Abstract
Pseudomonas aeruginosa is a Gram-negative bacterium and causes respiratory infection especially in elderly patients. Royal jelly has been used worldwide as a traditional remedy and as a nutrient; however, the effect against P. aeruginosa is unclear. The aim of this study was to analyze antibacterial, antiadherent, and anti-inflammatory effects of royal jelly against P. aeruginosa. Wild-type strain PAO1 and clinical isolates of P. aeruginosa were used for antibacterial assay and antiadherent assay to abiotic surface and epithelial cells, which are pharynx (Detroit 562) and lung (NCI-H292) epithelial cells. In anti-inflammatory assay, epithelial cells were pretreated with royal jelly before bacterial exposure to investigate its inhibitory effect on interleukin (IL-8) and macrophage inflammatory protein-3α/CCL20 overproduction. Although royal jelly did not have antibacterial activity at concentration of 50% w/v, antiadherent activity was confirmed on the abiotic surface and epithelial cells under concentration of 25%. Pretreatment with royal jelly significantly inhibited overproduction of IL-8 and CCL20 from both cells. These results demonstrated that royal jelly inhibits P. aeruginosa adherence and protects epithelial cells from excessive inflammatory responses against P. aeruginosa infection. Our findings suggested that royal jelly may be a useful supplement as complementary and alternative medicine for preventing respiratory infection caused by P. aeruginosa.
1. Introduction
Pseudomonas aeruginosa is an opportunistic pathogen in human and is a Gram-negative rod which is abundantly found in soil, plant, decaying matter, and water. This bacterium is able to survive in various habitats, because it can use various organic compounds as carbon and nitrogen resources. Although it is classified as an obligate aerobe, this species can grow in anaerobic environment such as in periapical lesion caused by dental infection [1, 2]. In chronic periapical lesion, P. aeruginosa was identified in mixed population together with other species [2]. It was also one of frequent microorganisms which survive in persistent or secondary periapical infection and root canal tissue [3, 4]. It sometimes causes respiratory infection especially in elderly patients. Our previous report showed that P. aeruginosa colonized in oral cavity could be a risk factor of aspiration pneumonia in the elderly patients with cerebrovascular diseases and dysphagia [5]. It has also been known that P. aeruginosa is the most important etiological factor causing fatal nosocomial infections and found to be resistant to several antibiotics. Previous study has reported that this pathogen was identified as the second rank of species isolated from monomicrobial nosocomial bloodstream infection and caused 47.9% and 27.6% of mortality in intensive-care unit (ICU) patients and in non-ICU patients, respectively [6]. Some isolates were resistant to piperacillin, ticarcillin-clavulanate (Tic-Clv), ceftazidime, imipenem (IPM), aztreonam, ciprofloxacin (CPFX), gentamycin, and tobramycin [6]. The increasing of antibiotic resistant P. aeruginosa has become a worldwide problem.
Bacterial adherence to the surface of epithelial cells is an initial step in bacterial colonization and induction of pathological responses on host tissue [7]. Virulence factor molecules determine the ability of P. aeruginosa to induce pathological responses. Those factors also play important roles in bacterial colonization, survival, and their invasion into host tissue [8].
Royal jelly is a secretion produced from the hypopharyngeal and mandibular glands of young worker honeybees and contains all the nutrients to develop the queen honeybee from the larva. It is composed of water, carbohydrate, lipids, proteins, vitamins (mainly riboflavin, niacin, and thiamin), some minerals (mainly calcium and iron), and other components and has been used worldwide as a traditional and ethopharmacological nutrient and remedy [9]. A number of studies demonstrated that it possesses antimicrobial activities and antitumor and anti-inflammatory activities. However, the effect of royal jelly against P. aeruginosa was studied only to a limited extent and the results do not allow drawing definitive conclusions [10–12].
We made a hypothesis that increased adherence correlates to an increase in the production of proinflammatory cytokines and the concentrations of royal jelly inhibiting adherence are also inhibiting inflammation. Therefore, the aim of this study was to analyze the effect of royal jelly on the adherence of P. aeruginosa to abiotic surface and human pharyngeal and lung epithelial cell lines, Detroit 562 and NCI-H292, as good experimental model for in vitro P. aeruginosa adherence, and to examine cytotoxicity and anti-inflammatory effects of royal jelly on these human epithelial cells stimulated with P. aeruginosa.
2. Materials and Methods
2.1. Bacterial Strains, Growth Condition, and Antibiotics
P. aeruginosa PAO1, wild-type strain, and four clinical isolates, TUH-54, TUH-124, TUH-188, and TUH-213, were used in this study. Four clinical isolates were isolated from oral cavity or respiratory tract. IPM and CPFX were purchased from Wako Pure Chemical Industries (Osaka, Japan). Amikacin (AMK) was purchased from Sigma-Aldrich (St. Louis, MO, USA). All P. aeruginosa strains were grown at 37°C in lysogeny broth (LB) or on LB agar plates. For each experiment, bacterial cells were picked up from single colony, inoculated in LB broth, and incubated at 37°C for 16 h of shaking.
