Abstract
The present study sought to elucidate the prognosis of adult T‐cell leukemia–lymphoma (ATL) patients receiving mogamulizumab, a defucosylated anti‐CCR4 monoclonal antibody. Progression‐free survival (PFS) and overall survival (OS) of ATL patients enrolled in two studies are herein updated, namely NCT00355472 (phase I study of mogamulizumab in relapsed patients with ATL and peripheral T‐cell lymphoma) and NCT00920790 (phase II study for relapsed ATL). Of 13 patients with relapsed aggressive ATL in the phase I study, four (31%) survived >3 years. For 26 relapsed patients with aggressive ATL in the phase II study, median PFS was 5.2 months and 1‐year PFS was 26%, whereas median OS was 14.4 months, and 3‐year OS was 23%. For patients without a rash or who developed a grade 1 rash only, median PFS was 0.8 months, and 1‐year PFS was zero, with a median OS of 6.0 months, and 3‐year OS of 8%. In contrast, for patients who developed a rash ≥grade 2, median PFS was 11.7 months, and 1‐year PFS was 50%, with a median OS of 25.6 months, and 3‐year OS of 36%. Thus, we conclude that mogamulizumab monotherapy may improve PFS and OS in some patients with relapsed aggressive ATL, especially those who develop a skin rash as a moderate immune‐related adverse event. Therefore, further investigation is warranted to validate the present observations and to clarify the mechanisms involved in the activity of mogamulizumab.
Keywords: Adult T‐cell leukemia–lymphoma, CC chemokine receptor 4, mogamulizumab, rash, regulatory T cell
Mature T‐cell/natural killer cell neoplasms comprise approximately 20 subclassified heterogeneous groups of non‐Hodgkin lymphomas and, in general, they have a worse prognosis compared to mature B‐cell neoplasms.1, 2 Of these, ATL, caused by human T‐cell lymphotropic virus type I, has a poor prognosis.3, 4, 5, 6 With respect to PTCL‐NOS, this also has a miserable prognosis.1, 2
CCR4 is expressed on tumor cells derived from most ATL patients,7, 8 as well as on tumor cells from a subgroup of PTCL‐NOS, which has an unfavorable prognosis.9, 10, 11 Therefore, we postulated that CCR4 may represent a novel molecular target for immunotherapy in ATL and other types of PTCL. Accordingly, mogamulizumab (KW‐0761), a humanized, anti‐CCR4 mAb with a defucosylated Fc region, which markedly enhanced ADCC, was developed,12, 13 and a phase I study of mogamulizumab in relapsed patients with CCR4‐positive ATL and PTCL was conducted (NCT00355472).14 A phase II study of mogamulizumab in patients with CCR4‐positive relapsed, aggressive ATL (acute, lymphoma, or unfavorable chronic types)3, 4 was subsequently carried out (NCT00920790).15 In addition, a phase II study of mogamulizumab in patients with relapsed PTCL and CTCL was conducted (NCT01192984).16
Based on the results of these studies, in 2012, mogamulizumab was approved for the treatment of relapsed/refractory ATL, and in 2014 for PTCL/CTCL in Japan. As a result, mogamulizumab is currently being used in clinical practice for the treatment of patients with CCR4‐positive ATL, and those with CCR4‐positive relapsed/refractory PTCL and CTCL in Japan. However, long‐term, follow‐up information on the OS and the PFS of patients who received mogamulizumab remains unclear. In addition, useful biomarkers predicting the therapeutic efficacies of mogamulizumab remain unidentified, whereas fatal AE caused by mogamulizumab have been reported in some patients.17, 18 Herein, we report an up‐to‐date, follow‐up analysis of our prior two prospective clinical trials of mogamulizumab.
Materials and Methods
Patients and study design
This study describes the results of a follow‐up analysis of patients with relapsed ATL enrolled in two studies, namely a phase I study of mogamulizumab in relapsed patients with ATL and PTCL (NCT00355472),14 and a phase II study of mogamulizumab for relapsed ATL (NCT00920790).15
Details of patient eligibilities have been previously described.14, 15 In summary, for the phase I study, patients were eligible if aged between 20 and 69 years with aggressive ATL or PTCL with CCR4 expression, and with relapse occurring after at least one prior chemotherapy regimen. For the phase II study, patients were eligible if they were aged 20 years or older with aggressive CCR4‐positive ATL who had relapsed after at least one prior chemotherapy regimen.
The former study was conducted from February 2007 to December 2008, and the latter study was conducted from June 2009 to February 2011. Among the 16 patients enrolled in the phase I study, 10 were still alive at data cut‐off (December 2008). Among the 26 patients enrolled in the phase II study and who could be evaluated for efficacy, 12 were still alive at data cut‐off (February 2011). Subsequent clinical data for 10 and 12 patients in the former and latter studies, respectively, including disease status (progression date or progression‐free date at last follow up) and survival (date alive at last follow up, or date of death), were updated in October 2014.
Statistical analysis
Probability of PFS and OS was estimated by the Kaplan–Meier method. PFS and OS were compared using the log–rank test. PFS was defined as the time from first mogamulizumab dose to progression, relapse, or death from any cause, whichever occurred first. OS was measured from the day of first mogamulizumab dose to death from any cause.
For PFS and OS analyses, ATL patients were divided into two groups according to several factors, including ECOG PS, age, and serum Alb level, which were unfavorable prognostic factors for patients with newly diagnosed acute and lymphoma ATL subtypes.19 In addition, ATL patients were divided into two or four groups according to the presence of an AE in the form of a skin rash, graded according to the National Cancer Institute Common Terminology Criteria for AE, version 3.0, and which was determined as mogamulizumab‐related in the previous study.15
Differences between the two groups were examined by Mann–Whitney U‐test or Fisher's exact test. Analyses were conducted at the Innovative Clinical Research Center, Kanazawa University, and carried out using SAS version 9.2 or higher (SAS, Cary, NC, USA). Two‐sided P < 0.05 was considered statistically significant.
Study overview
The study was compliant with the Ethical Guidelines for Medical and Health Research Involving Human Subjects, and was conducted in accordance with the Declaration of Helsinki. This study provided clinical data, which were obtained from two completed studies (NCT00355472 and NCT00920790). The academic investigators and the company were jointly responsible for the study design. The protocol was approved by the institutional review board at each participating site. All of the applicable patients provided written, informed consent.
