Abstract
CD155 is the fifth member in the nectin‐like molecule family, and functions as the receptor of poliovirus; therefore, CD155 is also referred to as necl‐5, or PVR. As an immunoglobulin‐like adhesion molecule, CD155 is involved in cell motility, and natural killer and T cell‐mediated immunity. CD155 is barely or weakly expressed in various normal human tissues, but frequently overexpressed in human malignant tumors. CD155 overexpression promotes tumor cell invasion and migration, and is associated with tumor progression and poor prognosis. As the ligand for both costimulatory receptor CD226 and coinhibitory receptor TIGIT and CD96 on natural killer and T cells, CD155 seems to play a dual role in oncoimmunity. However, some recent studies indicate that CD155 overexpression may induce tumor immune escape. Taken together, CD155 may be considered as a target for the treatment of tumors with CD155 overexpression.
Keywords: CD155, CD226, CD96, natural killer cell, TIGIT
CD155 was originally identified as a poliovirus receptor (PVR), with conserved amino acids and domain structure characteristics similar to the immunoglobulin superfamily.1, 2 CD155 is also referred to as necl‐5, as it belongs to the nectin‐like molecule family. These molecules harbor domain structures similar to nectins, which play critical roles in cell adhesion and polarization.3
CD155 heterophilically trans‐interacts with nectin‐3, colocalizing to epithelial cell–cell junctions.4, 5 CD155 overexpression promotes cell migration in a nectin‐3‐dependent or nectin‐3‐independent manner.4, 6 Moreover, CD155 overexpression stimulates cell proliferation, and enhances growth factor‐induced cell proliferation.7 In fact, CD155 overexpression can reduce the expression of nectin‐3, and disrupt contact inhibition.8
Regulation of CD155 Expression
DNA damage
DNA damage or DNA‐replication inhibitors induce CD155 expression through the activation of ataxia telangiectasia mutated (ATM) and ATM and Rad3‐related (ATR) protein kinases. For instance, some chemotherapeutic agents for multiple myeloma patients can upregulate CD155 on human fibroblasts and mouse tumor cells (Fig. 1a)9 Moreover, reactive oxygen species and reactive nitrogen species can also stimulate CD155 expression through DNA damage response (DDR) (Fig. 1a).10, 11 Viruses have been extensively demonstrated to induce DDR, leading to genomic instability and the development of human cancers.12, 13 Therefore, it is reasonable to ask whether viruses can induce CD155. However, until recently, only HIV‐1 Vpr protein was reported to upregulate CD155 involving the activation of ATR kinase and DDR.14 It remains unclear whether CD155 is involved in virus‐related human cancers. Interestingly, cytomegalovirus has been observed to downregulate CD155, which is considered to be a mechanism for this virus to escape immune surveillance.15
Figure 1.
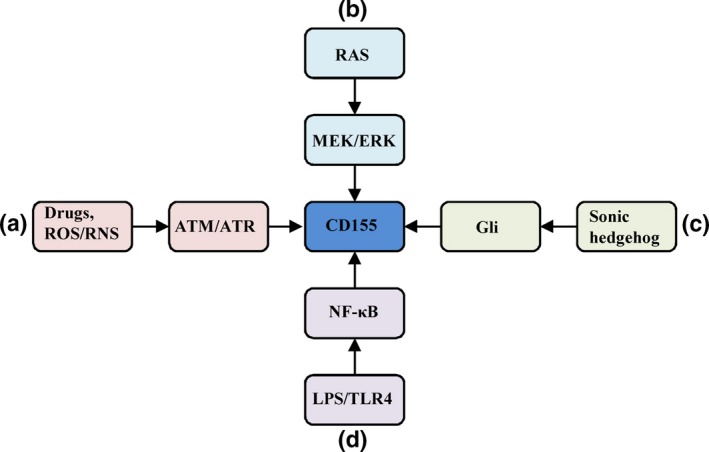
Regulation of CD155 expression. (a) Chemotherapeutic agents and reactive oxygen species (ROS)/reactive nitrogen species (RNS) activate ataxia telangiectasia mutated (ATM) and ATM and Rad3‐related (ATR) protein kinases, which upregulate CD155 expression. (b) RAS signaling induces CD155 expression via MEK/ERK signaling. (c) Aberrant activation of sonic hedgehog induces CD155 expression. (d) Combination of LPS with TLR4 activates NF‐κB via adaptor protein MYD88 and TRIF, which stimulates CD155 expression.