2.2. Cell Line Culture
Detroit 562 (American Type culture collection; ATCC, Manassas, VA, USA) and NCI-H292 (ATCC, Manassas, VA, USA) epithelial cell lines derived from pharynx and lung, respectively, were used. Detroit 562 cells were cultured in minimum essential medium alpha supplemented with 2 mM glutamine, 1% nonessential amino acids, 1 mM sodium pyruvate, 0.1% lactalbumin hydrolysate, 10% (vol/vol) fetal bovine serum (FBS), 100 μg mL−1 streptomycin, and 100 units mL−1 penicillin, and NCI-H292 cells were cultured in RPMI1640 medium supplemented with 2 mM glutamine, 10% (vol/vol) FBS, 100 μg mL−1 streptomycin, and 100 units mL−1 penicillin, in a water-saturated atmosphere of 95% air and 5% CO2 at 37°C. Both cells in medium were seeded in wells of 24-well tissue culture plate and incubated until confluent monolayers developed. Confluent monolayers were used in all experiments.
2.3. Royal Jelly Preparation
Royal jelly was purchased from Yamada Bee Farm (Okayama, Japan). Royal jelly was suspended in phosphate buffered saline (PBS) and stirred overnight at 4°C. The suspension was then centrifuged at 12,000g for 15 min at 4°C followed by filtration using 0.45 μm pore filter and kept at 4°C until just before use. For bacterial susceptibility test, fresh working solution of royal jelly was prepared in PBS or LB broth.
2.4. Susceptibility Assay
The minimum inhibitory concentrations (MICs) of antibiotics and royal jelly were assessed by the standard microbroth dilution method. Approximately 1 × 106 cells mL−1 of bacterial culture was inoculated into 100 μl of LB broth containing a twofold serial dilution of antibiotics or royal jelly suspension in 96-well culture plate (TPP, Trasadingen, Switzerland) and incubated for 24 h at 37°C. The MIC was defined as the lowest concentration showing no bacterial growth.
2.5. Bacterial Adherence Assay
Bacterial adherence assay for abiotic surface was performed using 96-well plates. Royal jelly was added in the culture medium before bacterial inoculation at the concentration of 25%. Approximately 1 × 106 cells mL−1 of P. aeruginosa culture was inoculated into 100 μl of LB broth and then incubated for 6 h at 37°C. After incubation, adherent bacteria were washed with purified water twice without disturbing the adherent bacteria and stained with 0.1% crystal violet for 10 min at room temperature, and excess stain was removed by gentle washes with purified water twice. After being dried, stained adherent bacteria were extracted from well by adding 150 μl of ethanol and the absorbance of the extract from stained adherent bacteria was measured at 595 nm using a microplate reader (model 680; Bio-Rad Laboratories, Hercules, CA, USA). For dose-dependent study, PAO1 and representative clinical isolate TUH-54 were cultured with 5, 12.5, 20, and 25% royal jelly suspension in culture medium.
Bacterial adherence assay for epithelial cells was also performed. Confluent Detroit 562 and NCI-H292 cell monolayers were preincubated with the various concentrations of royal jelly (12.5, 20, and 25%) for 30 min at 37°C. And then, P. aeruginosa PAO1 or TUH-54 was directly added to each epithelial cell in 24-well tissue culture plates at final concentration of 1 × 108 cells mL−1 and incubated for 1 h. As a positive control, both epithelial cells were stimulated with bacteria without pretreatment of royal jelly. All experiments were done using antibiotic-free culture medium. P. aeruginosa adherence to royal jelly-pretreated epithelial cells was quantified by determining the number of colony-forming units (CFUs) after washing with PBS twice. Adherent bacterial cells from epithelial cells were isolated by lysis with water prior to spreading on LB agar plates. We confirmed that there is no effect of osmotic shock caused by pure water on the bacterial viability (data not shown). The plates were incubated at 37°C for 24 h and CFU counts of adherent P. aeruginosa were determined and adherence was expressed as the percentage of bacteria recovered from cell lysis to those of the initial inoculums.
2.6. Lactate Dehydrogenase (LDH) Cytotoxicity Assay
The effect of royal jelly on cell cytotoxicity was determined using LDH assay. Confluent Detroit 562 and NCI-H292 cell monolayers in 24-well plates were cultured with the various concentrations of royal jelly (5, 12.5, 20, and 25%) for 4 h at 37°C in a water-saturated atmosphere of 95% air and 5% CO2. As a positive control, the cells were treated with 0.1% Triton X-100 and gently shaken for 10 min at room temperature. For the cytotoxicity assay, the levels of LDH in the recovered cell culture supernatants were determined using LDH cytotoxicity assay kit (Cayman Chemical, Ann Arbor, MI, USA) in accordance with the manufacturer's instructions. Absorbance was measured at 490 nm using a microplate reader (Bio-Rad Laboratories).
