Abstract
To evaluate the efficacy and safety of carbon‐ion radiotherapy for non‐squamous cell carcinoma of the head and neck, 35 patients were enrolled in this prospective study. The primary end‐point was the 3‐year local control rate, and the secondary end‐points included the 3‐year overall survival rate and adverse events. Acute and late adverse events were evaluated according to the Common Terminology Criteria for Adverse Events, version 4.0. The median follow‐up time for all patients was 39 months. Thirty‐two and three patients received 64.0 Gy (relative biological effectiveness) and 57.6 Gy (relative biological effectiveness) in 16 fractions, respectively. Adenoid cystic carcinoma was dominant (60%). Four patients had local recurrence and five patients died. The 3‐year local control and overall survival rates were 93% and 88%, respectively. Acute grade 2–3 radiation mucositis (65%) and dermatitis (31%) was common, which improved immediately with conservative therapy. Late mucositis of grade 2, grade 3, and grade 4 were observed in 11, one, and no patients, respectively. There were no adverse events of grade 5. Carbon‐ion radiotherapy achieved excellent local control and overall survival rates for non‐squamous cell carcinoma. However, the late mucosal adverse events were not rare, and meticulous treatment planning is required. Trial registration no. UMIN000007886.
Keywords: Carbon‐ion radiotherapy, head and neck tumor, non‐squamous cell carcinoma, particle beam therapy, prospective study
The major pathological type of head and neck cancer is squamous cell carcinoma, for which the standard treatment is multimodal therapy including surgery and concurrent chemoradiotherapy.1 Although non‐squamous cell carcinoma (NSCC) is usually resistant to photon therapy and chemotherapy, carbon‐ion radiotherapy is reportedly efficacious for these refractory diseases.2, 3 Carbon‐ion radiotherapy provides highly concentrated dose distributions because of the Bragg peak. Furthermore, carbon‐ion is classified as high linear energy transfer radiation, offering high biological effectiveness.4 Carbon‐ion radiotherapy has been applied at the National Institute of Radiological Sciences (NIRS) (Chiba, Japan) since 1994.3, 4 At NIRS, carbon‐ion radiotherapy has shown excellent outcomes for inoperable patients with NSCC, such as adenoid cystic carcinoma,5 adenocarcinoma,6 and basal cell adenocarcinoma.7 However, these reports have all originated from a single institution, and some clinicians have expressed concerns that reported outcomes may not be reproducible at other facilities. Therefore, we undertook a prospective trial to establish the efficacy and safety of carbon‐ion radiotherapy for NSCC of the head and neck.
Materials and Methods
Patients and tumor characteristics
All patients with NSCC were prospectively treated following a protocol for carbon‐ion radiotherapy approved by our Institutional Review Board. The inclusion criteria were as follows: (i) histologically confirmed NSCC; (ii) N0−1 M0; (iii) measurable tumor; (iv) age 16–80 years; and (v) performance status 0−2. The exclusion criteria were as follows: (i) history of irradiation to the head and neck; (ii) history of chemotherapy within 1 month before carbon‐ion radiotherapy; (iii) uncontrolled infection; (iv) severe concomitant disease; and (v) active double cancers. All biopsy specimens were centrally re‐evaluated by one pathologist (J.H.) at Gunma University Hospital (Maebashi, Japan). The current study did not enroll patients with malignant melanoma or sarcoma because they were being accrued for other prospective studies. Evaluations included physical examination, laryngoscopy, computed tomography (CT), MRI, and 18‐fluorodeoxyglucose PET within 1 month before treatment. The primary end‐point was the 3‐year local control rate. Secondary end‐points included the 3‐year overall survival (OS) rate, progression‐free survival (PFS) rate, health‐related quality of life (QOL), and adverse events. Acute and late adverse events were evaluated according to the Common Terminology Criteria for Adverse Events, version 4.0.