Results
Long‐term follow up in the phase I study
Patients who survived in the phase I study were followed up for 5 years and 10 months after the study data cut‐off (December 2008).14 Among 13 patients with relapsed ATL, three (patient numbers 102, 204, 412) showed longer PFS at the data cut‐off;14 of these, a patient with acute type ATL (number 102) was progression free until October 2014. PFS of this patient was over 2830 days (Table 1). In the present study, two patients (numbers 102 and 412) showed continued OS, and that of the latter patient with acute type ATL was over 2048 days. Among 13 patients with relapsed ATL, 10 (numbers 102 and 412 survived until October 2014; number 204 died of post‐allogeneic HCT complications) died of ATL progression. Thus, in the phase I study, a total of four patients (102, 204, 403, and 412, 31% [4/13]) survived for more than 3 years (Table 1).
Table 1.
Summary of the long‐term follow up of patients enrolled in the phase I study
| Patient no. by cohort | Sex | Age (years) | Disease | Overall response to mogamulizumab | PFS (days) | OS (days) | Rash (grade) |
|---|---|---|---|---|---|---|---|
| 1 | |||||||
| 101 | M | 46 | MF tumor stage | PD | 29 | 1166+ | 2 |
| 102 | M | 60 | ATL acute | SD→CRa | 2830+ | 2830+ | None |
| 103 | F | 68 | ATL acute | PR | 85 | 732 | None |
| 2 | |||||||
| 201 | M | 55 | ATL acute | SD | 50+ | 230 | None |
| 202 | F | 66 | ATL acute | SD | 36 | 201 | None |
| 203 | M | 66 | ATL acute | PD | 8+ | 61 | None |
| 204 | F | 57 | ATL acute | CR | 1268 | 2447 | None |
| 3 | |||||||
| 301 | M | 60 | ATL acute | PD | 36 | 298 | None |
| 302 | M | 64 | ATL acute | PD | 29 | 270 | None |
| 303 | F | 69 | ATL lymphoma | PD | 29 | 260 | None |
| 4 | |||||||
| 401 | F | 64 | PTCL‐NOS | PR | 2507+ | 2507+ | 2 |
| 402 | F | 62 | ATL acute | PR | 64 | 207 | None |
| 403 | F | 64 | ATL lymphoma | SD | 43 | 1103 | None |
| Expanded | |||||||
| 411 | M | 55 | ATL acute | PD | 28 | 506 | None |
| 412 | M | 62 | ATL acute | CR | 506 | 2048+ | 3 |
| 413 | F | 58 | PTCL‐NOS | SD | 1272 | 2230+ | 2 |
Disease had disappeared by 1 year after treatment; patient 102 was categorized as showing a CR. ATL, adult T‐cell leukemia‐lymphoma; CR, complete response; MF, mycosis fungoides; OS, overall survival; PD, progressive disease; PFS, progression‐free survival; PR, partial response; PTCL‐NOS, peripheral T‐cell lymphoma, not otherwise specified; SD, stable disease.
PFS and OS of relapsed ATL patients enrolled in the phase II study
Patients who survived in the phase II study were followed up for 3 years and 8 months after the study data cut‐off (February 2011).15 For 26 patients with relapsed aggressive ATL, median PFS was 5.2 months (95% CI, 0.9–10.3 months). Six (patient numbers 301, 306, 321, 616, 1114, 1323) of these patients showed a longer PFS, giving a PFS rate at 1 year of 26% (95% CI, 11%–45%; Table 2; Fig. 1a). Three patients were of an unfavorable chronic ATL subtype, and the remaining three were acute ATL subtypes. Thus, the PFS rate at 1 year was 21% (3/14 patients) for those with the acute subtype, and 50% (3/6 patients) for those with the unfavorable chronic subtype (Table 2).
Table 2.
Summary of the follow up of patients enrolled in the phase II study
| Patient number | Sex | Age (years) | Disease subtype | Overall response to mogamulizumab | PFS (days) | OS (days) |
|---|---|---|---|---|---|---|
| 202 | F | 61 | Acute | PD | 28 | 269 |
| 217 | F | 75 | Lymphoma | CR | 339 | 621 |
| 301 | F | 71 | Acute | SD | 555 | 602 |
| 306 | M | 55 | Acute | PR | 497 | 1857+ |
| 312 | F | 66 | Unfavorable Chronic | PR | 158 | 1740+ |
| 321 | M | 56 | Unfavorable Chronic | CR | 701 | 1687+ |
| 322 | F | 63 | Acute | PD | 36 | 330 |
| 427 | M | 64 | Acute | PD | 17 | 134 |
| 505 | F | 71 | Lymphoma | PD | 25 | 178 |
| 509 | M | 64 | Acute | PD | 12 | 101 |
| 519 | M | 73 | Lymphoma | SD | 31+ | 185 |
| 616 | F | 64 | Unfavorable Chronic | PR | 968 | 992 |
| 710 | M | 65 | Unfavorable Chronic | CR | 313 | 416 |
| 715 | F | 60 | Acute | CR | 167 | 323 |
| 824 | F | 76 | Acute | PD | 15 | 116 |
| 904 | F | 58 | Lymphoma | PD | 23 | 106 |
| 907 | M | 60 | Acute | CR | 213 | 820 |
| 918 | F | 65 | Acute | CR | 203 | 722 |
| 1108 | M | 68 | Acute | PD | 25 | 225 |
| 1114 | M | 66 | Unfavorable Chronic | PR | 1757+ | 1757+ |
| 1226 | M | 49 | Acute | PD | 17 | 164 |
| 1320 | M | 61 | Acute | PD | 26 | 234 |
| 1323 | F | 75 | Acute | CR | 372 | 739 |
| 1411 | F | 83 | Lymphoma | SD | 55+ | 463 |
| 1503 | F | 62 | Unfavorable Chronic | PR | 50 | 1958+ |
| 1513 | F | 69 | Lymphoma | PR | 139 | 1779+ |
CR, complete response; OS, overall survival; PD, progressive disease; PFS, progression‐free survival; PR, partial response; SD, stable disease.
Figure 1.