2.7. Royal Jelly Protection Assay
Detroit 562 monolayers cultured in a 24-well plate were pretreated with 25% royal jelly or PBS for 30 s at room temperature, washed with PBS twice, and irritated with 0.1% Triton X-100 for 30 sec at room temperature. The levels of LDH in the recovered cell culture supernatants were determined using LDH cytotoxicity assay kit and absorbance was measured at 490 nm using a microplate reader.
2.8. Chemokine Stimulation Assay
Confluent monolayers of Detroit 562 and NCI-H292 cells were preincubated with various concentrations of royal jelly (5, 12.5, 20, and 25%) in antibiotic-free culture medium for 30 min at 37°C. After 30 min incubation, the cells were washed with PBS twice. And then P. aeruginosa PAO1 or TUH-54 was directly added to each epithelial cell in 24-well tissue culture plates at final concentration of 2.0 × 107 cells mL−1 and incubated for 4 h. After 4 h incubations, the culture medium was collected and stored at −20°C until being assayed. Total RNA from the epithelial cells was isolated with NucleoSpin RNA II (MACHEREY-NAGEL, Duren, Germany).
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA kits (R&D Systems, Minneapolis, MN) were used to quantify IL-8 and CCL20 in cell culture supernatants collected after P. aeruginosa infection.
2.10. Reverse-Transcription-Polymerase Chain Reaction (RT-PCR)
RT and PCR were performed in two steps as follows. cDNA synthesis was performed with an RNA PCR Kit (TaKaRa, Shiga, Japan) and specific gene transcripts were amplified with ReddyMix PCR Mix (ABgene, Surrey, UK). The primers and PCR conditions for amplification of IL-8, CCL20, and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) mRNA were described previously [13]. GAPDH was used as an internal control. PCR products were analyzed by agarose gel electrophoresis and ethidium bromide staining.
2.11. Statistical Analysis
All experiments were conducted in triplicate or quadruplicate and statistical analyses were performed using the multifactorial one-way analysis of variance (ANOVA) with Tukey's Multiple Comparison test. Differences were considered significant when probability values were less than 1% (p < 0.01).
3. Results and Discussion
3.1. Studying the Comparative Susceptibility of P. aeruginosa to Antibiotics and Royal Jelly
The antibacterial activities of antibiotics and royal jelly against P. aeruginosa were shown in Table 1. According to the Clinical and Laboratory Standard Institute guideline M100-S22 break-point, TUH-54 exhibited resistance to IPM. Royal jelly did not exhibit antibacterial activities (≤50% w/v) against all tested bacteria. Our results are consistent with results reported by Boukraa stating that four kinds of royal jelly had antimicrobial activities against P. aeruginosa; however, the MICs were from 60 to 100% [14]. From these results, we conclude that the royal jelly may have quite low antimicrobial activity against P. aeruginosa. Major Royal Jelly Protein-1 (MRJP-1), which is one of components in beehive product that is Jelleine glycoproteins, has been shown to inhibit the growth of multidrug resistant P. aeruginosa [15]. Regarding the potency of royal jelly, our research then was focused on investigating antiadhesion potential and protective function of royal jelly on host cells.
Table 1.
Susceptibility to antibiotics and royal jelly for P. aeruginosa.
| Strain | Origin | MIC (μg mL−1) | MIC (%) | ||
|---|---|---|---|---|---|
| IPM | CPFX | AMK | Royal jelly | ||
| PAO1 | Wild type | 1 | 0.25 | 2 | ≥50 |
| TUH-54 | Pharyngeal secretions | 8 | 0.5 | 16 | ≥50 |
| TUH-122 | Coughed-up sputum | 2 | 0.5 | 4 | ≥50 |
| TUH-188 | Oral abscess | 2 | 0.125 | 4 | ≥50 |
| TUH-213 | Oral abscess | 2 | 0.125 | 4 | ≥50 |
3.2. The Effect of Royal Jelly on the Attachment of P. aeruginosa
The results of microtiter plate biofilm assay demonstrated that 25% royal jelly almost completely inhibited the bacterial adherence in PAO1, TUH-54, TUH-124, TUH-190, and TUH-213 (99-100% inhibition, data not shown). Furthermore, in order to determine the effective concentration of royal jelly to inhibit bacterial adherence, we performed dose-dependent experiments. Figure 1 shows that royal jelly could inhibit bacterial adherence at the concentration of 5% to both PAO1 and representative isolate, TUH-54. This inhibitory effect was increased as higher concentration of royal jelly. These results suggest that royal jelly has the potential to inhibit biofilm formation of P. aeruginosa by the inhibition of initial attachment on abiotic surface, such as medical devices. The ability of royal jelly to inhibit the attachment of bacteria closely related to its antibacterial components. Among the components of royal jelly including sugar proteins and lipids, it has been proven that glycoproteins Jelleine-I–III have antibacterial activity against both Gram-positive and Gram-negative bacteria [16]. P. aeruginosa virulence factor, Lectin B, which functions as the bacterial adhesin, could be blocked by royal jelly so that this bacterium cannot attach to the substrate [17].