Ras/FGF
Transformed V12Ras‐NIH3T3 cells express higher level of CD155 than wild‐type NIH3T3 cells, indicating that oncogenic Ras can upregulate CD155 expression (Fig. 1b).4 This induction involves Raf‐MEK‐ERK‐AP1 signaling, and fibroblast growth factor (FGF) can also upregulate CD155 expression through this signaling.16
Sonic hedgehog signaling
The sonic hedgehog (Shh) signaling pathway plays a critical role in cell differentiation, cell proliferation and tissue polarity. Aberrant Shh‐Gli activation is involved in a series of human cancers.17, 18 CD155 expression in human Ntera2 cells can be upregulated by treatment with purified shh protein; an intact Gli binding site has been found in CD155 core promoter, which is required for CD155 upregulation (Fig. 1c).19
TLR4 signaling
CD155 expression on dendritic cells (DC) can be upregulated by a series of toll‐like receptor (TLR) agonists, including LPS.20, 21 This induction involves TLR adaptor molecules MYD88 and TRIF, and depends on the activation of NF‐κB (Fig. 1d).22 In addition to DC, CD155 expression on neutrophils and macrophages can also be upregulated by LPS.23 It is unknown whether TLR signaling in the tumor microenvironment attributes to CD155 overexpression on tumor cells. IFN‐γ, induced by TLR4 activation, has been shown to upregulate CD155 expression on melanoma cells.24
Overexpression of CD155 in human malignancies
CD155 expression is barely detected in most normal tissues, but upregulated in a series of human malignancies, including colon cancer, lung adenocarcinoma, melanoma, pancreatic cancer and glioblastoma.25, 26, 27, 28, 29 Clinicopathological analysis indicates that CD155 overexpression is correlated with tumor progression and unfavorable prognosis.26, 27, 28 Moreover, levels of soluble isoforms of CD155 in the sera of cancer patients are significantly higher than those of healthy donors, and are positively correlated with cancer stage.30 These studies suggest that CD155 may be used as a biomarker for cancer progression and prognosis.
CD155 plays a key role in tumor cell invasion and migration.29, 31, 32 During migration, CD155 is recruited to the leading edge of migrating tumor cells, where it is colocalized with actin and αvβ3 integrin.29 CD155 overexpression reduces substrate adhesion, but enhances cell dispersal, which is dependent on the activation of Src/focal adhesion kinase (FAK) signaling.32 CD155 also plays a positive role in tumor growth. CD155 overexpression in ras‐transformed cells upregulates cyclin D2, downregulates p27 and shortens the G0/G1 phase.33 In contrast, CD155 downregulation suppresses tumor cell proliferation and induces cell‐cycle arrest at G2/M phase.28 Moreover, CD155 expression is correlated with tumor VEGF expression and intratumoral vessel density (Fig. 2).28
Figure 2.
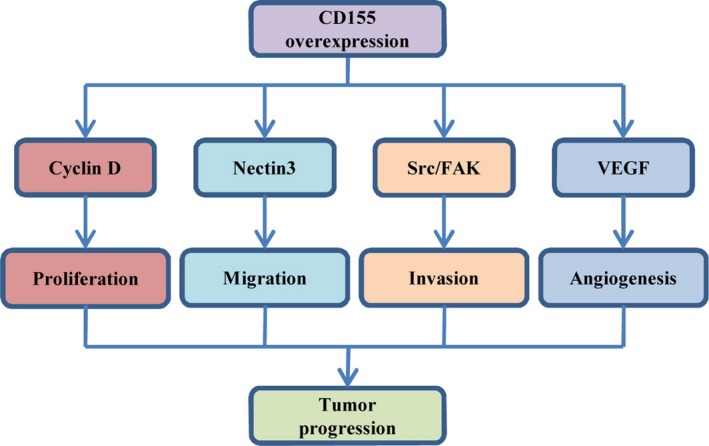
The role of CD155 in tumor development and its underlying molecular mechanisms. Some oncogenic signals induce CD155 overexpression, which regulates tumor cell proliferation, migration and invasion, and tumor angiogenesis.