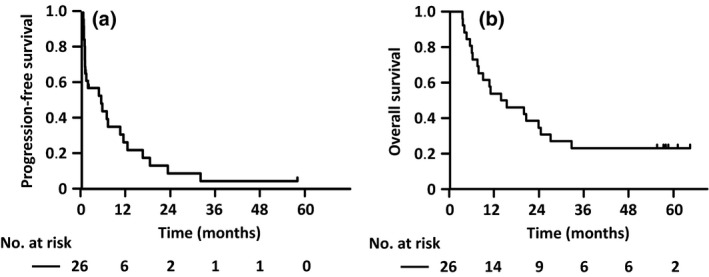
Progression‐free survival (PFS) and overall survival (OS) of all relapsed adult T‐cell leukemia–lymphoma (ATL) patients enrolled in the phase II study. (a) PFS curves for all relapsed ATL patients enrolled in the phase II study. Median PFS was 5.2 months (95% confidence interval [CI], 0.9–10.3 months), and the PFS rate at 1 year was 26% (95% CI, 11%–45%). (b) OS curves of all relapsed ATL patients enrolled in the phase II study. Median OS was 14.4 months (95% CI, 7.4–24.3 months), and the OS rate at 3 years was 23% (95% CI, 9%–40%).
For the same patients, median OS was 14.4 months (95% CI, 7.4–24.3 months), with six (patient numbers 306, 312, 321, 1114, 1503, 1513) of these patients showing longer OS, yielding an OS rate at 3 years of 23% (95% CI, 9%–40%; Table 2; Fig. 1b). Four such patients were of an unfavorable chronic subtype and the remaining patients consisted of one acute and one lymphoma subtype. Thus, the OS rate at 3 years was 7% (1/14 patients) for acute, 17% (1/6 patients) for lymphoma, and 67% (4/6 patients) for unfavorable chronic subtypes (Table 2).
PFS and OS according to unfavorable prognostic factors in relapsed ATL patients
Among ATL patients, of the unfavorable prognostic factors, PS (0–1 vs 2–4; Fig. S1a, b), age (≤70 vs ≥71 years; Fig. S1c, d), and serum Alb (<3.5 vs ≥3.5 g/dL; Fig. S1e, f), none was significantly associated with PFS and OS.
PFS and OS according to the presence of rashes
PFS and OS of patients who did not develop a rash were significantly shorter than those of patients who developed a rash (median PFS: 0.8 months vs 10.3 months, P < 0.001; Fig. 2a, and median OS: 5.4 months vs 23.7 months, P = 0.005; Fig. 2b, respectively). According to a breakdown by severity of skin rash, PFS of patients who did not develop a rash or who developed a grade 1 rash was significantly shorter than that of patients who developed a grade 2 or higher rash (median PFS: 0.8 months vs 11.1 months, P < 0.001; Fig. 2c). OS of patients who did not develop a rash or who developed a grade 1 rash was significantly shorter than that of patients who developed a grade 2 or higher rash (median OS: 6.0 months vs 25.6 months, P < 0.001; Fig. 2d). However, there were no significant differences between both PFS and OS in patients who did not develop a rash or who developed grade 1–2 rashes, and those who developed a grade 3 or higher rash (median PFS: 1.6 months vs 7.0 months; Fig. S2a, and median OS: 10.6 months vs 27.0 months, Fig. S2b, respectively).
Figure 2.
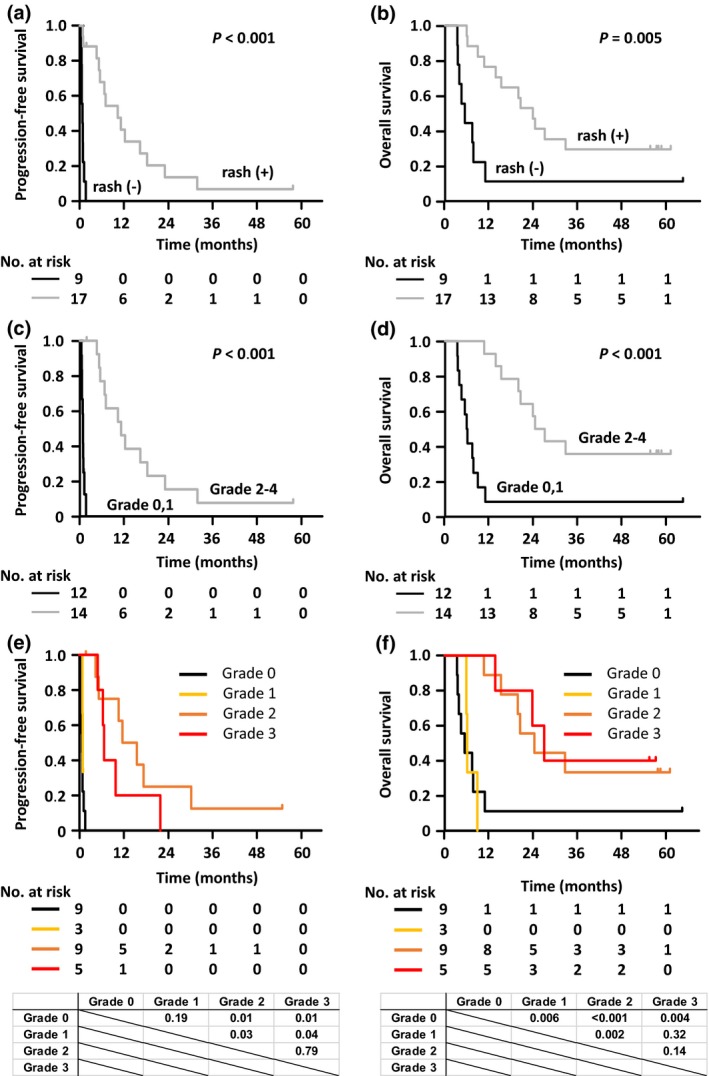
Progression‐free survival (PFS) and overall survival (OS) in relapsed adult T‐cell leukemia–lymphoma (ATL) patients according to the presence of skin rash. (a) PFS curves for relapsed ATL patients who did (+)/did not (–) develop a skin rash are shown. For patients who did not develop a rash after 1 year, median PFS was 0.8 months (95% confidence interval [CI], 0.4–1.2 months), and PFS rate was 0%. For patients who developed rashes after 1 year, median PFS was 10.3 months (95% CI, 5.2–18.2 months), and PFS rate was 41% (95% CI, 17%–63%). (b) OS curves for ATL patients who did/did not develop a skin rash are shown. For patients who did not develop a rash after 3 years, median OS was 5.4 months (95% CI, 3.3–10.8 months), and OS rate was 11% (95% CI, 1%–39%). For patients who developed rashes after 3 years, median OS was 23.7 months (95% CI, 10.6 months–not estimated [NE]), and the OS rate was 29% (95% CI, 11%–51%). (c) PFS curves for ATL patients who did not develop a rash or who developed a grade 1, or a grade 2 or higher skin rash are shown. For patients who did not develop a rash or who developed grade 1 rashes after 1 year, median PFS was 0.8 months (95% CI, 0.4–1.2 months), and PFS rate was 0%. For patients who developed a grade 2 or higher rash after 1 year, median PFS was 11.1 months (95% CI, 5.5–18.2 months), and PFS rate was 46% (95% CI, 19%–70%). (d) OS curves for ATL patients who did not develop a rash or who developed a grade 1, or a grade 2 or higher skin rash are shown. For patients who did not develop a rash or who developed a grade 1 rash after 3 years, median OS was 6.0 months (95% CI, 3.5–8.8 months), and OS rate was 8% (95% CI, 1%–31%). For patients who developed a grade 2 or higher rash after 3 years, median OS was 25.6 months (95% CI, 15.2 months–NE), and OS rate was 36% (95% CI, 13%–59%). (e) PFS curves for relapsed ATL patients who did not develop a rash or who developed grade 1, 2, or 3 skin rashes are shown. Median PFS and PFS rates at 1 year in patients who did not develop a rash or who developed grade 1, 2, or 3 rashes were 0.8 months (95% CI, 0.4–1.2 months) and 0%, 0.9 months (95% CI, 0.8 months–NE) and 