Figure 1.
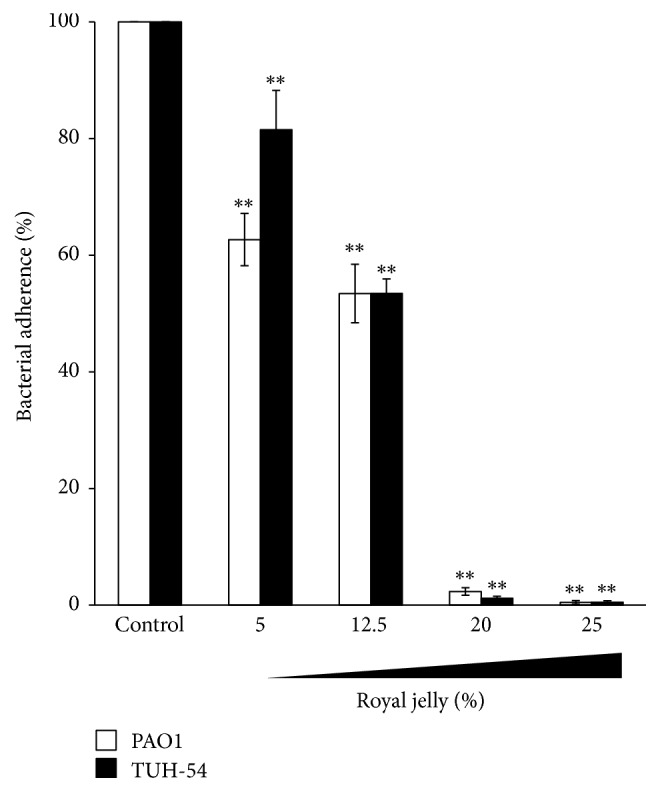
The inhibition of royal jelly to P. aeruginosa bacterial adherence. The inhibitory effect of royal jelly to the adherence of P. aeruginosa PAO1 and TUH-54 on abiotic surface. Data represent the means ± SDs of 4 independent experiments. Asterisks indicate significant differences (∗∗p < 0.001) versus control group (0% royal jelly).
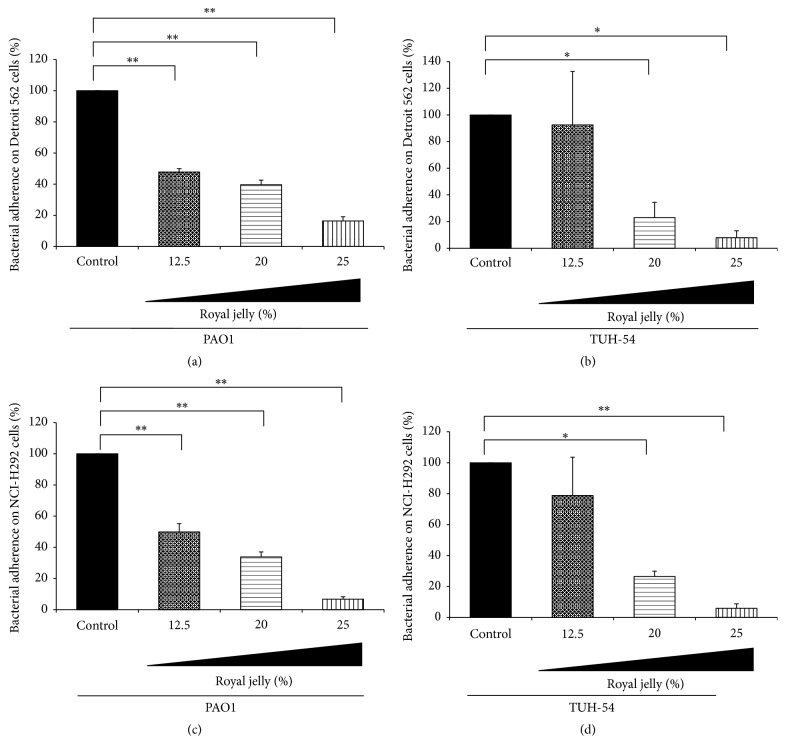
In addition to the adherence on abiotic surface, we next examine whether the royal jelly has the ability to inhibit the adhesion of P. aeruginosa to the epithelial cells. The bacterial adherence assay on epithelium cells demonstrated that the adherence of both PAO1 and the representative clinical isolate, TUH-54, was inhibited by 30 min pretreatment with various concentrations of royal jelly (Figure 2). Figure 2(a) shows that the adherence of PAO1 on Detroit 562 cells was inhibited by pretreatment of royal jelly in dose-dependent manner. The adherence of TUH-54 on the same cells was also significantly inhibited in 20% and 25% royal jelly-treated groups (Figure 2(b)). Furthermore, the effect of royal jelly on the adherence of PAO1 and TUH-54 to NCI-H292 cells showed similar results to those on Detroit 562 cell (Figures 2(c) and 2(d)).