Interaction of CD155 with CD226 in antitumor immunity
Costimulatory molecule CD226 (DNAX accessory molecule‐1, DNAM‐1) is mainly expressed on T cells, natural killer (NK) cells, monocytes and B cells. Anti‐CD226 monoclonal antibody (MAb) suppresses T and NK cell‐mediated cytotoxicity against some tumor cells, whereas it has no effect on the cytolysis of others, suggesting that CD226‐induced cytotoxicity depends on specific ligands from tumor cells.34 A study using CD226‐deficient mice has shown that CD226 is required for the activation of CD8+ cytotoxic T lymphocytes (CTL) by antigens from tumor cells.20
CD155 and CD112 have been identified as the ligands for CD226.35, 36 Interaction of CD226 with CD155/CD112 triggers NK or T cell‐mediated cytotoxicity, accompanied by an increase in cytokine production. Further in vitro studies have shown that tumor cells with higher CD155 expression are more susceptible to CD226‐induced killing.37, 38 In contrast, compared with wild‐type mice, CD226‐deficient NK or T cells display significantly less cytotoxic activity against CD155‐positive tumor cells than wild‐type cells, and CD226‐deficient mice show increased tumor development and mortality after transplantation of CD155‐positive tumor cells.39 Moreover, CD226 has been revealed to mediate the inhibition of CD155‐positive tumor metastasis by NK cells.40
Surprisingly, some later studies have shown that CD226 expression in the tumor microenvironment is downregulated. Peripheral blood NK (p‐NK) cells and tumor‐infiltrating NK (Ti‐NK) cells from breast cancer patients express decreased CD226 and some other activating receptors, with weakened cytotoxicity.41 Factors such as TGF‐β1 and PGE2 secreted by stromal cells and/or cancer cells are responsible the reduction of NK cell CD226 expression and cytotoxicity.41 These results have also been observed in a cancer MMTV‐Neu transgenic mouse model.42 Moreover, CD226 expression is downregulated on NK cells from myeloma patients with active disease compared with patients in remission and healthy controls.43
In addition, interaction between CD155 and CD226 can downregulate CD226 expression.44 Similarly, CD112, another ligand for CD226, is also involved in CD226 downregulation.45 CD226 expression on T and NK cells is increased remarkably in CD155 knockdown mice or mice treated with CD155‐neutralizing antibody.46 This regulation is post‐transcriptional based on the fact that CD226 mRNA has not been affected.46 One mechanism underlying CD226 downregulation may be related to CD155/CD112‐induced CD226 internalization.
Interaction of CD155 with TIGIT in tumor immune evasion
TIGIT contains an immunoreceptor tyrosine‐based inhibitory motif (ITIM) and is expressed on T cells and NK cells. Yu and colleagues found that TIGIT can bind to CD155 on DC with high affinity and suppress T cell activation indirectly by modulating DC activity.47 Stanietsky and colleagues further demonstrate that TIGIT is a coinhibitory receptor on NK cells for CD155 and CD112.48
Studies using TIGIT knockdown mice have revealed that TIGIT inhibits T cell proliferation and activation independent of antigen‐presenting cell (APC)‐derived CD155.49 However, the intrinsic inhibitory effect of TIGIT may be still related to CD155. As shown by Lozano and colleagues, T cell‐derived CD155 can bind to CD226 to activate T cells under the condition of TIGIT deficiency.50 Moreover, TIGIT has been demonstrated to directly disrupt CD226 homodimerization, suppressing CD8+ T cell response by interfering with CD155/CD226 interaction.51
Contrary to CD226, TIGIT is significantly upregulated on tumor‐infiltrating T cells, and its expression parallels with that of other coinhibitory receptors, most notably PD‐1, indicating that TIGIT also acts as a marker of chronically stimulated or exhausted tumor‐infiltrating T cells.51 Moreover, high TIGIT expression on CD8+ T cells is associated with refractory leukemia and leukemia relapse.52