Treatment planning
All patients provided written informed consent before carbon‐ion radiotherapy. Briefly, patients were positioned in customized cradles (Moldcare; Alcare, Tokyo, Japan) and immobilized using thermoplastic shells (Shellfitter; Kuraray, Osaka, Japan). A mouthpiece was created to maintain the lower jaw's position. Computed tomography simulation (thickness, 2 mm) was acquired for treatment planning, and MRI was carried out for reference imaging. The XiO‐N system (Elekta, Stockholm, Sweden) was used for treatment planning. Delineation of the gross tumor volume (GTV) was based on contrast‐enhanced MRI. Basically, the clinical target volume (CTV) had at least a 5‐mm margin around the GTV. Clinical target volume 1 included whole anatomic sites, such as the nasal cavity and maxillary sinus, where the tumors were located, while CTV2 was limited around the GTV. Planning target volume (PTV) 1 and PTV2 had 2‐mm margins around CTV1 and CTV2. Clinical target volume and PTV margins were modified as necessary when the targets were close to organs at risks (OARs). Based on previous studies, the dose constraints of OARs were defined as follows: maximum dose of 30 Gy (relative biological effectiveness [RBE]) for the spinal cord,6 maximum dose of 57 Gy (RBE) for the optic nerve,8 and 60 Gy (RBE) <20 cm2 for the skin.9 The radiation dose was prescribed at the isocenter of the PTVs. The PTVs were encompassed by the 95% isodose line of the prescribed dose. The dose of carbon‐ion radiotherapy was expressed as Gy (RBE). Although 64.0 Gy (RBE) was generally administered in 16 fractions, when tumors were close to skin or mucosa, 57.6 Gy (RBE) was administered in 16 fractions. The PTV1 was irradiated with 36 Gy (RBE) and PTV2 was irradiated with the remaining dose.
Quality of life
Health‐related QOL was evaluated using short form (SF)‐8 questionnaires,10 which include eight domains that are summarized by the physical components score and mental components score (MCS). Higher numerical scores indicate better QOL. The SF‐8 questionnaires were completed before and at 1, 3, 6, 12, and 24 months after treatment.
Follow‐up
Patients were seen every month for the first 6 months and every 3 months thereafter. Magnetic resonance imaging and CT were carried out alternately every 3 months, and 18‐fluorodeoxyglucose PET was carried out every year.
Statistical analysis
The 3‐year local control rate of photon therapy for NSCC was estimated to be 65%,11 and that of carbon‐ion radiotherapy was expected to be 90%. To detect a difference between these treatments by using α error of 0.05 and β error of 0.20, 23 patients were required. Considering the possibility of patient dropout and the presence of various pathologies, 35 patients were deemed necessary for this study. Local control, PFS, and OS rates were estimated using the Kaplan–Meier method and compared using log–rank tests. Differences between groups were assessed using t‐tests. Statistical significance was defined as P < 0.05. Statistical analyses were undertaken using IBM spss Statistics for Mac, version 23.0 (SPSS, Armonk, NY, USA). Local control was defined as no evidence of tumor regrowth in the PTV. Furthermore, local control was followed until death, and patients were not censored even when they developed lymph node or distant metastasis.
Results
Between June 2010 and November 2014, 35 patients with NSCC prospectively underwent carbon‐ion radiotherapy at Gunma University Heavy Ion Medical Center. Their characteristics are summarized in Table 1, and a representative case is shown in Figure 1. The median follow‐up time for all patients was 39 months (range, 6–70 months). There were 21 adenoid cystic carcinomas, five olfactory neuroblastomas (ONB), four mucoepidermoid carcinomas, two adenocarcinomas, and three other pathologies (basal cell adenocarcinoma, transitional cell carcinoma, and carcinoma ex pleomorphic adenoma). There were no patients with lymph node metastasis. Fifteen patients were operable and 20 patients were surgically inoperable because of aspects of advance disease, such as invasion of the brain, basal skull, carotid artery, or base of the tongue. Operability was discussed in the Cancer Board of the hospital, including the radiation oncologist, otolaryngologist, stomatology and maxillofacial surgeon, and medical oncologist.
Table 1.
Patient and tumor characteristics
| Characteristics | n = 35 (%) |
|---|---|
| Age | |
| Median (years) | 59 (range: 31−77) |
| Sex | |
| Male | 15 (43) |
| Female | 20 (57) |
| Performance status | |
| 0 | 11 (31) |
| 1/2 | 24 (69) |
| Histology | |
| Adenoid cystic carcinoma | 21 (60) |
| Olfactory neuroblastoma | 5 (14) |
| Mucoepidermoid carcinoma | 4 (11) |
| Adenocarcinoma | 2 (6) |
| Others | 3 (9) |
| Location of primary tumor | |
| Maxillary sinus | 9 (26) |
| Nasal cavity | 9 (26) |
| Parotid gland | 6 (17) |
| Oral cavity | 5 (14) |
| Pharynx | 4 (11) |
| External auditory canal | 2 (6) |
| Operability | |
| Operable | 15 (43) |
| Inoperable | 20 (57) |
| Disease | |
| Primary tumor | 29 (83) |
| Postoperative recurrence | 6 (17) |
| T stage | |
| T2 | 5 (14) |
| T3 | 8 (23) |
| T4 | 22 (63) |
| Radiation dose | |
| 64.0 Gy (RBE)/16 fractions | 32 (91) |
| 57.6 Gy (RBE)/16 fractions | 3 (9) |
Figure 1.