Figure 2.
The inhibitory effect of royal jelly on the adherence of PAO1 and TUH-54 on human pharyngeal and lung epithelial cells. The inhibitory effect of royal jelly on the adherence of PAO1 (a, c) and TUH-54 (b, d) to Detroit 562 (a, b) and NCI-H292 (c, d) cells. Data represent the means ± SDs of 4 independent experiments. Asterisks indicate significant differences (∗∗p < 0.001 and ∗p < 0.01) versus control group (0% royal jelly).
Bacterial adhesion is an initial step in the pathogenic mechanism of P. aeruginosa infection. Oligosaccharide-mediated bacterium-cell recognition and adhesion are crucial for their colonization. Lectins play important role in glyconic recognition pattern as adhesins. There are two soluble lectins, Lec-A (PA-IL) and Lec-B (PA-IIL), in P. aeruginosa. These lectins are present on bacterial outer membrane and bind to galactose and fucose, respectively. They contribute important role in tissue damage caused by P. aeruginosa [18–21]. Some inventions have found the possibility of using specific lectins inhibitor as an alternative way to control the growth of P. aeruginosa and to reduce lung injury and as well as mortality caused by the same case, and therefore, lectins inhibition by specific carbohydrates proposed new perspective in therapy of P. aeruginosa infection [21]. Previous studies have confirmed that LecB binds to royal jelly and its hemagglutination activity was also inhibited by this compound [17]. Besides LecB, there are other adheren components, such as flagella and type IV pili, which are virulence factors and play a role in the attachment of bacteria to lung epithelial cells [22]. The bind between the adhesins with a membrane receptor initiates bacterial attachment to the epithelial cells [22]. Apart from the important role of flagella and pili, lectins, LecA and LecB, recently become a new target in the development of new antibacterials [23]. So far, royal jelly is known to inhibit the activity of LecB, but its effect on flagella and pili is still unknown. The findings from this study showed the potential inhibitory effect of royal jelly on adherence of P. aeruginosa to the human pharyngeal and lung epithelial cells and provide valuable information for the management of P. aeruginosa infections in clinical work since bacterial adhesion and biofilm formation play important roles in the initial bacterial infection. These results are also supported by previous study using antiadhesion-active components of edible seeds [24]. Siryaporn et al. showed that virulence activated through surface attachment required quorum sensing (QS) system (mediated by LasR) [25]. However, after 16 h incubation with 25% royal jelly, the expressions of lasR gene were not affected by royal jelly using qRT-PCR, suggesting that royal jelly has no effect on QS system (Supplemental Figure in Supplementary Material available online at https://doi.org/10.1155/2017/3191752). The action mechanisms of royal jelly for the inhibition of P. aeruginosa adherence are unknown and then the study regarding the influence of royal jelly on various adhesins of P. aeruginosa, such as LecB, flagellin, and pili, to epithelial cells is now under investigation.
3.3. Cytotoxicity and Protective Effect of Royal Jelly on Epithelial Cells
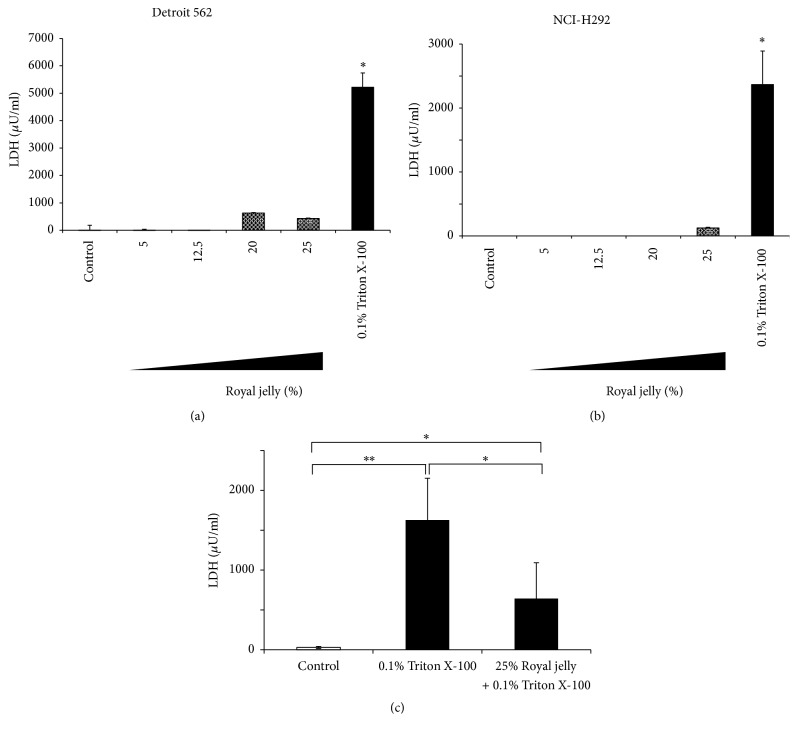
To confirm the absence of the effect of royal jelly on human pharyngeal and lung epithelial cells, we measured the level of LDH released from two epithelial cell lines, Detroit 562 cells and NCI-H292. Figure 3 shows that there are no significant differences in released LDH activity even after treatment with 25% royal jelly. Royal jelly is one of nutritional supplements that are safe and widely consumed. Giving royal jelly orally, as much as 10 g kg−1, did not cause acute cytotoxic reactions in rats [26]. Our data also support the safety of royal jelly to use for pharynx and lung epithelial cells.