One recent study by Inozume and colleagues has shown that CD155 overexpression in melanoma cells inhibits CTL response in the presence of CD226, and this inhibition depends on the interaction between CD155 and TIGIT.24 It is reasonable to consider that CD155 overexpression in tumor cells may result in increased CD155 combination with coinhibitory molecules, including TIGIT. However, TIGIT blockade alone or TIGIT knockdown has no obvious effect on tumor growth and metastasis, suggesting that other inhibitory molecules may compensate for TIGIT.51, 53
Interaction of CD155 with CD96 in tumor immune evasion
CD96 was originally identified by Wang and colleagues and named as Tactile (T cell activation, increased late expression).54 Like TIGIT, CD96 also contains an ITIM and is expressed on T cells and NK cells. By using NK92 cells, Fuchs and colleagues have revealed CD96 as another receptor for CD155, and that interaction between CD96 and CD155 mediates cell adhesion.55
Chan et al. (2014) demonstrate that CD96 is a coinhibitory receptor on NK cells by generating CD96 knockdown mice.23 Their study has indicated that CD96 not only competes with CD226 for CD155 binding, but also directly suppresses NK cell function via binding with CD155. CD226 knockdown mice are susceptible to melanoma lung metastasis, but CD96 knockdown mice are resistant to it.23 CD96 blockade can further suppress melanoma metastasis in TIGIT knockdown mice.23, 53
CD155 Ligands‐Mediated Signals
CD155‐CD226 ligation
CD226 ligation is associated with CD226 tyrosine phosphorylation, indicating that CD226 is a signal‐transducing molecule.34 CD226‐mediated adhesion and cytotoxicity by NK cells involves PKC, which phosphorylates CD226 at Ser329.56 CD226 Ser329 phosphorylation facilitates the association of CD226 with LFA‐1 (CD11a/CD18), inducing CD226 tyrosine phosphorylation via fyn protein tyrosine kinase.57
CD155‐TIGIT ligation
Ligation of CD155 with TIGIT results in TIGIT phosphorylation at Tyr225, recruiting SHIP1 (SH2‐containing inositol phosphatase 1) via adaptor molecule Grb2; SHIP1 further blocks phosphatidylinositol 3‐kinase (PI3K) and ERK signaling, and inhibits NK cell‐mediated cytotoxicity.58 Phosphorylated TIGIT can also recruit SHIP1 via adaptor molecule β‐arrestin 2, suppressing NF‐κB activity and IFN‐γ production in NK cells.59 These findings indicate that SHIP1 is a key molecule in CD155‐TIGIT signaling (Fig. 3). Indeed, SHIP1 silencing opposes TIGIT/CD155‐mediated inhibitory signaling and restores NK cell cytotoxicity.59
Figure 3.
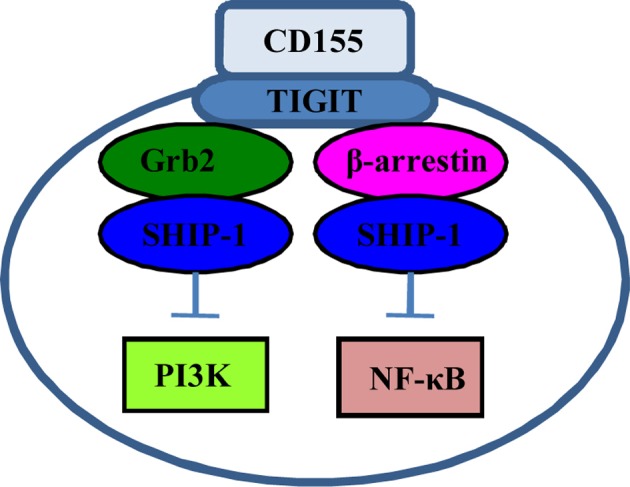
Signalings initiated by CD155‐TIGIT ligation. Combination of CD155 with TIGIT recruits SHIP‐1 via Grb2 or β‐arrestin 2, blocking PI3K signaling or NF‐κB activity to suppress NK cell‐mediated cytotoxicity.
CD155‐CD96 ligation
CD96 has been demonstrated to suppress NK cell function via binding with CD155; however, CD96‐mediated signaling remains unknown.23
CD155‐nectin‐3 ligation
Similar to interaction between nectins, interaction of CD155 with nectin‐3 also induces the activation of Cdc42 and Rac, involving molecules including c‐Src, Rap1, FRG and Vav2.3, 60
Conclusion and Perspective
As an immunoregulatory molecule, CD155 can combine with costimulatory molecule CD226, and coinhibitory molecules TIGIT and CD96, having a dual function in oncoimmunity. Accordingly, the balance between CD155/CD226 and CD155/TIGIT or CD155/CD96 maintains normal NK and T cell function. However, this balance may be disrupted in the tumor microenvironment. On one hand, tumor cells usually overexpress CD155; on the other hand, tumor‐infiltrating immune cells may express decreased CD226 and increased TIGIT. Therefore, CD155/TIGIT or CD155/CD96‐mediated inhibitory signaling may be dominant in the tumor microenvironment (Fig. 4).
Figure 4.
