 |
| Meat consumption almost every day, n (%) | 6,823 (15.8) | 848 (10.9) | 2372 (14.7) | 2640 (18.1) | 688 (20.5) | 275 (22.1) | <0.001 |
| Fish consumption almost every day, n (%) | 10 052 (23.3) | 2029 (26.1) | 4058 (25.1) | 3016 (20.6) | 681 (20.3) | 268 (21.5) | <0.001 |
| Egg consumption almost every day, n (%) | 12 762 (29.6) | 2066 (26.6) | 4645 (28.8) | 4488 (30.7) | 1116 (33.3) | 447 (35.9) | <0.001 |
| Milk consumption almost every day, n (%) | 14 170 (32.9) | 2609 (33.6) | 5317 (32.9) | 4767 (32.6) | 1042 (31.1) | 435 (34.9) | <0.001 |
| Green and yellow vegetable consumption almost every day, n (%) | 20 341 (47.2) | 4004 (51.5) | 7900 (48.9) | 6488 (44.4) | 1403 (41.9) | 546 (43.9) | <0.001 |
| Non‐green and non‐yellow vegetable consumption almost every day, n (%) | 26 180 (60.7) | 4902 (63.1) | 10 108 (62.6) | 8597 (58.9) | 1862 (55.6) | 711 (57.1) | <0.001 |
| Fruit consumption almost every day, n (%) | 25 487 (59.1) | 4844 (62.3) | 9958 (61.7) | 8331 (57.0) | 1686 (50.3) | 668 (53.7) | <0.001 |
| Miso soup consumption almost every day, n (%) | 22 847 (53.0) | 4585 (59.0) | 9416 (58.3) | 6865 (47.0) | 1416 (42.3) | 565 (45.4) | <0.001 |
| Pickled vegetable consumption almost every day, n (%) | 27 400 (63.5) | 4934 (63.5) | 10 507 (65.1) | 9163 (62.7) | 2000 (59.7) | 796 (63.9) | <0.001 |
| Black tea consumption ≥1 cup/day, n (%) | 3110 (7.2) | 491 (6.3) | 1224 (7.6) | 1043 (7.1) | 239 (7.1) | 113 (9.1) | <0.001 |
| Green tea consumption ≥1 cup/day, n (%) | 31 991 (74.2) | 5887 (75.7) | 12 651 (78.3) | 10 350 (70.9) | 2268 (67.7) | 835 (67.1) | <0.001 |
| Employed, n (%) | 19 904 (46.2) | 3323 (42.8) | 7091 (43.9) | 7184 (49.2) | 1748 (52.2) | 558 (44.8) | <0.001 |
Table 3 shows HRs of all‐sites cancer incidence by coffee consumption frequency and sex. The risk of all‐sites cancer incidence significantly decreased with increasing coffee consumption frequency among men (P for trend < 0.001) and women (P for trend = 0.020). The adjusted HR was significantly lower among men with coffee consumption ≥5 cups/day than among those with who never consumed coffee (adjusted HR, 0.74 [95% CI, 0.62–0.88]). The adjusted HRs were marginally lower in women with coffee consumption ≥5 cups/day than among those who never consumed coffee (adjusted HR, 0.76 [95% CI, 0.58–1.02]). Table S3 shows HRs of all‐sites cancer incidence by coffee consumption frequency and sex in each prefecture. The risk of all‐sites cancer incidence tended to be lower in the coffee consumption group than in the non‐coffee consumption group in each prefecture.
Table 3.