Figure 3.
No cytotoxicity effect of royal jelly extract on pharyngeal, Detroit 562 (a) and lung and NCI-H292 (b), epithelial cells. As a positive control, epithelial cells were treated with 0.1% Triton X-100 and shaken gently for 10 min at room temperature. Epithelial protective effect of royal jelly against 0.1% Triton X-100 (c). Detroit 562 monolayers cultured in a 24-well plate were pretreated with 25% royal jelly or PBS for 30 s at room temperature, washed with PBS twice, and irritated with 0.1% Triton X-100 for 30 sec at room temperature. Data represent the means ± SDs of 3 independent experiments. Asterisks indicate significant differences (∗∗p < 0.001 and ∗p < 0.01) versus control group (0% royal jelly).
To evaluate the effect of royal jelly for preventing cell damage, LDH assay was performed by the addition of 0.1% Triton X-100 after pretreatment with 25% royal jelly. As shown in Figure 3(c), royal jelly reduced LDH release by Triton X, suggesting that royal jelly has physically protective effect for epithelial cells.
3.4. Royal Jelly Inhibits Overproduction of Chemokines in Human Pharyngeal and Lung Epithelial Cells
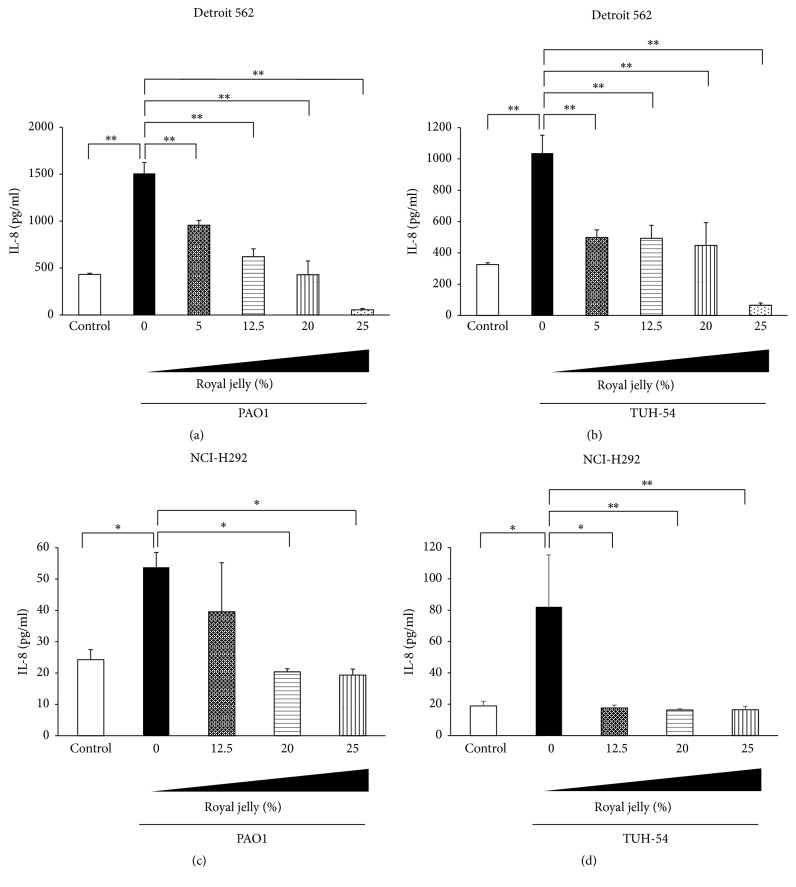
We next investigated the preventive effects of pretreatment with royal jelly on chemokines productions in P. aeruginosa PAO1 or TUH-54-stimulated human pharyngeal and lung epithelial cells. P. aeruginosa PAO1 and TUH-54 strains significantly induced IL-8 and CCL20 productions in Detroit 562 and NCI-H292 cells after 4 h incubation (Figures 4 and 5). Figures 4(a) and 4(b) show that the IL-8 overproduction in pharyngeal epithelial cells, Detroit 562, after the stimulation with PAO1 and TUH-54 strains was significantly reduced by the 30 min pretreatment with royal jelly in dose-dependent manner. Moreover, the pretreatment with 20% and 25% royal jelly significantly inhibited IL-8 production in PAO1-stimulated NCI-H292 cells (Figure 4(c)) and more than 12.5% royal jelly significantly reduced IL-8 production in TUH-54-stimulated NCI-H292 cells (Figure 4(d)). It is interesting that only 30 min pretreatment of epithelial cells is effective for inhibition of IL-8 overproduction by P. aeruginosa infection.