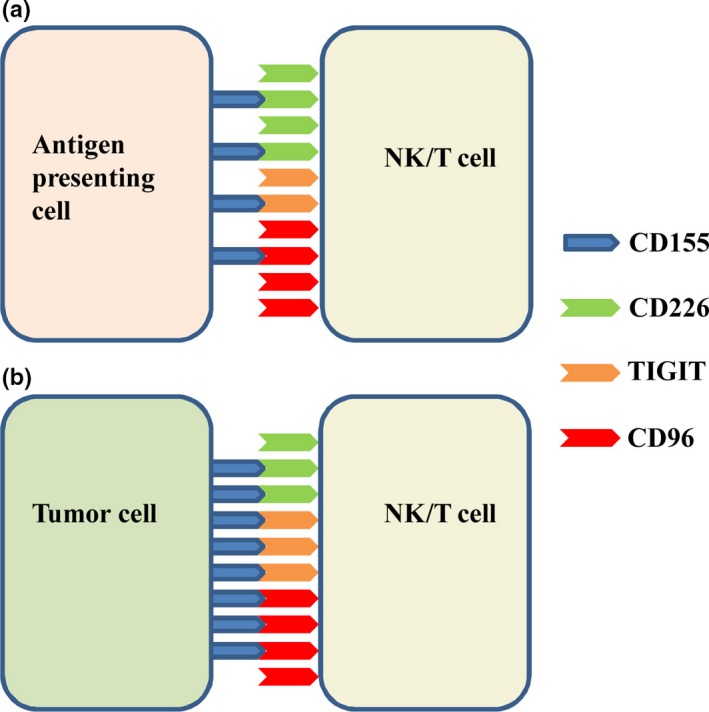
The potential role of CD155 in tumor immune regulation. (a) CD155 from antigen presenting cells (APC) combines with costimulatory CD226, and coinhibitory TIGIT and CD96 in normal conditions. (b) In the tumor microenvironment, CD155 is overexpressed on tumor cells; CD226 is downregulated and TIGIT is upregulated on NK and T cells. Therefore, CD155‐triggered inhibitory signaling is likely predominant in the tumor microenvironment.
Some other findings also lead us to deliberately consider the role of CD155 in human tumors, such as the overexpression of CD155 on human tumor cells, involvement of some oncogenic signaling in the regulation of CD155 expression, attribution of CD155 to tumor growth and metastasis, and correlation of CD155 expression with tumor progression and prognosis. Thus, more investigations should be undertaken to determine the effect of anti‐CD155 treatment for tumors. It has been demonstrated that chemotherapeutic agents can induce CD155 expression on tumor cells. Due to the possibility that CD155 overexpression may counteract the efficiency of chemotherapeutic agents, combination of anti‐CD155 therapy with chemotherapy could be considered under this condition.
Disclosure Statement
The authors have no conflicts of interest to declare.
Cancer Sci 108 (2017) 1934–1938
Funding information
Natural Scientific Foundation of Liaoning Province (2014021036).
References
- 1. Mendelsohn CL, Wimmer E, Racaniello VR. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell 1989; 56: 855–65. [DOI] [PubMed] [Google Scholar]
- 2. He Y, Bowman VD, Mueller S et al Interaction of the poliovirus receptor with poliovirus. Proc Nat Acad Sci USA 2000; 97: 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Takai Y, Irie K, Shimizu K, Sakisaka T, Ikeda W. Nectins and nectin‐like molecules: roles in cell adhesion, migration, and polarization. Cancer Sci 2003; 94: 655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ikeda W, Kakunaga S, Itoh S et al Tage4/Nectin‐like molecule‐5 heterophilically trans‐interacts with cell adhesion molecule Nectin‐3 and enhances cell migration. J Biol Chem 2003; 278: 28167–72. [DOI] [PubMed] [Google Scholar]
- 5. Mueller S, Wimmer E. Recruitment of nectin‐3 to cell–cell junctions through trans‐heterophilic interaction with CD155, a vitronectin and poliovirus receptor that localizes to alpha(v)beta3 integrin‐containing membrane microdomains. J Biol Chem y 2003; 278: 31251–60. [DOI] [PubMed] [Google Scholar]
- 6. Ikeda W, Kakunaga S, Takekuni K et al Nectin‐like molecule‐5/Tage4 enhances cell migration in an integrin‐dependent, Nectin‐3‐independent manner. J Biol Chem 2004; 279: 18015–25. [DOI] [PubMed] [Google Scholar]
- 7. Kakunaga S, Ikeda W, Shingai T et al Enhancement of serum‐ and platelet‐derived growth factor‐induced cell proliferation by Necl‐5/Tage4/poliovirus receptor/CD155 through the Ras‐Raf‐MEK‐ERK signaling. J Biol Chem 2004; 279: 36419–25. [DOI] [PubMed] [Google Scholar]
- 8. Minami Y, Ikeda W, Kajita M, Fujito T, Monden M, Takai Y. Involvement of up‐regulated Necl‐5/Tage4/PVR/CD155 in the loss of contact inhibition in transformed NIH3T3 cells. Biochem Biophys Res 2007; 352: 856–60. [DOI] [PubMed] [Google Scholar]
- 9. Soriani A, Zingoni A, Cerboni C et al ATM‐ATR‐dependent up‐regulation of DNAM‐1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK‐cell susceptibility and is associated with a senescent phenotype. Blood 2009; 113: 3503–11. [DOI] [PubMed] [Google Scholar]
- 10. Ardolino M, Zingoni A, Cerboni C et al DNAM‐1 ligand expression on Ag‐stimulated T lymphocytes is mediated by ROS‐dependent activation of DNA‐damage response: relevance for NK‐T cell interaction. Blood 2011; 117: 4778–86. [DOI] [PubMed] [Google Scholar]