Hazard ratios (HR) and 95% confidence intervals (CI) of all‐sites cancer incidence according to coffee consumption category and sex in a Japanese cohort
| Never | Sometimes | 1–2 cups/day | 3–4 cups/day | ≥5 cups/day | P‐value for trend | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | |||
| Men | ||||||||||||||
| Participants (n = 39 480), n | 5023 | 12 410 | 14 702 | 5371 | 1974 | |||||||||
| All‐sites cancer incidence | ||||||||||||||
| Person‐years (n = 411 341) | 48 172 | 124 527 | 158 084 | 59 439 | 21 119 | |||||||||
| Cases (n = 4244), n | 628 | 1393 | 1552 | 494 | 177 | |||||||||
| Model 1 adjusted HRs (95% CI) | Ref. | 0.88 | (0.80–0.97) | 0.009 | 0.91 | (0.83–1.00) | 0.046 | 0.90 | (0.79–1.01) | 0.074 | 0.84 | (0.71–0.99) | 0.041 | 0.095 |
| Model 2 adjusted HRs (95% CI) | Ref. | 0.89 | (0.81–0.99) | 0.031 | 0.86 | (0.78–0.96) | 0.006 | 0.83 | (0.73–0.95) | 0.005 | 0.74 | (0.62–0.88) | 0.001 | <0.001 |
| Women | ||||||||||||||
| Participants (n = 42 913), n | 7698 | 16 060 | 14 569 | 3345 | 1241 | |||||||||
| All‐sites cancer incidence | ||||||||||||||
| Person‐years (n = 472 433) | 78 736 | 172 954 | 167 518 | 39 270 | 13 955 | |||||||||
| Cases (n = 2601), n | 552 | 1041 | 778 | 173 | 57 | |||||||||
| Model 1 adjusted HRs (95% CI) | Ref. | 1.02 | (0.92–1.13) | 0.682 | 0.93 | (0.83–1.04) | 0.184 | 1.02 | (0.85–1.21) | 0.848 | 0.84 | (0.64–1.11) | 0.225 | 0.153 |
| Model 2 adjusted HRs (95% CI) | Ref. | 1.03 | (0.92–1.15) | 0.642 | 0.89 | (0.79–1.01) | 0.083 | 0.95 | (0.79–1.15) | 0.613 | 0.76 | (0.58–1.02) | 0.064 | 0.020 |
Model 1, adjusted for age‐group, sex, and region. Model 2, adjusted for age‐group, sex, region, history of hypertension, history of diabetes mellitus, body mass index, smoking status, alcohol drinking, type of job, rice consumption, bread consumption, meat consumption, fish consumption, egg consumption, milk consumption, green and yellow vegetable consumption, non‐green and non‐yellow vegetable consumption, fruit consumption, miso soup consumption, pickled vegetable, black tea consumption, and green tea consumption. Ref., reference.
The adjusted HRs of all‐sites cancer mortality rates by coffee consumption frequency and sex are presented in Table 4. An inverse association was observed between coffee consumption frequency and all‐sites cancer mortality rates among men (P for trend < 0.001) and women (P for trend = 0.047). In men with high frequency of coffee consumption, a lower HR was observed for all‐sites cancer mortality (adjusted HR for ≥5 cups/day vs never, 0.71 [95% CI, 0.58–0.88]). In women, adjusted HRs of all‐sites cancer mortality were marginally lower among those with a coffee consumption ≥5 cups/day than among those who never consumed coffee (adjusted HR, 0.77 [95% CI, 0.54–1.10]). In addition, in an analysis to exclude subjects who died within 3 years from baseline, a similar inverse association was observed between coffee consumption frequency and all‐sites cancer mortality among men (P for trend = 0.002) but not among women (P for trend = 0.299).
Table 4.