Figure 4.
Inhibitory effect of royal jelly on IL-8 overproduction in human pharyngeal, Detroit 562 (a, b) and lung and NCI-H292 (c, d), epithelial cells stimulated with P. aeruginosa PAO1 (a, c) and TUH-54 (b, d) for 4 h. Data represent the means ± SDs of 4 independent experiments. Asterisks indicate significant differences (∗∗p < 0.001 and ∗p < 0.01) between the indicated groups.
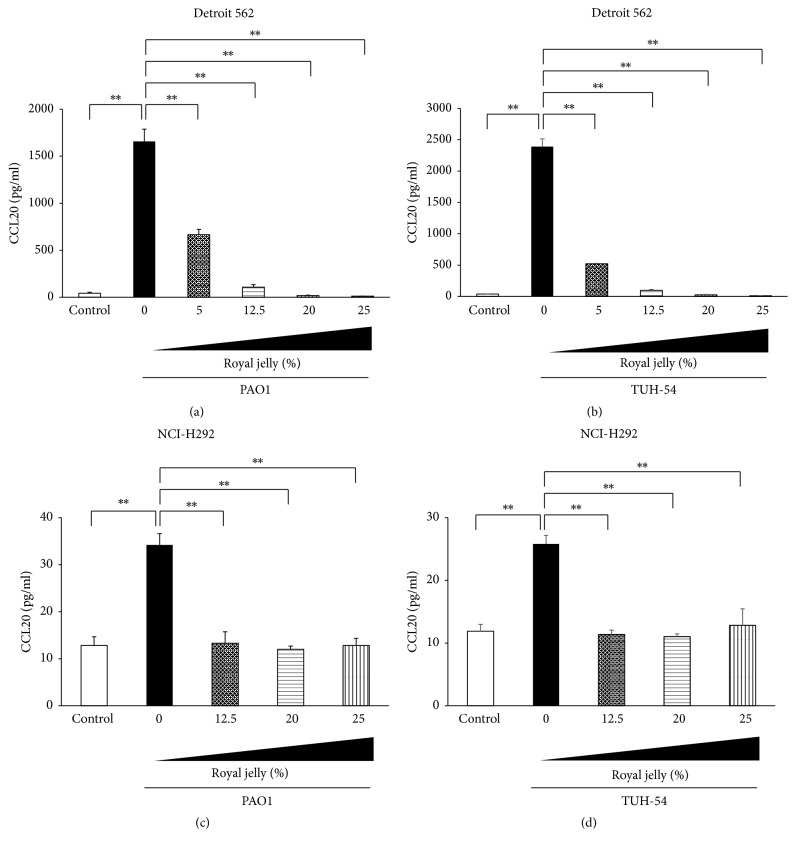
Figure 5.
Inhibitory effect of royal jelly on CCL20 overproduction in human pharyngeal, Detroit 562 (a, b) and lung and NCI-H292 (c, d), epithelial cells stimulated with P. aeruginosa PAO1 (a, c) and TUH-54 (b, d) for 4 h. Data represent the means ± SDs of 4 independent experiments. Asterisks indicate significant differences (∗∗p < 0.001) between the indicated groups.
Figure 5 also shows the inhibitory effect of 30 min pretreatment with royal jelly on CCL20 overproduction in human pharyngeal and lung epithelial cells after stimulation with PAO1 or TUH-54 strain for 4 h. This inhibitory effect on CCL20 production was similar to that on IL-8.
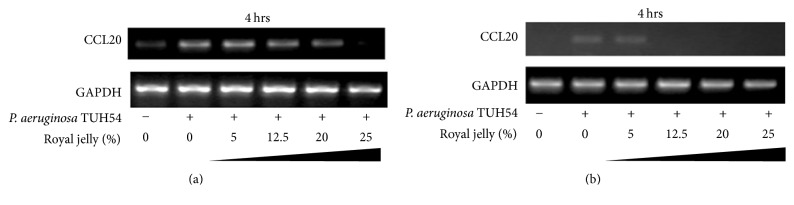
We finally confirmed the mRNA expression levels of CCL20 and IL-8 in human pharyngeal and lung epithelial cells stimulated with P. aeruginosa TUH-54 or PAO1 for 4 h by RT-PCR. Figure 6 shows that mRNA expression level of CCL20 in P. aeruginosa TUH-54-stimulated Detroit 562 and NCI-H292 cells for 4 h was inhibited by 30 min pretreatment with more than 12.5% royal jelly in both epithelial cells. Similar results were seen in the mRNA expression of IL-8 in both epithelial cells exposed to PAO1 and TUH-54 strains (data not shown). These results are consistent and supported by previous study showing that royal jelly has anti-inflammatory effects to suppress LPS-induced IL-6 and CXC chemokine ligand 10 from the periodontal ligament cell line [27].