- 11. Fionda C, Abruzzese MP, Zingoni A et al Nitric oxide donors increase PVR/CD155 DNAM‐1 ligand expression in multiple myeloma cells: role of DNA damage response activation. BMC Cancer 2015; 15: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cerboni C, Fionda C, Soriani A et al The DNA damage response: a common pathway in the regulation of NKG2D and DNAM‐1 ligand expression in normal, infected, and cancer cells. Front Immunol 2014; 4: 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hollingworth R, Grand RJ. Modulation of DNA damage and repair pathways by human tumour viruses. Viruses 2015; 7: 2542–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vassena L, Giuliani E, Matusali G, Cohen EA, Doria M. The human immunodeficiency virus type 1 Vpr protein upregulates PVR via activation of the ATR‐mediated DNA damage response pathway. J Gen Virol 2013; 94: 2664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tomasec P, Wang EC, Davison AJ et al Downregulation of natural killer cell‐activating ligand CD155 by human cytomegalovirus UL141. Nat Immunol 2005; 6: 181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hirota T, Irie K, Okamoto R, Ikeda W, Takai Y. Transcriptional activation of the mouse Necl‐5/Tage4/PVR/CD155 gene by fibroblast growth factor or oncogenic Ras through the Raf‐MEK‐ERK‐AP‐1 pathway. Oncogene 2005; 24: 2229–35. [DOI] [PubMed] [Google Scholar]
- 17. Rimkus TK, Carpenter RL, Qasem S, Chan M, Lo HW. Targeting the sonic hedgehog signaling pathway: review of smoothened and GLI inhibitors. Cancers 2016; 8: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Athar M, Li C, Kim AL, Spiegelman VS, Bickers DR. Sonic hedgehog signaling in Basal cell nevus syndrome. Cancer Res 2014; 74: 4967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Solecki DJ, Gromeier M, Mueller S, Bernhardt G, Wimmer E. Expression of the human poliovirus receptor/CD155 gene is activated by sonic hedgehog. J Biol Chem 2002; 277: 25697–702. [DOI] [PubMed] [Google Scholar]
- 20. Gilfillan S, Chan CJ, Cella M et al DNAM‐1 promotes activation of cytotoxic lymphocytes by nonprofessional antigen‐presenting cells and tumors. J Exp Med 2008; 205: 2965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pende D, Castriconi R, Romagnani P et al Expression of the DNAM‐1 ligands, Nectin‐2 (CD112) and poliovirus receptor (CD155), on dendritic cells: relevance for natural killer‐dendritic cell interaction. Blood 2006; 107: 2030–6. [DOI] [PubMed] [Google Scholar]
- 22. Kamran N, Takai Y, Miyoshi J, Biswas SK, Wong JS, Gasser S. Toll‐like receptor ligands induce expression of the costimulatory molecule CD155 on antigen‐presenting cells. PLoS One 2013; 8: e54406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chan CJ, Martinet L, Gilfillan S et al The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions. Nat Immunol 2014; 15: 431–8. [DOI] [PubMed] [Google Scholar]
- 24. Inozume T, Yaguchi T, Furuta J, Harada K, Kawakami Y, Shimada S. Melanoma cells control antimelanoma CTL responses via interaction between TIGIT and CD155 in the effector phase. J Invest Dermatol 2016; 136: 255–63. [DOI] [PubMed] [Google Scholar]