Hazard ratios (HR) and 95% confidence interval (CI) of all‐sites cancer death according to coffee consumption frequency and sex in a Japanese cohort
| Never | Sometimes | 1–2 cups/day | 3–4 cups/day | ≥5 cups/day | P‐value for trend | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | |||
| Men | ||||||||||||||
| Participants (n = 39 685), n | 5072 | 12 497 | 14 760 | 5380 | 1976 | |||||||||
| Person‐years (n = 461 699), n | 56 932 | 145 657 | 172 737 | 63 304 | 23 069 | |||||||||
| Cases (n = 3021), n | 495 | 1,018 | 1049 | 333 | 126 | |||||||||
| Model 1 adjusted HRs (95% CI) | Ref. | 0.82 | (0.74–0.91) | <0.001 | 0.83 | (0.74–0.92) | 0.001 | 0.85 | (0.74–0.98) | 0.026 | 0.82 | (0.67–1.00) | 0.045 | 0.037 |
| Model 2 adjusted HRs (95% CI) | Ref. | 0.85 | (0.76–0.96) | 0.006 | 0.79 | (0.70–0.90) | <0.001 | 0.78 | (0.67–0.92) | 0.002 | 0.71 | (0.58–0.88) | 0.002 | <0.001 |
| Model 3 adjusted HRs (95% CI) | Ref. | 0.85 | (0.75–0.96) | 0.010 | 0.80 | (0.70–0.91) | 0.001 | 0.77 | (0.65–0.91) | 0.003 | 0.75 | (0.61–0.94) | 0.012 | 0.002 |
| Women | ||||||||||||||
| Participants (n = 43 124), n | 7772 | 16 148 | 14 608 | 3351 | 1245 | |||||||||
| Person‐years (n = 527 319) | 91 386 | 198 576 | 182 891 | 41 818 | 15 359 | |||||||||
| Cases (n = 1635), n | 392 | 651 | 459 | 97 | 36 | |||||||||
| Model 1 adjusted HRs (95% CI) | Ref. | 0.98 | (0.86–1.11) | 0.777 | 0.92 | (0.80–1.06) | 0.268 | 1.03 | (0.82–1.30) | 0.781 | 0.92 | (0.65–1.29) | 0.617 | 0.472 |
| Model 2 adjusted HRs (95% CI) | Ref. | 0.99 | (0.86–1.14) | 0.918 | 0.86 | (0.74–1.01) | 0.070 | 0.93 | (0.73–1.18) | 0.558 | 0.77 | (0.54–1.10) | 0.156 | 0.047 |
| Model 3 adjusted HRs (95% CI) | Ref. | 0.98 | (0.84–1.15) | 0.847 | 0.91 | (0.77–1.08) | 0.295 | 0.96 | (0.74–1.25) | 0.765 | 0.87 | (0.60–1.26) | 0.461 | 0.299 |
Model 1, adjusted for age‐group, sex, and region. Model 2, adjusted for age‐group, sex, region, history of hypertension, history of diabetes mellitus, body mass index, smoking status, alcohol drinking, type of job, rice consumption, bread consumption, meat consumption, fish consumption, egg consumption, milk consumption, green and yellow vegetable consumption, non‐green and non‐yellow vegetable consumption, fruit consumption, miso soup consumption, pickled vegetable, black tea consumption, and green tea consumption. Model 3, adjusted for age‐group, sex, region, history of hypertension, history of diabetes mellitus, body mass index, smoking status, alcohol drinking, type of job, rice consumption, bread consumption, meat consumption, fish consumption, egg consumption, milk consumption, green and yellow vegetable consumption, non‐green and non‐yellow vegetable consumption, fruit consumption, miso soup consumption, pickled vegetable, black tea consumption, and green tea consumption. Men and women who had all‐cause death within the first 3 years of follow‐up were excluded. Ref., reference.
For subgroup analysis by smoking status, the adjusted HRs between coffee consumption frequency and all‐sites cancer incidence, according to sex and smoking status, are presented in Table 5. Among current smokers of both sexes, increased coffee consumption frequency was significantly associated with a reduced risk of all‐sites cancer incidence (P for trend = 0.002); however, this association was not found in men or women with no history of smoking. The interaction between coffee consumption frequency and smoking status was statistically significant for women (P for interaction = 0.025).
Table 5.