0%, 14.3 months (95% CI, 4.6–31.8 months) and 63% (95% CI, 23%–86%), and 7.0 months (95% CI, 5.2–23.0 months) and 20% (95% CI, 1%–58%), respectively. (f) OS curves for ATL patients who did not develop a rash or who developed grade 1, 2, or 3 rashes are shown. Median OS and OS rates at 3 years in patients who did not develop a rash or who developed grade 1, 2, or 3 rashes were 5.4 months (95% CI, 3.3–10.8 months) and 11% (95% CI, 1%–39%), 6.1 months (95% CI, 5.9–8.8 months) and 0%, 24.3 months (95% CI, 10.6 months–NE) and 33% (95% CI, 8%–62%), and 27.0 months (95% CI, 13.7 months–NE) and 40% (95% CI, 5%–75%), respectively. PFS and OS curves were compared using a log–rank test and the P‐values calculated between each curve are indicated in the lower panel.
We then analyzed PFS and OS according to each grade of rash (no rash, rash grades 1, 2 vs 3). PFS of patients who did not develop a rash was significantly shorter than those of patients who developed a grade 1 (median PFS of 0.9 months, P = 0.001), 2 (median PFS of 14.3 months, P < 0.001), or 3 (median PFS of 7.0 months, P = 0.004) rash. PFS of patients who developed a grade 1 rash was also significantly shorter than that of patients who developed a grade 2 rash (P = 0.004). However, there was no significant difference in the PFS between patients who developed a grade 1 and 3, or a grade 2 and 3 rash (Fig. 2e). In terms of OS, that of patients who did not develop a rash was significantly shorter than that of patients who developed a grade 2 (median OS of 24.3 months, P = 0.01), or 3 (median OS of 27.0 months, P = 0.01) rash. OS of patients who developed a grade 1 rash (median OS of 6.1 months) was also significantly shorter than that of patients who developed a grade 2 (P = 0.03) or 3 (P = 0.04) rash. In contrast, there was no significant difference in the OS between patients who did not develop a rash or who developed a grade 1 rash (P = 0.19), or a grade 2 and 3 rash (P = 0.79; Fig. 2f).
Clinical characteristics of ATL patients according to the presence of a rash
Clinical characteristics, before mogamulizumab treatment, of patients who subsequently developed a grade 2 or higher rash were analyzed. WBC of ATL patients who developed a grade 2 or higher rash was higher compared to that of patients who did not develop a rash or who developed a grade I rash (P = 0.05, Table 3). An abnormal lymphocyte count was also significantly higher in patients who developed a grade 2 or higher rash (P = 0.04). In contrast, blood hemoglobin levels and platelet counts were not significantly associated with the presence or absence of a grade 2 or higher rash. Age (≥71 vs ≤70 years), sex (male vs female), PS (0–1 vs 2–4), presence or absence of ATL skin lesions, serum Alb (≥3.5 g/dL vs <3.5 g/dL), serum LDH (>2N vs ≤2N; LDH = 2N signifies an LDH level twice the upper limit of normal according to hospital laboratory guidelines) and an eosinophil count (≤500/μL vs >500/μL)20 were also not significantly associated with the presence or absence of a grade 2 or higher rash (Table 3).
Table 3.
Characteristics of relapsed ATL patients according to a rash induced by mogamulizumab
| Patients’ characteristics before mogamulizumab treatment | Rash grade | P‐value | |
|---|---|---|---|
| 0, 1 | 2, 3, 4 | ||
| Total patients, number (%) | 12 (46) | 14 (54) | |
| Age, years | |||
| ≤70 | 9 (47) | 10 (53) | 1.00 |
| ≥71 | 3 (43) | 4 (57) | |
| Sex | |||
| Female | 6 (40) | 9 (60) | 0.69 |
| Male | 6 (55) | 5 (45) | |
| ECOG PS | |||
| 0, 1 | 10 (48) | 11 (52) | 1.00 |
| 2, 3, 4 | 2 (40) | 3 (60) | |
| ATL skin lesion | |||
| Absent | 8 (44) | 10 (56) | 1.00 |
| Present | 4 (50) | 4 (50) | |
| Serum Alb, g/dL | |||
| ≥3.5 | 10 (43) | 13 (57) | 0.58 |
| <3.5 | 2 (67) | 1 (33) | |
| Serum LDHa | |||
| ≤2N | 6 (35) | 11 (65) | 0.22 |
| >2N | 6 (67) | 3 (33) | |
| Eosinophil count/μL | |||
| ≤500 | 11 (46) | 13 (54) | 1.00 |
| >500 | 1 (50) | 1 (50) | |
| WBC/μL | |||
| Mean | 6373 | 12 995 | 0.05 |
| Median | 4510 | 7850 | |
| Range | 2900–16 030 | 3700–40 250 | |
| Abnormal lymphocyte count/μL | |||
| Mean | 1,045 | 6108 | 0.04 |
| Median | 672 | 3587 | |
| Range | 0–4324 | 0–30 188 | |
| Hb, g/dL | |||
| Mean | 11.6 | 12.1 | 0.49 |
| Median | 11.9 | 12.1 | |
| Range | 9.0–15.3 | 8.9–15.9 | |
| Plt, × 103/μL | |||
| Mean | 192 | 174 | 0.43 |
| Median | 185 | 152 | |
| Range | 70–338 | 90–328 | |
LDH is expressed as a ratio in which the patient's LDH level was divided by the upper limit of normal for LDH as set by the respective hospital laboratory. Alb, albumin; ATL, adult T‐cell leukemia‐lymphoma; ECOG, Eastern Cooperative Oncology Group; Hb, hemoglobin; LDH, lactate dehydrogenase; Plt, platelet count; PS, performance status; WBC, white blood cell count.