- 25. Masson D, Jarry A, Baury B et al Overexpression of the CD155 gene in human colorectal carcinoma. Gut 2001; 49: 236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakai R, Maniwa Y, Tanaka Y et al Overexpression of Necl‐5 correlates with unfavorable prognosis in patients with lung adenocarcinoma. Cancer Sci 2010; 101: 1326–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bevelacqua V, Bevelacqua Y, Candido S et al Nectin like‐5 overexpression correlates with the malignant phenotype in cutaneous melanoma. Oncotarget 2012; 3: 882–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nishiwada S, Sho M, Yasuda S et al Clinical significance of CD155 expression in human pancreatic cancer. Anticancer Res 2015; 35: 2287–97. [PubMed] [Google Scholar]
- 29. Sloan KE, Eustace BK, Stewart JK et al CD155/PVR plays a key role in cell motility during tumor cell invasion and migration. BMC Cancer 2004; 4: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Iguchi‐Manaka A, Okumura G, Kojima H et al Increased soluble CD155 in the serum of cancer patients. PLoS One 2016; 11: e0152982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morimoto K, Satoh‐Yamaguchi K, Hamaguchi A et al Interaction of cancer cells with platelets mediated by Necl‐5/poliovirus receptor enhances cancer cell metastasis to the lungs. Oncogene 2008; 27: 264–73. [DOI] [PubMed] [Google Scholar]
- 32. Sloan KE, Stewart JK, Treloar AF, Matthews RT, Jay DG. CD155/PVR enhances glioma cell dispersal by regulating adhesion signaling and focal adhesion dynamics. Cancer Res 2005; 65: 10930–7. [DOI] [PubMed] [Google Scholar]
- 33. Kono T, Imai Y, Yasuda S et al The CD155/poliovirus receptor enhances the proliferation of ras‐mutated cells. Int J Cancer 2008; 122: 317–24. [DOI] [PubMed] [Google Scholar]
- 34. Shibuya A, Campbell D, Hannum C et al DNAM‐1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity 1996; 4: 573–81. [DOI] [PubMed] [Google Scholar]
- 35. Bottino C, Castriconi R, Pende D et al Identification of PVR (CD155) and Nectin‐2 (CD112) as cell surface ligands for the human DNAM‐1 (CD226) activating molecule. J Exp Med 2003; 198: 557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tahara‐Hanaoka S, Shibuya K, Onoda Y et al Functional characterization of DNAM‐1 (CD226) interaction with its ligands PVR (CD155) and nectin‐2 (PRR‐2/CD112). Int Immunol 2004; 16: 533–8. [DOI] [PubMed] [Google Scholar]
- 37. Pende D, Spaggiari GM, Marcenaro S et al Analysis of the receptor‐ligand interactions in the natural killer‐mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the poliovirus receptor (CD155) and nectin‐2 (CD112). Blood 2005; 105: 2066–73. [DOI] [PubMed] [Google Scholar]
- 38. Castriconi R, Dondero A, Corrias MV et al Natural killer cell‐mediated killing of freshly isolated neuroblastoma cells: critical role of DNAX accessory molecule‐1‐poliovirus receptor interaction. Cancer Res 2004; 64: 9180–4. [DOI] [PubMed] [Google Scholar]
- 39. Iguchi‐Manaka A, Kai H, Yamashita Y et al Accelerated tumor growth in mice deficient in DNAM‐1 receptor. J Exp Med 2008; 205: 2959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chan CJ, Andrews DM, McLaughlin NM et al DNAM‐1/CD155 interactions promote cytokine and NK cell‐mediated suppression of poorly immunogenic melanoma metastases. J Immunol 2010; 184: 902–11. [DOI] [PubMed] [Google Scholar]
- 41. Mamessier E, Sylvain A, Thibult ML et al Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest 2011; 121: 3609–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mamessier E, Sylvain A, Bertucci F et al Human breast tumor cells induce self‐tolerance mechanisms to avoid NKG2D‐mediated and DNAM‐mediated NK cell recognition. Cancer Res 2011; 71: 6621–32. [DOI] [PubMed] [Google Scholar]
- 43. El‐Sherbiny YM, Meade JL, Holmes TD et al The requirement for DNAM‐1, NKG2D, and NKp46 in the natural killer cell‐mediated killing of myeloma cells. Cancer Res 2007; 67: 8444–9. [DOI] [PubMed] [Google Scholar]