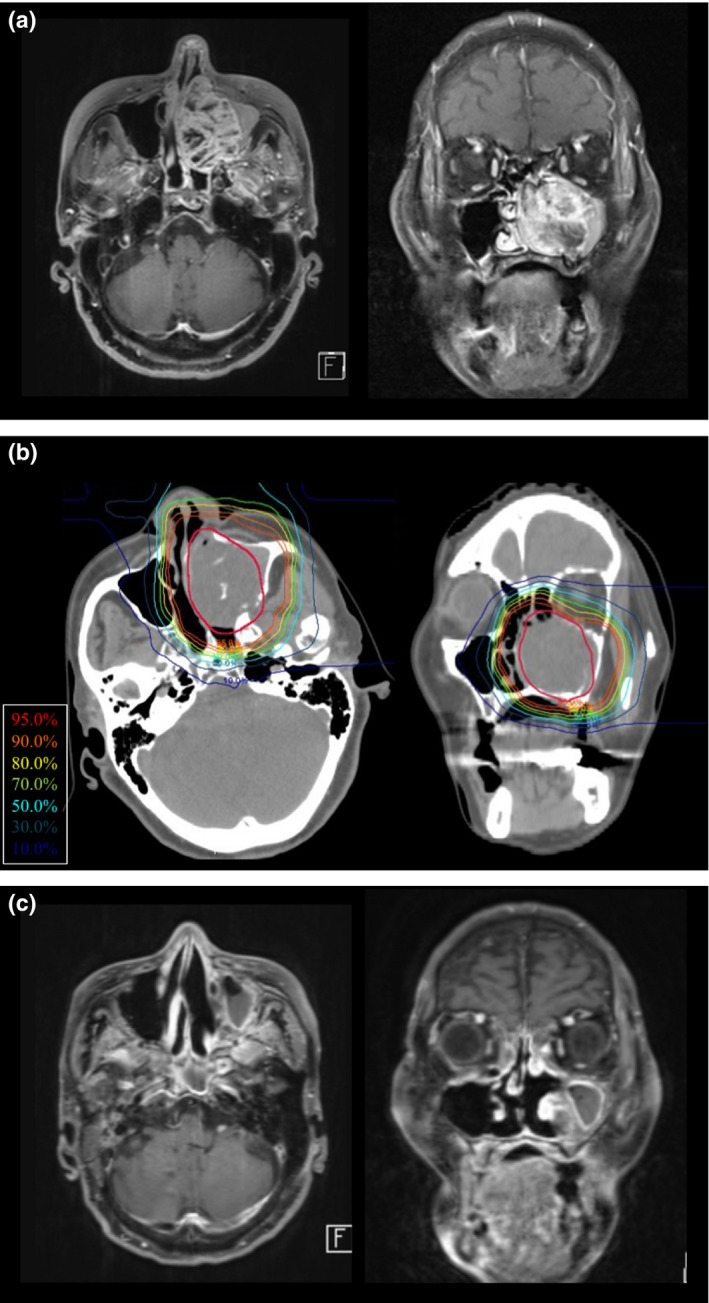
Representative case of nasal cavity adenoid cystic carcinoma treated with carbon‐ion radiotherapy. The 77‐year‐old female patient had a T4aN0M0 left maxillary sinus tumor. (a) MRI contrast‐enhanced T1‐weighted images revealed the maxillary sinus tumor with the extension to the nasal cavity. The patient refused surgery and hoped to receive carbon‐ion radiotherapy. (b) Dose distribution of carbon‐ion radiotherapy using 64.0 Gy (relative biological effectiveness) in 16 fractions. The gross tumor volume is shown in red. (c) Twenty‐four months after treatment, the tumor had shrunk on MRI. There were no late adverse events of grade 2 or higher. The patient was alive at 3 years after treatment without disease progression.
During follow‐up, four patients had local recurrence in the parotid gland (n = 2) and maxillary sinus (n = 2). Salvage surgery was carried out in these patients, who were alive without disease progression at last follow up (1, 6, 31, and 41 months after surgery). There were no severe postoperative complications. The 3‐year local control rate for all patients was 93% (95% confidence interval [CI], 84%–100%) (Fig. 2). The 3‐year local control rates for T2, T3, and T4 tumors were 100%, 86%, and 94%, respectively (Table 2, P = 0.08). The local control rate was 90% for patients with adenoid cystic carcinomas (n = 21) and 100% for all other pathologies (n = 14). Other clinical factors were not significantly associated with local control.
Figure 2.