Discussion
The present long‐term follow up of a phase I study of mogamulizumab monotherapy showed that among 13 patients with relapsed aggressive ATL, four (31%) patients survived for more than 3 years, of whom three (23%) survived for at least 5 years. As for 26 patients with relapsed aggressive ATL enrolled in the phase II study, a median PFS of 5.2 months, a PFS rate at 1 year of 26%, a median OS of 14.4 months, and an OS rate at 3 years of 23% were achieved. To date, there have been no prescribed standards of care for patients with relapsed or refractory aggressive ATL, although several studies have been conducted to improve their prognosis. In a phase II study of bortezomib in relapsed or refractory aggressive ATL, a median PFS of 38 days was reported (n = 15).21 Antiviral therapy, consisting of a combination of zidovudine and interferon, which has been proposed as a standard first‐line therapy in leukemic subtypes of ATL,22 demonstrated a median OS of 3.0 months in acute or lymphoma subtypes of ATL patients who had been previously treated (n = 7) or untreated (n = 12).23 A phase II study of alemtuzumab in patients with ATL, who had been either previously treated (n = 20) or untreated (n = 9), has been reported. Patients consisted of 15 acute, 11 lymphoma, and three chronic subtypes, with a median PFS and OS of 2.0 and 5.9 months, respectively.24 Collectively, although a patient selection bias may have occurred at study enrolment,14, 15 and a direct comparison between the two different studies may not be appropriate, some patients with relapsed aggressive ATL who were enrolled in phase I and II studies and who received mogamulizumab monotherapy actually survived for a long time. Most recently, in a phase II study of lenalidomide in relapsed or recurrent ATL in Japan, median PFS and OS of 3.8 and 20.3 months, respectively, were observed.25 The lenalidomide study showed a relatively longer OS despite a relatively shorter PFS and, in this context, the fact that this study was conducted in the era after mogamulizumab approval, should be kept in mind.
Although the sample size of the present study was small, a poor PS, older age, and lower serum Alb levels were not significantly associated with PFS and OS in patients with relapsed aggressive ATL. A poor PS, older age, and lower serum Alb levels were previously identified as unfavorable prognostic factors in acute or lymphoma subtype ATL patients in a large‐scale retrospective analysis (ATL–PI).19 A poor PS was also one of the components of the JCOG‐PI,26 which was established by three independent JCOG prospective clinical trials,27, 28, 29 and applied to aggressive ATL. Although these two PI were established based on ATL patients who were previously untreated, the unfavorable prognostic factors of these widely acceptable PI19, 26 were not applicable to the present, relapsed patients. We think that the most probable reason for this is that the treatment strategies applied to the patients were different. This indicates that in the era of mogamulizumab the establishment of a novel PI in ATL patients is warranted.
The present study demonstrated that the development of rashes had a positive impact not only on PFS, but also on OS. In this context, with respect to a phase I study among 13 ATL patients, one (number 412) patient developed a rash, with this patient surviving more than 5 years. These findings seem to be important, and almost consistent with other reports of retrospective analyses.30, 31 Because CCR4 is highly expressed on effector Treg,32, 33, 34 mogamulizumab also has Treg depletion activities.35 Such Treg depletion by mogamulizumab is likely to lead to stimulation of various types of immunity such as antitumor immune responses targeting ATL‐related antigens,36, 37, 38 possibly including the neoantigens caused by the abundant mutations of ATL cells,39, 40 in addition to autoimmune responses targeting autoantigens. It appears that the former results in therapeutic efficacies such as prolonged PFS and OS, and the latter results in immune‐related AE, including rashes. We should pay particular attention to the fact that severe skin‐related AE, such as Stevens‐Johnson syndrome/toxic epidermal necrolysis, are themselves sometimes fatal.17 Additionally, when severe immune‐related AE occur, we are forced to implement intensive immunosuppressive therapies such as systemic steroids, which not only attenuate antitumor immune responses but can also lead to various types of severe infectious complications. Therefore, a not too mild and not too severe, but moderate, provocation of immune response seems to be important, and is consistent with the current observations that PFS in patients who developed a grade 2 rash showed a longer trend compared to the PFS of patients who developed a grade 3 rash. The finding that the induction of a moderate immune reaction is best for patient survival is similar to GVHD after allogeneic HCT for ATL. That is, the hazard ratios for the OS of ATL patients with grade I, II, III, and IV acute GVHD compared with the absence of acute GVHD were 0.568, 0.688, 1.199, and 2.245, respectively.41 This type of positive effect of cutaneous immune‐related AE on OS has also been reported for nivolumab (an immune checkpoint inhibitor, antiprogrammed cell death protein‐1 mAb) treatment of metastatic melanoma.42
Among the clinical characteristics of patients, prior mogamulizumab treatment, a higher WBC and higher abnormal lymphocyte count were associated with the development of a grade 2 or higher rash. Although the causal mechanisms are not fully clarified, we must pay special attention to an immune‐related AE, the development of rashes, when we treat ATL patients that have high WBC and high abnormal lymphocyte counts with mogamulizumab. Simultaneously, these characteristics indicate such patients are more likely to benefit from mogamulizumab monotherapy.