Subgroup analysis of associations between coffee consumption and all‐sites cancer incidence in a Japanese cohort
| Never | Sometimes | 1–2 cups/day | 3–4 cups/day | ≥5 cups/day | P for trend | P for interaction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | ||||
| Men | |||||||||||||||
| Never smoker | |||||||||||||||
| Cases (n = 438), n | 92 | 193 | 119 | 23 | 11 | 0.437 | |||||||||
| Model 1 adjusted HRs (95% CI) | Ref. | 1.04 | (0.81–1.33) | 0.778 | 1.01 | (0.76–1.33) | 0.970 | 0.88 | (0.55–1.39) | 0.579 | 0.87 | (0.46–1.63) | 0.663 | 0.577 | |
| Model 2 adjusted HRs (95% CI) | Ref. | 1.14 | (0.87–1.51) | 0.347 | 1.15 | (0.84–1.57) | 0.384 | 1.03 | (0.63–1.67) | 0.917 | 0.92 | (0.48–1.78) | 0.812 | 0.921 | |
| Current smoker | |||||||||||||||
| Cases (n = 2723), n | 349 | 793 | 1042 | 397 | 142 | ||||||||||
| Model 1 adjusted HRs (95% CI) | Ref. | 0.83 | (0.73–0.94) | 0.003 | 0.78 | (0.69–0.89) | 0.000 | 0.78 | (0.67–0.90) | 0.001 | 0.70 | (0.58–0.86) | 0.001 | 0.000 | |
| Model 2 adjusted HRs (95% CI) | Ref. | 0.85 | (0.75–0.98) | 0.023 | 0.80 | (0.70–0.92) | 0.002 | 0.82 | (0.70–0.96) | 0.015 | 0.71 | (0.57–0.87) | 0.001 | 0.002 | |
| Women | |||||||||||||||
| Never smoker | |||||||||||||||
| Cases (n = 1838), n | 407 | 752 | 526 | 114 | 39 | 0.025 | |||||||||
| Model 1 adjusted HRs (95% CI) | Ref. | 1.05 | (0.93–1.19) | 0.420 | 0.95 | (0.83–1.08) | 0.415 | 1.09 | (0.88–1.35) | 0.436 | 1.04 | (0.75–1.45) | 0.803 | 0.808 | |
| Model 2 adjusted HRs (95% CI) | Ref. | 1.09 | (0.95–1.24) | 0.222 | 0.96 | (0.83–1.12) | 0.607 | 1.10 | (0.88–1.38) | 0.394 | 1.05 | (0.75–1.47) | 0.783 | 0.905 | |
| Current smoker | |||||||||||||||
| Cases (n = 356), n | 68 | 103 | 140 | 35 | 10 | ||||||||||
| Model 1 adjusted HRs (95% CI) | Ref. | 0.86 | (0.63–1.18) | 0.358 | 0.80 | (0.59–1.09) | 0.159 | 0.66 | (0.43–1.02) | 0.060 | 0.41 | (0.21–0.81) | 0.010 | 0.005 | |
| Model 2 adjusted HRs (95% CI) | Ref. | 0.83 | (0.59–1.17) | 0.295 | 0.72 | (0.51–1.02) | 0.067 | 0.63 | (0.40–0.99) | 0.044 | 0.37 | (0.18–0.75) | 0.006 | 0.002 | |
Model 1, adjusted for age‐group, sex, and region; Model 2, adjusted for age‐group, sex, region, history of hypertension, history of diabetes mellitus, body mass index, smoking status, alcohol drinking, type of job, rice consumption, bread consumption, meat consumption, fish consumption, egg consumption, milk consumption, green and yellow vegetable consumption, non‐green and non‐yellow vegetable consumption, fruit consumption, miso soup consumption, pickled vegetable, black tea consumption, and green tea consumption. CI, confidence interval; HR, hazard ratio; Ref., reference.
The adjusted HRs between coffee consumption frequency and all‐sites cancer mortality by sex and smoking status are also shown in Table 6. Higher coffee consumption frequency was significantly associated with lower all‐sites cancer mortality among current male smokers (P for trend = 0.005), but no significant association was found for men with no history of smoking. Higher coffee consumption frequency in women was significantly associated with lower all‐sites cancer mortality among women with current smoking (P for trend = 0.003), but was not associated with lower all‐sites cancer mortality among women who never smoked. The interaction between coffee consumption frequency and smoking status was marginally significant for women (P for interaction = 0.055).
Table 6.