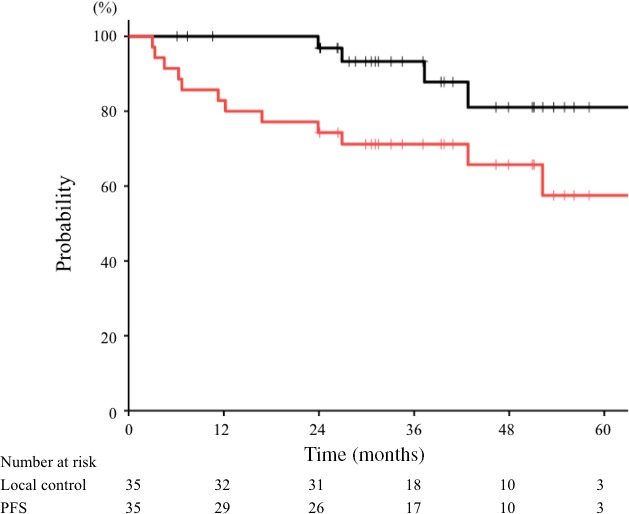
Local control and progression‐free survival (PFS) curves for non‐squamous cell carcinoma treated with carbon‐ion radiotherapy. The 3‐year local control (black line) and PFS (red line) rates for all patients (n = 35) were 93% and 71%, respectively.
Table 2.
Univariate analysis for local control and overall survival (OS)
| Characteristics | n = 35 | Local control | Overall survival | ||
|---|---|---|---|---|---|
| 3‐year (%) | P value | 3‐year (%) | P value | ||
| Age | |||||
| ≧59 | 18 | 93 | 0.45 | 83 | 0.26 |
| <59 | 17 | 94 | 93 | ||
| Sex | |||||
| Male | 15 | 94 | 0.85 | 93 | 0.34 |
| Female | 20 | 92 | 84 | ||
| Performance status | |||||
| 0 | 11 | 91 | 0.69 | 100 | 0.05 |
| 1/2 | 24 | 94 | 82 | ||
| Histology | |||||
| Adenoid cystic carcinoma | 21 | 90 | 0.64 | 90 | 0.66 |
| Olfactory neuroblastoma | 5 | 100 | 100 | ||
| Mucoepidermoid carcinoma | 4 | 100 | 67 | ||
| Adenocarcinoma | 2 | 100 | – | ||
| Others | 3 | 100 | 67 | ||
| Location of primary tumor | |||||
| Maxillary sinus/nasal cavity | 18 | 93 | 0.17 | 88 | <0.01 |
| Oral cavity/pharynx | 9 | 100 | 100 | ||
| Parotid gland | 6 | 83 | 100 | ||
| External auditory canal | 2 | – | 0 | ||
| Operability | |||||
| Operable | 15 | 93 | 0.85 | 80 | 0.14 |
| Inoperable | 20 | 93 | 95 | ||
| Disease | |||||
| Primary tumor | 29 | 92 | 0.74 | 89 | 0.24 |
| Postoperative recurrence | 6 | 100 | 83 | ||
| T stage | |||||
| T2 | 5 | 100 | 0.08 | 100 | 0.95 |
| T3 | 8 | 86 | 88 | ||
| T4 | 22 | 94 | 85 | ||
| Radiation dose | |||||
| 64.0 Gy (RBE)/16 fractions | 32 | 93 | 0.39 | 100 | 0.47 |
| 57.6 Gy (RBE)/16 fractions | 3 | 100 | 87 | ||
Eleven patients had disease progression, and the 3‐year PFS for all patients was 71% (95% CI, 56%–86%) (Fig. 2). The PFS rates for T2, T3, and T4 tumors were 100%, 63%, and 68% (P = 0.53). The first progressive site was local disease in three patients, lymph node metastasis in one, and distant metastasis in seven (bone, n = 3; lung, n = 1; multiple sites, n = 3). Tumor location was significantly associated with PFS (P < 0.01). The 3‐year PFS rate was 77% for the maxillary sinus and nasal cavity (n = 18), 89% for the oral cavity and pharynx (n = 9), 50% for the parotid gland (n = 6), and 0% for the external auditory canal (n = 2). Other clinical factors were not significantly associated with PFS.
During follow‐up, four patients died of disease progression and one died of intercurrent disease (gastric cancer). Overall, the 3‐year OS rate for all patients was 88% (95% CI, 77%–99%) (Fig. 3). Overall survival rates for T2, T3, and T4 tumors were 100%, 88%, and 85%, respectively (Table 2, P = 0.95). Regarding pathology, the 3‐year OS rates were 90% for adenoid cystic carcinoma, 100% for ONB, 67% for mucoepidermoid carcinoma, and 67% for other pathologies (P = 0.66). Tumor location was significantly associated with OS (P < 0.01). The 3‐year OS rates were 88% for the maxillary sinus and nasal cavity (n = 18), 100% for the oral cavity and pharynx (n = 9), 100% for the parotid gland (n = 6), and 0% for the external auditory canal (n = 2). Other clinical factors were not significantly associated with OS.