- 44. Carlsten M, Norell H, Bryceson YT et al Primary human tumor cells expressing CD155 impair tumor targeting by down‐regulating DNAM‐1 on NK cells. J Immunol 2009; 183: 4921–30. [DOI] [PubMed] [Google Scholar]
- 45. Sanchez‐Correa B, Gayoso I, Bergua JM et al Decreased expression of DNAM‐1 on NK cells from acute myeloid leukemia patients. Immunol Cell Biol 2012; 90: 109–15. [DOI] [PubMed] [Google Scholar]
- 46. Seth S, Qiu Q, Danisch S et al Intranodal interaction with dendritic cells dynamically regulates surface expression of the co‐stimulatory receptor CD226 protein on murine T cells. J Biol Chem 2011; 286: 39153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yu X, Harden K, Gonzalez LC et al The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol 2009; 10: 48–57. [DOI] [PubMed] [Google Scholar]
- 48. Stanietsky N, Simic H, Arapovic J et al The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Nat Acad Sci USA 2009; 106: 17858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Joller N, Hafler JP, Brynedal B et al Cutting edge: TIGIT has T cell‐intrinsic inhibitory functions. J Immunol 2011; 186: 1338–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lozano E, Dominguez‐Villar M, Kuchroo V, Hafler DA. The TIGIT/CD226 axis regulates human T cell function. J Immunol 2012; 188: 3869–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Johnston RJ, Comps‐Agrar L, Hackney J et al The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell 2014; 26: 923–37. [DOI] [PubMed] [Google Scholar]
- 52. Kong Y, Zhu L, Schell TD et al T‐Cell Immunoglobulin and ITIM domain (TIGIT) associates with CD8+ T‐cell exhaustion and poor clinical outcome in AML patients. Clin Cancer Res 2016; 22: 3057–66. [DOI] [PubMed] [Google Scholar]
- 53. Blake SJ, Stannard K, Liu J et al Suppression of metastases using a new lymphocyte checkpoint target for cancer immunotherapy. Cancer Discov 2016; 6: 446–59. [DOI] [PubMed] [Google Scholar]
- 54. Wang PL, O'Farrell S, Clayberger C, Krensky AM. Identification and molecular cloning of tactile. A novel human T cell activation antigen that is a member of the Ig gene superfamily. J Immunol 1992; 148: 2600–8. [PubMed] [Google Scholar]
- 55. Fuchs A, Cella M, Giurisato E, Shaw AS, Colonna M. Cutting edge: CD96 (tactile) promotes NK cell‐target cell adhesion by interacting with the poliovirus receptor (CD155). J Immunol 2004; 172: 3994–8. [DOI] [PubMed] [Google Scholar]
- 56. Shibuya A, Lanier LL, Phillips JH. Protein kinase C is involved in the regulation of both signaling and adhesion mediated by DNAX accessory molecule‐1 receptor. J Immunol 1998; 161: 1671–6. [PubMed] [Google Scholar]
- 57. Shibuya K, Lanier LL, Phillips JH et al Physical and functional association of LFA‐1 with DNAM‐1 adhesion molecule. Immunity 1999; 11: 615–23. [DOI] [PubMed] [Google Scholar]
- 58. Liu S, Zhang H, Li M et al Recruitment of Grb2 and SHIP1 by the ITT‐like motif of TIGIT suppresses granule polarization and cytotoxicity of NK cells. Cell Death Diff 2013; 20: 456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li M, Xia P, Du Y et al T‐cell immunoglobulin and ITIM domain (TIGIT) receptor/poliovirus receptor (PVR) ligand engagement suppresses interferon‐gamma production of natural killer cells via beta‐arrestin 2‐mediated negative signaling. J Biol Chem 2014; 289: 17647–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sato T, Irie K, Okamoto R, Ooshio T, Fujita N, Takai Y. Common signaling pathway is used by the trans‐interaction of Necl‐5/Tage4/PVR/CD155 and nectin, and of nectin and nectin during the formation of cell‐cell adhesion. Cancer Sci 2005; 96: 578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]