Subgroup analysis of associations between consumption frequency and all‐sites cancer death in a Japanese cohort
| Never | Sometimes | 1–2 cups/day | 3–4 cups/day | ≥5 cups/day | P for trend | P for interaction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | ||||
| Men | |||||||||||||||
| Smoking status | |||||||||||||||
| Never smoker | |||||||||||||||
| Cases (n = 299), n | 65 | 137 | 74 | 16 | 7 | 0.449 | |||||||||
| Model 1 adjusted HRs (95% CI) | Ref. | 1.03 | (0.77–1.39) | 0.824 | 0.97 | (0.69–1.36) | 0.862 | 1.01 | (0.58–1.76) | 0.975 | 0.84 | (0.39–1.84) | 0.666 | 0.712 | |
| Model 2 adjusted HRs (95% CI) | Ref. | 1.21 | (0.86–1.69) | 0.273 | 1.15 | (0.78–1.69) | 0.479 | 1.26 | (0.70–2.28) | 0.436 | 1.01 | (0.44–2.31) | 0.980 | 0.644 | |
| Current smoker | |||||||||||||||
| Cases (n = 4788), n | 743 | 1553 | 1679 | 576 | 237 | ||||||||||
| Model 1 adjusted HRs (95% CI) | Ref. | 0.76 | (0.66‐0.88) | <0.001 | 0.73 | (0.63‐0.84) | <0.001 | 0.75 | (0.63‐0.89) | 0.001 | 0.67 | (0.53‐0.85) | 0.001 | <0.001 | |
| Model 2 adjusted HRs (95% CI) | Ref. | 0.82 | (0.70‐0.96) | 0.011 | 0.77 | (0.65‐0.90) | 0.001 | 0.80 | (0.67‐0.97) | 0.020 | 0.69 | (0.54‐0.88) | 0.003 | 0.005 | |
| Women | |||||||||||||||
| Smoking status | |||||||||||||||
| Never smoker | |||||||||||||||
| Cases (n = 1089), n | 278 | 437 | 285 | 65 | 24 | 0.055 | |||||||||
| Model 1 adjusted HRs (95% CI) | Ref. | 0.99 | (0.85–1.15) | 0.857 | 0.90 | (0.76–1.08) | 0.254 | 1.16 | (0.88–1.53) | 0.301 | 1.13 | (0.74–1.72) | 0.565 | 0.950 | |
| Model 2 adjusted HRs (95% CI) | Ref. | 1.05 | (0.88–1.24) | 0.591 | 0.93 | (0.77–1.14) | 0.501 | 1.18 | (0.88–1.59) | 0.259 | 1.12 | (0.72–1.72) | 0.618 | 0.789 | |
| Current smoker | |||||||||||||||
| Cases (n = 806), n | 189 | 251 | 276 | 58 | 32 | ||||||||||
| Model 1 adjusted HRs (95% CI) | Ref. | 0.93 | (0.65–1.32) | 0.674 | 0.83 | (0.58–1.18) | 0.293 | 0.69 | (0.41–1.17) | 0.168 | 0.52 | (0.24–1.11) | 0.090 | 0.041 | |
| Model 2 adjusted HRs (95% CI) | Ref. | 0.89 | (0.59–1.32) | 0.555 | 0.68 | (0.45–1.02) | 0.064 | 0.56 | (0.32–0.99) | 0.045 | 0.41 | (0.18–0.91) | 0.028 | 0.003 | |
Model 1, adjusted for age‐group, sex, and region. Model 2, adjusted for age‐group, sex, region, history of hypertension, history of diabetes mellitus, history of stroke, history of heart disease, body mass index, smoking status, alcohol drinking, type of job, rice consumption, bread consumption, meat consumption, fish consumption, egg consumption, milk consumption, green and yellow vegetable consumption, non‐green and non‐yellow vegetable consumption, fruit consumption, miso soup consumption, pickled vegetable, black tea consumption, and green tea consumption. CI, confidence interval; HR, hazard ratio; Ref., reference.
Discussion
In the Three‐Prefecture Cohort Study, there was an inverse association between coffee consumption frequency and the risk of all‐sites cancer incidence and mortality, among men and women. The association between a greater frequency of coffee consumption and reduced all‐sites cancer incidence was similar to the results from a previous meta‐analysis of 40 prospective cohort studies, suggesting that coffee drinking had no harmful effect and that coffee consumption frequency was inversely associated with the risk of all‐sites cancer.23 Our results showing the inverse association between coffee consumption frequency and all‐sites cancer incidence and mortality provide valuable information for the prevention of cancer incidence and mortality in the general Japanese population. Importantly, our results also indicated that coffee consumption did not negatively affect cancer incidence in the Japanese population.