Figure 3.
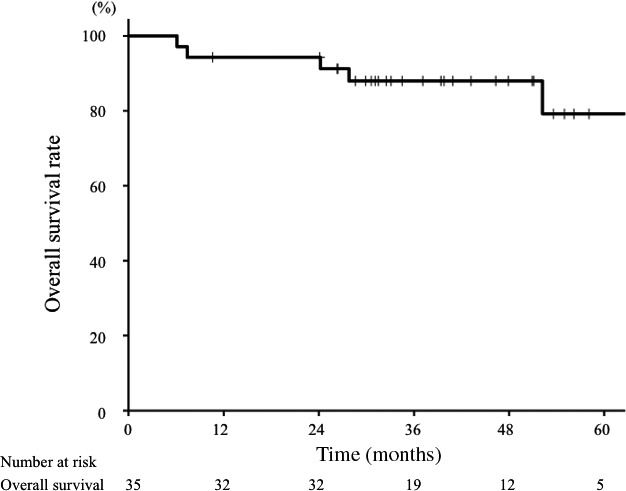
Overall survival curve for patients with non‐squamous cell carcinoma treated with carbon‐ion radiotherapy. The 3‐year overall survival rate for all patients (n = 35) was 88%.
The characteristics of ONB and non‐ONB head and neck NSCCs can differ substantially.12, 13, 14 Accordingly, we compared clinical results between ONB (n = 5) and non‐ONB (n = 30). The 3‐year local control rates for ONB and non‐ONB were 100% and 92%, respectively (P = 0.41). The 3‐year PFS rates for ONB and non‐ONB were 100% and 67%, respectively (P = 0.13). The 3‐year OS rates for ONB and non‐ONB were 100% and 86%, respectively (P = 0.37). Acute and late adverse events are shown in Table 3. Acute grade 2 to 3 radiation mucositis was common (65%). Grade 2 dermatitis was observed in 31% of patients, but no grade 3 dermatitis was evident. These acute adverse events improved immediately with conservative therapy. Chronic mucositis of grade 2 was observed in 31% of patients, and 1 patient (3%) suffered from grade 3 mucositis requiring hospitalization, analgesic, and gastrostoma. There were two cases of grade 2 brain necrosis requiring steroids and two cases of grade 3 cataracts requiring surgery. There were five cases of grade 2 or higher visual impairment (one glaucoma, one optic nerve disorder, one vitreous hemorrhage, and two retinal hemorrhages), and these tumors invaded the orbital space and were close to the eye.
Table 3.
Acute and late adverse events for all patients (n = 35)
| Acute adverse events | Grade 2 (%) | Grade 3 (%) | Grade 4 (%) |
|---|---|---|---|
| Mucositis | 15 (43) | 8 (23) | 0 (0) |
| Dermatitis | 11 (31) | 0 (0) | 0 (0) |
| Conjunctivitis | 5 (14) | 0 (0) | 0 (0) |
| Dysgeusia | 1 (3) | 0 (0) | 0 (0) |
| Late adverse events | |||
| Mucositis | 11 (31) | 1 (3) | 0 (0) |
| Dermatitis | 0 (0) | 0 (0) | 0 (0) |
| Conjunctivitis | 1 (3) | 0 (0) | 0 (0) |
| Dysgeusia | 2 (6) | 0 (0) | 0 (0) |
| Brain necrosis | 2 (6) | 0 (0) | 0 (0) |
| Cataract | 0 (0) | 2 (6) | 0 (0) |
| Visual imparment | 2 (6) | 1 (3) | 2 (6) |
| Trismus | 3 (9) | 0 (0) | 0 (0) |
| Otitis media | 5 (14) | 0 (0) | 0 (0) |
| Olfactory nerve disorder | 4 (11) | 0 (0) | 0 (0) |
Health‐related QOL scores are shown in Figure 4. Radiotherapy resulted in temporary, non‐significant MCS impairment at 1 month, but MCS returned to baseline levels at 3 months after treatment. In contrast, the physical components score was improved by radiotherapy, with significance improvements observed at 6, 12, and 24 months after treatment, compared with pretreatment scores (P < 0.05).
Figure 4.