A recent phase II study of lenalidomide in relapsed or recurrent aggressive ATL demonstrated promising antitumor activity.25 Lenalidomide also has immunomodulatory effects, including stimulation of NK cell function;43 thus when combined with antibody agents in vitro, lenalidomide enhanced ADCC by augmenting NK cells.44 Combination therapy consisting of lenalidomide plus an antibody agent, rituximab, was active as an initial therapy for mantle cell lymphoma not only in in vitro preclinical experiments, but also in the clinic.45 In this context, because the antitumor activity of mogamulizumab is completely dependent on ADCC, mainly by NK cells as effector cells,12, 13 combination therapy consisting of mogamulizumab plus lenalidomide should be considered as having potential in the treatment of ATL.
Although this up‐to‐date analysis offers novel and important findings on mogamulizumab use for ATL, several limitations should also be borne in mind. First, the previous phase I and II studies14, 15 mentioned were relatively small and, thus, definitive conclusions about the long‐term efficacies on survival by mogamulizumab cannot be drawn. Second, although an allogeneic HCT is considered the only curative treatment strategy for aggressive ATL,6, 41, 46 the present study did not collect data about allogeneic HCT in corresponding patients. This information seems to be important as mogamulizumab treatment prior to allogeneic HCT has been reported to be associated with an unfavorable prognosis caused by increased transplantation‐related AE, mainly acute GVHD.47
In conclusion, this updated analysis suggests that mogamulizumab monotherapy may improve PFS and OS in some patients with relapsed aggressive ATL, especially in those with a moderate immune‐related AE in the form of a skin rash. Further investigation of mogamulizumab treatment for ATL is warranted to clarify the mechanisms involved in the present observations.
Disclosure Statement
Takashi Ishida: Research funding from Kyowa Hakko Kirin Co., Ltd, Bayer Pharma AG, J‐Pharma Co., Ltd, and Celgene K.K. Honoraria from Kyowa Hakko Kirin Co., Ltd, and Celgene K.K. Atae Utsunomiya: Research funding from Kyowa Hakko Kirin Co., Ltd, Honoraria from Kyowa Hakko Kirin Co., Ltd, Sumitomo Dainippon Pharma Co., Ltd, Immuno‐Biological Laboratories Co., Ltd, Japan Blood Products Organization, Roche Diagnostics K.K., Daiichi‐Sankyo Company, Siemens K.K., Bristol‐Myers Squibb, Pfizer Japan Inc., Astellas Pharma Inc., Novartis Pharma K.K., HUYA Bioscience International, Nippon Shinyaku Co., Ltd, Chugai Pharmaceutical Co., Ltd, Celgene K.K. Tatsuro Jo: Research funding from Kyowa Hakko Kirin Co., Ltd, Honoraria from Kyowa Hakko Kirin Co., Ltd, Celegene, Chugai. Kazuhito Yamamoto: Research funding from Kyowa Hakko Kirin Co., Ltd, AbbVie, MSD, Pfizer, Novartis, Celegene, Takeda, Chugai, Ono Pharmaceutical, ARIAD Pharmaceuticals, Inc./CMIC, Honoraria from Kyowa Hakko Kirin Co., Ltd, Pfizer, Novartis, Celegene, Takeda, Chugai, Ono Pharmaceutical, ARIAD Pharmaceuticals, Inc./CMIC, Janssen, Bristol‐Myers Squibb, Sumitomo Dainippon Pharma, Otsuka Pharmaceutical, Boehringer Ingelheim, Mundipharma. Koji Kato has no potential COI to disclose. Shinichiro Yoshida: Research funding from Kyowa Hakko Kirin Co., Ltd. Shigeki Takemoto has no potential COI to disclose. Hitoshi Suzushima has no potential COI to disclose. Yukio Kobayashi has no potential COI to disclose. Yoshitaka Imaizumi: Research funding from Kyowa Hakko Kirin Co., Ltd, Honoraria from Kyowa Hakko Kirin Co., Ltd. Kenichi Yoshimura has no potential COI to disclose. Kouichi Kawamura: an employee of Kyowa Hakko Kirin Co., Ltd. Stock or other ownership of Kyowa Hakko Kirin Co., Ltd. Takeshi Takahashi: an employee of Kyowa Hakko Kirin Co., Ltd. Kensei Tobinai: Research funding from Kyowa Hakko Kirin Co., Ltd, AbbVie, Celgene, Chugai, Eisai, GlaxoSmithKline, Janssen, Mundipharma, Ono Pharmaceutical, Servier, and Takeda. Honoraria from Eisai, Janssen, HUYA Bioscience, Takeda, and Zenyaku Kogyo. Ryuzo Ueda: Research funding from Kyowa Hakko Kirin Co., Ltd, Rikaken Co., Ltd, Medical & Biological Laboratories Co., Ltd, Chugai Pharmaceutical Co., Ltd. Honoraria from Chugai Pharmaceutical Co., Ltd, Kyowa Hakko Kirin Co., Ltd, Ono Pharmaceutical Co., Ltd. Consultancy with Mundipharma K.K., Ono Pharmaceutical Co., Ltd, Terumo Co., Ltd.
Abbreviations
- ADCC
antibody‐dependent cellular cytotoxicity
- AE
adverse event
- Alb
albumin
- ATL
adult T‐cell leukemia–lymphoma
- CCR4
CC chemokine receptor 4
- CI
confidence interval
- CTCL
cutaneous T‐cell lymphoma
- ECOG
Eastern Cooperative Oncology Group
- GVHD
graft‐versus‐host disease
- HCT
hematopoietic cell transplantation
- JCOG
Japan Clinical Oncology Group
- LDH
lactate dehydrogenase
- NK
natural killer
- OS
overall survival
- PFS
progression‐free survival
- PI
prognostic index
- PS
performance status
- PTCL‐NOS
peripheral T‐cell lymphoma, not otherwise specified
- Treg
regulatory T cells
- WBC
white blood cell count
Supporting information
Fig. S1. PFS and OS of relapsed ATL patients enrolled in the phase II study according to unfavorable prognostic factors.
Fig. S2. PFS and OS in relapsed ATL patients who developed no rash or a grade 1–2 skin rash, and a grade 3 or higher skin rash.
Acknowledgments
We thank all nurses and clinical research coordinators who were involved in this study for their excellent patient care and schedule management skills.
Cancer Sci 108 (2017) 2022–2029
Funding information
Kyowa Hakko Kirin Co. Ltd (Tokyo, Japan).