Our results showed that the risk of all‐sites cancer mortality decreased among men and women who had a higher frequency of coffee consumption. However, the association between coffee consumption frequency and all‐sites cancer mortality may differ by the cancer site. For example, coffee consumption frequency has been reported to be a protective factor for liver cancer.17, 18, 19 Therefore, we also undertook a subgroup analysis excluding liver cancer incidence and mortality. The results for all‐sites cancer mortality among men and all‐sites cancer mortality among women were similar to those in model 2 (Table 4). In contrast, the International Agency for Research on Cancer Monograph working group reported that coffee was classified as “possibly carcinogenic to humans” (Group 2B) in 1991, but was evaluated as unclassifiable as to its carcinogenicity to humans (Group 3) in 2016.6 Thus, the balance of benefit and carcinogenicity by coffee consumption should be considered. The conclusion regarding the effect of coffee consumption on all‐site cancer incidence and mortality would be also confirmed by further meta‐analysis and pooled analysis using large‐scale cohorts including our results.
The potential mechanism underlying the effect of coffee consumption on cancer incidence and mortality could be partly explained by the fact that coffee contains high concentrations of chlorogenic acid,24 which might have a beneficial effect on inflammatory diseases.25 An increase in plasma antioxidant levels3, 26 and a reduction in the biomarkers of oxidative stress24, 27 have been reported after drinking coffee. Long‐term inflammation in the body could be attributable to carcinogens,3 and high frequency of coffee consumption could decrease the risk of all‐sites cancer incidence by inhibiting inflammation. Furthermore, in a previous study,2, 3 and ours, the study subjects who did not consume coffee had a higher proportion of history of hypertension or diabetes than those with a high coffee intake. Therefore, the baseline health status of participants who never consumed coffee could be worse than that of participants with a high coffee intake, and the effect of coffee consumption may be greater among heavy coffee drinkers.3 However, subgroup analysis, excluding cases of death occurring within 3 years from baseline, indicated that coffee consumption frequency was, nevertheless, significantly associated with a reduced risk of all‐sites cancer mortality among men.
We carried out a subgroup analysis according to smoking status to determine any residual confounding by smoking. If smoking status were a residual confounder, the risk reduction with coffee consumption would be greater among non‐smokers than among smokers. However, there was strong inverse effect on all‐sites cancer incidence among smokers, and there was weak inverse effect or no effect on all‐sites cancer incidence among never smokers. A potential explanation for this interaction is the following mechanism. Caffeine stimulates the production of cytochrome P450 enzymes, such as CYP1A2 or NAT2 in the liver, and these enzymes increase the metabolic activation of carcinogens, such as polycyclic aromatic hydrocarbons, in cigarette smoking.28, 29 Therefore, caffeine may modify the increased all‐sites cancer incidence risk caused by smoking. In addition, other factors, including sex, dietary components of food, beverages, and fitness level, also interact with caffeine metabolism.30, 31 Although we could not explain the detailed mechanisms from our results, bioactive components as presented above may suggest the possibility of a strong decreasing effect among smokers.
Limitations
This study has several limitations. First, the three prefectures did not use the same questionnaire to assess coffee consumption, and the estimated coffee consumption could be higher than reported because the categories were combined. In the sensitivity analysis by prefecture, we also assessed the difference in the risk between prefectures; the risk of all‐sites cancer incidence tended to be lower in the coffee consumption group than in the non‐coffee consumption group in each prefecture. Thus, this difference by prefecture was small but the category combination was a limitation. Second, this study followed participants until 2000 and the lifestyles of this study might differ from current lifestyles in 2016. However, coffee consumption in Japan has remained at the same level since 2000.32 Finally, we could not adjust for unknown confounding factors affecting the association between coffee consumption and cancer incidence and mortality.