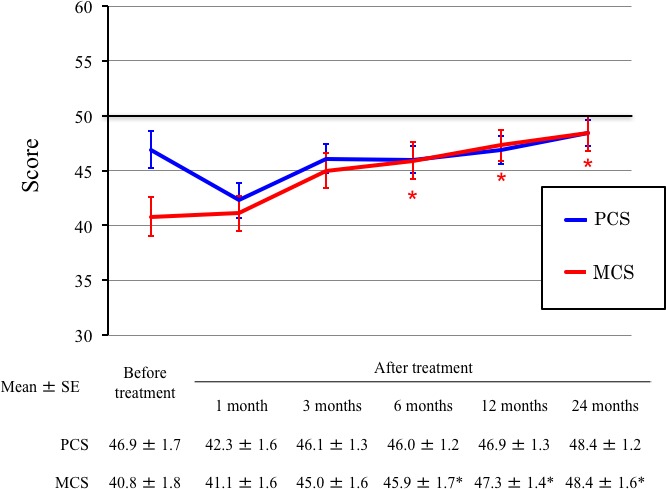
Changes of short form‐8 health survey values in patients with non‐squamous cell carcinoma, before and after carbon‐ion radiotherapy. *P < 0.05 for the comparison of values before and after treatments. MCS, mental component summary; PCS, physical component summary.
Discussion
The primary end‐point of this study was the 3‐year local control rate, which was 93% (95% CI, 84%–100%). This value is higher than our estimated value of 65% in photon therapy. Our results are comparable to previous studies of carbon‐ion radiotherapy at NIRS.2, 6 In a phase II study, Mizoe et al.2 reported that the 5‐year local control rate was 73% for adenoid cystic carcinoma. A retrospective study of 22 patients with adenocarcinoma showed a 3‐year local control rate of 84%.6 Recently, Jensen et al.15 studied intensity‐modulated radiotherapy plus carbon‐ion boost therapy, observing a 3‐year local control rate of 84% for adenoid cystic carcinoma at Heidelberg. Our prospective study showed that the favorable outcomes of carbon‐ion radiotherapy were reproducible for NSCC.
The standard treatment for NSCC, especially adenoid cystic carcinoma, is considered to be radical surgery and postoperative radiotherapy.14 van Weert et al.16 reported the clinical results of surgery for 105 adenoid cystic carcinoma patients. Postoperative radiotherapy was undertaken in 93% of the patients, and the 5‐year local control and OS rates were 82% and 68%, respectively. Shen et al.17 reported 101 adenoid cystic carcinoma patients treated with surgery. Twenty‐four percent of patients had T4 disease, and postoperative radiotherapy were carried out in 62% of patients. The 5‐year local control and OS rates were 71% and 91%, respectively. Our study showed 3‐year local control and OS rates of 93% and 88%, which are comparable to the previously reported results of surgery because 63% of patients had T4 disease in our study.
Our study included five patients with ONB, and their outcomes were excellent, with 3‐year local control and OS rates of 100% and 100%, respectively, which are comparable to previous results of carbon‐ion radiotherapy.2 Olfactory neuroblastoma has been reported to be sensitive to chemotherapy,13, 18 due to different characteristics compared to other NSCCs, such as adenoid cystic carcinoma. Because of its distinct features, ONB should generally be distinguished from other forms of NSCCs. There have been few reports on the efficacy of photon therapy for ONB. Therefore, carbon‐ion radiotherapy, with its superior dose distribution, could be a promising treatment because the diseases are close to OARs, such as the brain.
In this study, tumor location was significantly prognostic for PFS and OS; worse outcomes (OS, 0%; PFS, 0%) were observed for the two patients with external auditory canal tumor (adenoid cystic carcinoma and mucoepidermoid carcinoma). Although both patients died of disease progression with rapid distant metastasis, the small number of patients makes the outcomes of these tumors unclear. Previously, retrospective research on 13 patients with squamous cell carcinoma in the external auditory canal and middle ear showed a poor prognosis (3‐year OS, 40%).19 Further studies of this extremely rare disease are necessary.