References
- 1. Vose J, Armitage J, Weisenburger D. International peripheral T‐cell and natural killer/T‐cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol 2008; 26: 4124–30. [DOI] [PubMed] [Google Scholar]
- 2. Swerdlow SH, Campo E, Pileri SA et al The 2016 revision of the World Health Organization (WHO) classification of lymphoid neoplasms. Blood 2016; 127: 2375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T‐cell leukaemia‐lymphoma: a report from the Lymphoma Study Group (1984–87). Br J Haematol 1991; 79: 428–37. [DOI] [PubMed] [Google Scholar]
- 4. Tsukasaki K, Hermine O, Bazarbachi A et al Definition, prognostic factors, treatment, and response criteria of adult T‐cell leukemia‐lymphoma: a proposal from an international consensus meeting. J Clin Oncol 2009; 27: 453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T‐cell leukemia: clinical and hematologic features of 16 cases. Blood 1997; 50: 481–92. [PubMed] [Google Scholar]
- 6. Ishitsuka K, Tamura K. Human T‐cell leukaemia virus type I and adult T‐cell leukaemia‐lymphoma. Lancet Oncol 2014; 15: e517–26. [DOI] [PubMed] [Google Scholar]
- 7. Ishida T, Utsunomiya A, Iida S et al Clinical significance of CCR4 expression in adult T‐cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin Cancer Res 2003; 9: 3625–34. [PubMed] [Google Scholar]
- 8. Yoshie O, Fujisawa R, Nakayama T et al Frequent expression of CCR4 in adult T‐cell leukemia and human T‐cell leukemia virus type 1‐transformed T cells. Blood 2002; 99: 1505–11. [DOI] [PubMed] [Google Scholar]
- 9. Ishida T, Inagaki H, Utsunomiya A et al CXC chemokine receptor 3 and CC chemokine receptor 4 expression in T‐cell and NK‐cell lymphomas with special reference to clinicopathological significance for peripheral T‐cell lymphoma, unspecified. Clin Cancer Res 2004; 10: 5494–500. [DOI] [PubMed] [Google Scholar]
- 10. Nakagawa M, Nakagawa‐Oshiro A, Karnan S et al Array comparative genomic hybridization analysis of PTCL‐U reveals a distinct subgroup with genetic alterations similar to lymphoma‐type adult T‐cell leukemia/lymphoma. Clin Cancer Res 2009; 15: 30–8. [DOI] [PubMed] [Google Scholar]
- 11. Ohshima K, Karube K, Kawano R et al Classification of distinct subtypes of peripheral T‐cell lymphoma unspecified, identified by chemokine and chemokine receptor expression: analysis of prognosis. Int J Oncol 2004; 25: 605–13. [PubMed] [Google Scholar]
- 12. Shinkawa T, Nakamura K, Yamane N et al The absence of fucose but not the presence of galactose or bisecting N‐acetylglucosamine of human IgG1 complex‐type oligosaccharides shows the critical role of enhancing antibody‐dependent cellular cytotoxicity. J Biol Chem 2003; 278: 3466–73. [DOI] [PubMed] [Google Scholar]
- 13. Ishii T, Ishida T, Utsunomiya A et al Defucosylated humanized anti‐CCR4 monoclonal antibody KW‐0761 as a novel immunotherapeutic agent for adult T‐cell leukemia. Clin Cancer Res 2010; 16: 1520–31. [DOI] [PubMed] [Google Scholar]
- 14. Yamamoto K, Utsunomiya A, Tobinai K et al Phase I study of KW‐0761, a defucosylated humanized anti‐CCR4 antibody, in relapsed patients with adult T‐cell leukemia‐lymphoma and peripheral T‐cell lymphoma. J Clin Oncol 2010; 28: 1591–8. [DOI] [PubMed] [Google Scholar]
- 15. Ishida T, Joh T, Uike N et al Defucosylated anti‐CCR4 monoclonal antibody (KW‐0761) for relapsed adult T‐cell leukemia‐lymphoma: a multicenter phase II study. J Clin Oncol 2012; 30: 837–42. [DOI] [PubMed] [Google Scholar]
- 16. Ogura M, Ishida T, Hatake K et al Multicenter phase II study of mogamulizumab (KW‐0761), a defucosylated anti‐cc chemokine receptor 4 antibody, in patients with relapsed peripheral T‐cell lymphoma and cutaneous T‐cell lymphoma. J Clin Oncol 2014; 32: 1157–63. [DOI] [PubMed] [Google Scholar]
- 17. Ishida T, Ito A, Sato F et al Stevens‐Johnson Syndrome associated with mogamulizumab treatment of adult T‐cell leukemia/lymphoma. Cancer Sci 2013; 104: 647–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ifuku H, Kusumoto S, Tanaka Y et al Fatal reactivation of hepatitis B virus infection in a patient with adult T‐cell leukemia‐lymphoma receiving the anti‐CC chemokine receptor 4 antibody mogamulizumab. Hepatol Res 2015; 45: 1363–7. [DOI] [PubMed] [Google Scholar]
- 19. Katsuya H, Yamanaka T, Ishitsuka K et al Prognostic index for acute‐ and lymphoma‐type adult T‐cell leukemia/lymphoma. J Clin Oncol 2012; 30: 1635–40. [DOI] [PubMed] [Google Scholar]
- 20. Utsunomiya A, Ishida T, Inagaki A et al Clinical significance of a blood eosinophilia in adult T‐cell leukemia/lymphoma: a blood eosinophilia is a significant unfavorable prognostic factor. Leuk Res 2007; 31: 915–20. [DOI] [PubMed] [Google Scholar]
- 21. Ishitsuka K, Utsunomiya A, Katsuya H et al A phase II study of bortezomib in patients with relapsed or refractory aggressive adult T‐cell leukemia/lymphoma. Cancer Sci 2015; 106: 1219–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bazarbachi A, Plumelle Y, Carlos Ramos J et al Meta‐analysis on the use of zidovudine and interferon‐alfa in adult T‐cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J Clin Oncol 2010; 28: 4177–83. [DOI] [PubMed] [Google Scholar]
- 23. Gill PS, Harrington W Jr, Kaplan MH et al Treatment of adult T‐cell leukemia‐lymphoma with a combination of interferon alfa and zidovudine. N Engl J Med 1995; 332: 1744–8. [DOI] [PubMed] [Google Scholar]