In this cohort, increasing coffee consumption resulted in a decreased risk of all‐sites cancer incidence and mortality.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Table S1. Combination of instant coffee and brewed coffee consumption frequency in three Japanese prefectures
Table S2. Baseline persons by coffee consumption frequency in men and women in three Japanese prefectures
Table S3. Hazard ratios (HR) and 95% confidence intervals (CI) of all‐sites cancer according to coffee consumption category and sex in three Japanese prefectures
Acknowledgements
We sincerely thank the staff within each study area for their time and efforts in the collection and processing of data. We also express our gratitude to all study participants. This study was supported by a Grant‐in‐Aid for Scientific Research (25460752) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Cancer Sci 108 (2017) 2079–2087
Funding information
Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1. Freedman ND, Park Y, Abnet CC et al Association of coffee drinking with total and cause‐specific mortality. N Engl J Med 2012; 366: 1891–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saito E, Inoue M, Sawada N et al Association of coffee intake with total and cause‐specific mortality in a Japanese population: the Japan Public Health Center‐based Prospective Study. Am J Clin Nutr 2015; 101: 1029–37. [DOI] [PubMed] [Google Scholar]
- 3. Tamakoshi A, Lin Y, Kawado M et al Effect of coffee consumption on all‐cause and total cancer mortality: findings from the JACC study. Eur J Epidemiol 2011; 26: 285–93. [DOI] [PubMed] [Google Scholar]
- 4. Gomez‐Ruiz JA, Leake DS, Ames JM. In vitro antioxidant activity of coffee compounds and their metabolites. J Agric Food Chem 2007; 55: 6962–9. [DOI] [PubMed] [Google Scholar]
- 5. O'Keefe JH, Bhatti SK, Patil HR et al Effects of habitual coffee consumption on cardiometabolic disease, cardiovascular health, and all‐cause mortality. J Am Coll Cardiol 2013; 62: 1043–51. [DOI] [PubMed] [Google Scholar]
- 6. Loomis D, Guyton KZ, Grosse Y et al Carcinogenicity of drinking coffee, mate, and very hot beverages. Lancet Oncol 2016; 17: 877–8. [DOI] [PubMed] [Google Scholar]
- 7. Sugiyama K, Kuriyama S, Akhter M et al Coffee consumption and mortality due to all causes, cardiovascular disease, and cancer in Japanese women. J Nutr 2010; 140: 1007–13. [DOI] [PubMed] [Google Scholar]
- 8. Loftfield E, Freedman ND, Graubard BI et al Association of coffee consumption with overall and cause‐specific mortality in a large US Prospective Cohort Study. Am J Epidemiol 2015; 182: 1010–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gardener H, Rundek T, Wright CB et al Coffee and tea consumption are inversely associated with mortality in a multiethnic urban population. J Nutr 2013; 143: 1299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li XJ, Ren ZJ, Qin JW et al Coffee consumption and risk of breast cancer: an up‐to‐date meta‐analysis. PLoS One 2013; 8: e52681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bhoo‐Pathy N, Peeters PH, Uiterwaal CS et al Coffee and tea consumption and risk of pre‐ and postmenopausal breast cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study. Breast Cancer Res 2015; 17: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gan Y, Wu J, Zhang S et al Association of coffee consumption with risk of colorectal cancer: a meta‐analysis of prospective cohort studies. Oncotarget 2017; 8: 18699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee KJ, Inoue M, Otani T et al Coffee consumption and risk of colorectal cancer in a population‐based prospective cohort of Japanese men and women. Int J Cancer 2007; 121: 1312–8. [DOI] [PubMed] [Google Scholar]
- 14. Ran HQ, Wang JZ, Sun CQ. Coffee consumption and pancreatic cancer risk: An update meta‐analysis of cohort studies. Pak J Med Sci 2016; 32: 253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luo J, Inoue M, Iwasaki M et al Green tea and coffee intake and risk of pancreatic cancer in a large‐scale, population‐based cohort study in Japan (JPHC study). Eur J Cancer Prev 2007; 16: 542–8. [DOI] [PubMed] [Google Scholar]