Although carbon‐ion radiotherapy provides sharp dose distributions, it is difficult to avoid adverse events when tumors directly invade normal tissues. Acute dermatitis and mucositis were common in our study, and improved with conservative therapy. Because skin dose was strictly based on a previous dose constraint, 60 Gy (RBE) <20 cm2,9 severe late dermatitis was not observed. However, consideration should be given to chronic mucositis, particularly because 34% of patients had grade 2 or higher. In a previous phase II study by Mizoe et al.2 at NIRS, 4% of patients had grade 2 or higher late mucositis. The comparative rarity of severe late mucositis might relate to differences in total radiation doses. Based on a phase I/II study, 64 Gy (RBE) was defined as a maximum tolerated dose.4 Both our study and Mizoe et al.'s used similar criteria for 64 Gy (RBE), and selected 57.4 Gy (RBE) for tumors that were close to the skin or mucosal. Nonetheless, 64 Gy (RBE) was given to 90% of patients in our study and 8% in the previous study. Although lower doses generally reduce adverse event rates, local control can be affected. In fact, local control rates were 93% at 3 years in our study and 73% at 5 years for adenoid cystic carcinoma in Mizoe et al.'s study. Further studies are warranted to evaluate relationships between radiation doses and tumor control in NSCC of the head and neck. Recently, dose constraints have been established for several organs (including the optic nerve, brain, mucosa, and maxilla) in carbon‐ion radiotherapy of head and neck tumors.8, 20, 21, 22 Dose constraints need to be determined for late mucositis, to allow careful carbon‐ion radiotherapy planning.
Quality of life and functional status are important relative to clinical outcomes in patients with head and neck cancer. Although QOL studies of surgery and chemoradiotherapy have become more common for head and neck cancers,23, 24 no QOL study has examined carbon‐ion radiotherapy. Here, QOL was prospectively evaluated using SF‐8 questionnaires, which showed that mental status gradually improved after treatment. This suggests that pretreatment fears and anxiety may have disappeared following successful treatment. Physical scores temporally decreased after treatment, but rapidly improved to baseline levels during the 2 years after treatment. This suggests that acute adverse events had temporary effects, and late adverse events did not harm patients’ physical conditions. Additional study is required to assess QOL during longer follow‐up.
This study had several limitations. The median follow‐up period of 39 months was relatively short. Therefore, additional follow‐up is required to evaluate local control, OS, and late adverse events. Furthermore, the evaluation of individual pathologies was not robust because of the small number of enrolled patients. However, NSCC of the head and neck is rare, and we think it is important to evaluate outcomes prospectively. Each anatomical site and disease pathology should ideally be evaluated as a distinct disease, and an optimal strategy should be established for each of these diseases separately. Although the primary end‐point of this study was 3‐year local control, there was a concern that local control overestimates the treatment efficacy because of competing risk. However, in this study, local control was followed until death, and patients were not censored even when they developed metastasis. Furthermore, the patients survived well, with a 3‐year OS rate of 89%, indicating that the 3‐year local control was considered evaluable in this study.
In conclusion, this prospective study showed excellent local control and OS outcomes for NSCC. The outcomes were similar to those previously reported for carbon‐ion radiotherapy, showing that it has reproducible efficacy. Late adverse events are not rare; therefore, dose constraints for OARs are required to establish safer treatment planning in carbon‐ion radiotherapy and prevent adverse events such as late mucositis.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
We thank Editage for English language editing.
Cancer Sci 108 (2017) 2039–2044
Funding information
None.
References
- 1. Grégoire V, Langendijk JA, Nuyts S. Advances in radiotherapy for head and neck cancer. J Clin Oncol 2015; 33: 3277–84. [DOI] [PubMed] [Google Scholar]
- 2. Mizoe JE, Hasegawa A, Jingu K et al Results of carbon ion radiotherapy for head and neck cancer. Radiother Oncol 2012; 103: 32–7. [DOI] [PubMed] [Google Scholar]
- 3. Mizoe JE, Tsujii H, Kamada T et al Dose escalation study of carbon ion radiotherapy for locally advanced head‐and‐neck cancer. Int J Radiat Oncol Biol Phys 2004; 60: 358–64. [DOI] [PubMed] [Google Scholar]