- 24. Sharma K, Janik JE, O'Mahony D et al Phase II study of alemtuzumab(CAMPATH‐1®) in patients with HTLV‐1‐associated adult T‐cell leukemia/lymphoma. Clinical Cancer Res 2017; 23: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ishida T, Fujiwara H, Nosaka K et al Multicenter Phase II study of lenalidomide in relapsed or recurrent adult T‐cell leukemia‐lymphoma: ATLL‐002. J Clin Oncol 2016; 34: 4086–93. [DOI] [PubMed] [Google Scholar]
- 26. Fukushima T, Nomura S, Shimoyama M et al Japan Clinical Oncology Group (JCOG) prognostic index and characterization of long‐term survivors of aggressive adult T‐cell leukaemia‐lymphoma (JCOG0902A). Br J Haematol 2014; 166: 739–48. [DOI] [PubMed] [Google Scholar]
- 27. Tsukasaki K, Utsunomiya A, Fukuda H et al VCAP‐AMP‐VECP compared with biweekly CHOP for adult T‐cell leukemia‐lymphoma: Japan Clinical Oncology Group Study JCOG9801. J Clin Oncol 2007; 25: 5458–64. [DOI] [PubMed] [Google Scholar]
- 28. Tsukasaki K, Tobinai K, Shimoyama M et al Deoxycoformycin‐containing combination chemotherapy for adult T‐cell leukemia‐lymphoma: Japan Clinical Oncology Group Study (JCOG9109). Int J Hematol 2003; 77: 164–70. [DOI] [PubMed] [Google Scholar]
- 29. Yamada Y, Tomonaga M, Fukuda H et al A new G‐CSF‐supported combination chemotherapy, LSG15, for adult T‐cell leukaemia‐lymphoma: Japan Clinical Oncology Group Study 9303. Br J Haematol 2001; 113: 375–82. [DOI] [PubMed] [Google Scholar]
- 30. Yonekura K, Kanzaki T, Gunshin K et al Effect of anti‐CCR4 monoclonal antibody (mogamulizumab) on adult T‐cell leukemia‐lymphoma: cutaneous adverse reactions may predict the prognosis. J Dermatol 2014; 41: 239–44. [DOI] [PubMed] [Google Scholar]
- 31. Yonekura K, Tokunaga M, Kawakami N et al Cutaneous adverse reaction to Mogamulizumab may indicate favourable prognosis in adult T‐cell Leukaemia‐lymphoma. Acta Derm Venereol 2016; 96: 1000–2. [DOI] [PubMed] [Google Scholar]
- 32. Ishida T, Ueda R. CCR4 as a novel molecular target for immunotherapy of cancer. Cancer Sci 2006; 97: 1139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sugiyama D, Nishikawa H, Maeda Y et al Anti‐CCR4 mAb selectively depletes effector‐type FoxP3 + CD4 + regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci USA 2013; 110: 17945–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suzuki S, Ishida T, Yoshikawa K, Ueda R. Current status of immunotherapy. Jpn J Clin Oncol 2016; 46: 191–203. [DOI] [PubMed] [Google Scholar]
- 35. Kurose K, Ohue Y, Wada H et al Phase Ia study of FoxP3 + CD4 treg depletion by infusion of a humanized anti‐CCR4 antibody, KW‐0761, in cancer patients. Clin Cancer Res 2015; 21: 4327–36. [DOI] [PubMed] [Google Scholar]
- 36. Nishikawa H, Maeda Y, Ishida T et al Cancer/testis antigens are novel targets of immunotherapy for adult T‐cell leukemia/lymphoma. Blood 2012; 119: 3097–104. [DOI] [PubMed] [Google Scholar]
- 37. Masaki A, Ishida T, Suzuki S et al Autologous Tax‐specific CTL therapy in a primary adult T cell leukemia/lymphoma cell‐bearing NOD/Shi‐scid, IL‐2Rγnull mouse model. J Immunol 2013; 191: 135–44. [DOI] [PubMed] [Google Scholar]
- 38. Narita T, Ishida T, Masaki A et al HTLV‐1 bZIP factor‐specific CD4 T cell responses in adult T cell leukemia/lymphoma patients after allogeneic hematopoietic stem cell transplantation. J Immunol 2014; 192: 940–7. [DOI] [PubMed] [Google Scholar]
- 39. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015; 348: 69–74. [DOI] [PubMed] [Google Scholar]
- 40. Kataoka K, Nagata Y, Kitanaka A et al Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet 2015; 47: 1304–15. [DOI] [PubMed] [Google Scholar]
- 41. Ishida T, Hishizawa M, Kato K et al Impact of graft‐versus‐host disease on allogeneic hematopoietic cell transplantation for adult T cell leukemia‐lymphoma focusing on preconditioning regimens: nationwide retrospective study. Biol Blood Marrow Transplant 2013; 19: 1731–9. [DOI] [PubMed] [Google Scholar]
- 42. Freeman‐Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in resected and unresectable metastatic melanoma: Characteristics of immune‐related adverse events and association with outcomes. Clin Cancer Res 2016; 15: 886–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer 2004; 4: 314–22. [DOI] [PubMed] [Google Scholar]
- 44. Zhang L, Qian Z, Cai Z et al Synergistic antitumor effects of lenalidomide and rituximab on mantle cell lymphoma in vitro and in vivo. Am J Hematol 2009; 84: 553–9. [DOI] [PubMed] [Google Scholar]
- 45. Ruan J, Martin P, Shah B et al Lenalidomide plus rituximab as initial treatment for mantle‐cell lymphoma. N Engl J Med 2015; 373: 1835–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ishida T, Hishizawa M, Kato K et al Allogeneic hematopoietic stem cell transplantation for adult T‐cell leukemia‐lymphoma with special emphasis on preconditioning regimen: a nationwide retrospective study. Blood 2012; 120: 1734–41. [DOI] [PubMed] [Google Scholar]
- 47. Fuji S, Inoue Y, Utsunomiya A et al Pretransplantation anti‐CCR4 antibody mogamulizumab against adult T‐cell leukemia/lymphoma is associated with significantly increased risks of severe and corticosteroid‐refractory graft‐versus‐host disease, nonrelapse mortality, and overall mortality. J Clin Oncol 2016; 34: 3426–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. PFS and OS of relapsed ATL patients enrolled in the phase II study according to unfavorable prognostic factors.
Fig. S2. PFS and OS in relapsed ATL patients who developed no rash or a grade 1–2 skin rash, and a grade 3 or higher skin rash.