- 16. Lin Y, Tamakoshi A, Kawamura T et al Risk of pancreatic cancer in relation to alcohol drinking, coffee consumption and medical history: findings from the Japan collaborative cohort study for evaluation of cancer risk. Int J Cancer 2002; 99: 742–6. [DOI] [PubMed] [Google Scholar]
- 17. Larsson SC, Wolk A. Coffee consumption and risk of liver cancer: a meta‐analysis. Gastroenterology 2007; 132: 1740–5. [DOI] [PubMed] [Google Scholar]
- 18. Inoue M, Kurahashi N, Iwasaki M et al Effect of coffee and green tea consumption on the risk of liver cancer: cohort analysis by hepatitis virus infection status. Cancer Epidemiol Biomarkers Prev 2009; 18: 1746–53. [DOI] [PubMed] [Google Scholar]
- 19. Kurozawa Y, Ogimoto I, Shibata A et al Coffee and risk of death from hepatocellular carcinoma in a large cohort study in Japan. Br J Cancer 2005; 93: 607–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cao S, Liu L, Yin X et al Coffee consumption and risk of prostate cancer: a meta‐analysis of prospective cohort studies. Carcinogenesis 2014; 35: 256–61. [DOI] [PubMed] [Google Scholar]
- 21. Katanoda K, Sobue T, Satoh H et al An association between long‐term exposure to ambient air pollution and mortality from lung cancer and respiratory diseases in japan. J Epidemiol 2011; 21: 132–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sado J, Kitamura T, Kitamura Y et al Rationale, design, and profile of the Three‐Prefecture Cohort in Japan: a 15‐year follow‐up. J Epidemiol 2017; 27: 193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu X, Bao Z, Zou J et al Coffee consumption and risk of cancers: a meta‐analysis of cohort studies. BMC Cancer 2011; 11: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Svilaas A, Sakhi AK, Andersen LF et al Intakes of antioxidants in coffee, wine, and vegetables are correlated with plasma carotenoids in humans. J Nutr 2004; 134: 562–7. [DOI] [PubMed] [Google Scholar]
- 25. Andersen LF, Jacobs DR Jr, Carlsen MH et al Consumption of coffee is associated with reduced risk of death attributed to inflammatory and cardiovascular diseases in the Iowa Women's Health Study. Am J Clin Nutr 2006; 83: 1039–46. [DOI] [PubMed] [Google Scholar]
- 26. Olthof MR, Hollman PC, Katan MB. Chlorogenic acid and caffeic acid are absorbed in humans. J Nutr 2001; 131: 66–71. [DOI] [PubMed] [Google Scholar]
- 27. Lopez‐Garcia E, van Dam RM, Qi L et al Coffee consumption and markers of inflammation and endothelial dysfunction in healthy and diabetic women. Am J Clin Nutr 2006; 84: 888–93. [DOI] [PubMed] [Google Scholar]
- 28. Kurahashi N, Inoue M, Iwasaki M et al Coffee, green tea, and caffeine consumption and subsequent risk of bladder cancer in relation to smoking status: a prospective study in Japan. Cancer Sci 2009; 100: 294–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Porta M, Vioque J, Ayude D et al Coffee drinking: the rationale for treating it as a potential effect modifier of carcinogenic exposures. Eur J Epidemiol 2003; 18: 289–98. [DOI] [PubMed] [Google Scholar]
- 30. Kot M, Daniel WA. Caffeine as a marker substrate for testing cytochrome P450 activity in human and rat. Pharmacol Rep 2008; 60: 789–97. [PubMed] [Google Scholar]
- 31. Carrillo JA, Benitez J. Clinically significant pharmacokinetic interactions between dietary caffeine and medications. Clin Pharmacokinet 2000; 39: 127–53. [DOI] [PubMed] [Google Scholar]
- 32. All Japan Coffee Association . The trends in coffee consumption. [Cited 7 Jun 2017.] Available from URL: http://coffee.ajca.or.jp/wp-content/uploads/2011/08/data04_2015-06b.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Combination of instant coffee and brewed coffee consumption frequency in three Japanese prefectures
Table S2. Baseline persons by coffee consumption frequency in men and women in three Japanese prefectures
Table S3. Hazard ratios (HR) and 95% confidence intervals (CI) of all‐sites cancer according to coffee consumption category and sex in three Japanese prefectures