- 4. Kanai T, Endo M, Minohara S et al Biophysical characteristics of HIMAC clinical irradiation system for heavy‐ion radiation therapy. Int J Radiat Oncol Biol Phys 1999; 44: 201–10. [DOI] [PubMed] [Google Scholar]
- 5. Koto M, Hasegawa A, Takagi R et al Evaluation of the safety and efficacy of carbon ion radiotherapy for locally advanced adenoid cystic carcinoma of the tongue base. Head Neck 2016a; 38(Suppl 1): E2122–6. [DOI] [PubMed] [Google Scholar]
- 6. Koto M, Hasegawa A, Takagi R et al Feasibility of carbon ion radiotherapy for locally advanced sinonasal adenocarcinoma. Radiother Oncol 2014a; 113: 60–5. [DOI] [PubMed] [Google Scholar]
- 7. Jingu K, Hasegawa A, Mizo JE et al Carbon ion radiotherapy for basal cell adenocarcinoma of the head and neck: preliminary report of six cases and review of the literature. Radiat Oncol 2010; 5: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hasegawa A, Mizoe JE, Mizota A, Tsujii H. Outcomes of visual acuity in carbon ion radiotherapy: analysis of dose‐volume histograms and prognostic factors. Int J Radiat Oncol Biol Phys 2006; 64: 396–401. [DOI] [PubMed] [Google Scholar]
- 9. Yanagi T, Kamada T, Tsuji H et al Dose‐volume histogram and dose‐surface histogram analysis for skin reactions to carbon ion radiotherapy for bone and soft tissue sarcoma. Radiother Oncol 2010; 95: 60–5. [DOI] [PubMed] [Google Scholar]
- 10. Fukuhara S, Suzukamo Y. Manual of the SF‐8: Japanese Version. Kyoto: Institute for Health Outcomes & Process Evaluation Research, 2004. [Google Scholar]
- 11. Mendenhall WM, Morris CG, Amdur RJ et al Radiotherapy alone or combined with surgery for adenoid cystic carcinoma of the head and neck. Head Neck 2004; 26: 154–62. [DOI] [PubMed] [Google Scholar]
- 12. Noh OK, Lee SW, Yoon SM et al Radiotherapy for esthesioneuroblastoma: is elective nodal irradiation warranted in the multimodality treatment approach? Int J Radiat Oncol Biol Phys 2011; 79: 443–9. [DOI] [PubMed] [Google Scholar]
- 13. Su SY, Bell D, Ferrarotto R et al Outcomes for olfactory neuroblastoma treated with induction chemotherapy. Head Neck 2017; 39: 1671–9. [DOI] [PubMed] [Google Scholar]
- 14. Coca‐Pelaz A, Rodrigo JP, Bradley PJ et al Adenoid cystic carcinoma of the head and neck–An update. Oral Oncol 2015; 51: 652–61. [DOI] [PubMed] [Google Scholar]
- 15. Jensen AD, Poulakis M, Nikoghosyan AV et al High‐LET radiotherapy for adenoid cystic carcinoma of the head and neck: 15 years’ experience with raster‐scanned carbon ion therapy. Radiother Oncol 2016; 118: 272–80. [DOI] [PubMed] [Google Scholar]
- 16. van Weert S, Bloemena E, van der Waal I et al Adenoid cystic carcinoma of the head and neck: a single‐center analysis of 105 consecutive cases over a 30‐year period. Oral Oncol 2013; 49: 824–9. [DOI] [PubMed] [Google Scholar]
- 17. Shen C, Xu T, Huang C, Hu C, He S. Treatment outcomes and prognostic features in adenoid cystic carcinoma originated from the head and neck. Oral Oncol 2012; 48: 445–9. [DOI] [PubMed] [Google Scholar]
- 18. Patil VM, Joshi A, Noronha V et al Neoadjuvant chemotherapy in locally advanced and borderline resectable nonsquamous sinonasal tumors (esthesioneuroblastoma and sinonasal tumor with neuroendocrine differentiation). Int J Surg Oncol 2016; 2016: 6923730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koto M, Hasegawa A, Takagi R et al Carbon ion radiotherapy for locally advanced squamous cell carcinoma of the external auditory canal and middle ear. Head Neck 2016b; 38: 512–6. [DOI] [PubMed] [Google Scholar]
- 20. Koto M, Hasegawa A, Takagi R et al Risk factors for brain injury after carbon ion radiotherapy for skull base tumors. Radiother Oncol 2014b; 111: 25–9. [DOI] [PubMed] [Google Scholar]
- 21. Musha A, Shimada H, Shirai K et al Prediction of acute radiation mucositis using an oral mucosal dose surface model in carbon ion radiotherapy for head and neck tumors. PLoS One 2015; 10: e0141734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sasahara G, Koto M, Ikawa H et al Effects of the dose‐volume relationship on and risk factors for maxillary osteoradionecrosis after carbon ion radiotherapy. Radiat Oncol 2014; 9: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leonhardt FD, Quon H, Abrahão M, O'Malley BW Jr, Weinstein GS. Transoral robotic surgery for oropharyngeal carcinoma and its impact on patient‐reported quality of life and function. Head Neck 2012; 34: 146–54. [DOI] [PubMed] [Google Scholar]
- 24. Truong MT, Zhang Q, Rosenthal DI et al Quality of life and performance status from a substudy conducted within a prospective phase 3 randomized trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for locally advanced head and neck carcinoma: NRG Oncology Radiation Therapy Oncology Group 0522. Int J Radiat Oncol Biol Phys 2017; 97: 687–99. [DOI] [PMC free article] [PubMed] [Google Scholar]