Figure 6.
The inhibitory effect of royal jelly on CCL20 mRNA expression in human pharyngeal, Detroit 562 (a) and lung and NCI-H292 (b), epithelial cells stimulated with P. aeruginosa TUH-54 strain for 4 h.
IL-8 is a proinflammatory cytokine that can be induced in the event of bacterial infections and has a key role in the recruitment and activity of neutrophils, which are considered major contributors to the tissue damage during inflammatory diseases [28–30]. The production of this molecule is increased in cystic fibrosis mouse model infected with P. aeruginosa LPS and bacterial culture supernatant [31]. The involvement of P. aeruginosa in the oropharyngeal infection is very important to consider. Other studies also proved that LPS and bacterial culture supernatant induce the production of IL-8 [32] and human bronchial epithelial cells increased the production of IL-8 by 5 h exposure with P. aeruginosa LPS [33]. Regarding novel mechanism involved in this induction, it has been suggested that the outer membrane vesicle, which is common in all Gram-negative bacteria, plays an important role to deliver short RNA into host cells [33]. CCL20 has been shown to act as a chemotactic factor that attracts strongly lymphocytes and slightly neutrophils into inflammatory lesions [34]. This study demonstrated that royal jelly can inhibit the increased production of IL-8 and CCL20 by stimulation with P. aeruginosa and these interesting findings encourage us to clarify this inhibitory mechanism. Collectively, our results suggest that royal jelly can reduce the inflammatory response against P. aeruginosa infection in pharyngeal and lung epithelial cells and may be a useful supplement as complementary and alternative medicine for preventing respiratory infection caused by P. aeruginosa.
4. Conclusions
Our results demonstrated that royal jelly can inhibit the adherence of P. aeruginosa PAO1 and TUH-54 on abiotic surface in a dose-dependent manner. In addition, we elucidated that 30 min pretreatment with royal jelly can inhibit the adhesion of both P. aeruginosa strains in pharynx and lung and significantly reduce the overproduction of IL-8 and CCL20 in both P. aeruginosa-stimulated epithelial cells. Royal jelly could suppress the production of proinflammatory cytokines by inhibiting the adherence of bacteria to epithelial cells. Furthermore, these findings suggest that certain components of royal jelly, when identified and studied in much more detail, might be used as complementary medicine.
Supplementary Material
No inhibitory effect of royal jelly on lasR gene expression in P. aeruginosa PAO1 and TUH-54. Data represent the means ± SDs of 3 independent experiments.
Acknowledgments
This work was supported by the Fellowship Fund for Foreign Researcher, Fujii-Othuka Fund for International Education and Research Exchanges for HS from 2014 to 2015, and JSPS KAKENHI Grant no. JP 17K11708.
Disclosure
This work was previously presented at IADR. This manuscript is including the core data; however, some new data were added to it.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Fujii R., Saito Y., Tokura Y., Nakagawa K.-I., Okuda K., Ishihara K. Characterization of bacterial flora in persistent apical periodontitis lesions. Oral Microbiology and Immunology. 2009;24(6):502–505. doi: 10.1111/j.1399-302X.2009.00534.x. [DOI] [PubMed] [Google Scholar]
- 2.Muray P. R., Rosenthal K. S., Pfaller M. A. Medical Microbiology. 7th. Philadelphia, Pa, USA: Elsevier Saunders; 2013. [Google Scholar]
- 3.Barnett F., Axelrod P., Tronstad L., Slots J., Graziani A., Talbot G. Ciprofloxacin treatment of periapical Pseudomonas aeruginosa infection. Dental Traumatology. 1988;4(3):132–137. doi: 10.1111/j.1600-9657.1988.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 4.Siqueira J. F., Jr., Rôças I. N. Clinical implications and microbiology of bacterial persistence after treatment procedures. Journal of Endodontics. 2008;34(11):1291–1301. doi: 10.1016/j.joen.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 5.Hirota K., Yoneyama T., Sakamoto M., et al. High prevalence of Pseudomonas aeruginosa from oropharyngeal biofilm in patients with cerebrovascular infarction and dysphagia. Chest. 2010;138(1):237–238. doi: 10.1378/chest.10-0240. [DOI] [PubMed] [Google Scholar]
- 6.Wisplinghoff H., Bischoff T., Tallent S. M., Seifert H., Wenzel R. P., Edmond M. B. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clinical Infectious Diseases. 2004;39(3):309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 7.Karlsson K. A. Pathogen-host protein-carbohydrate interactions as the basis of important infections. Advances in Experimental Medicine and Biology. 2001;491:431–443. doi: 10.1007/978-1-4615-1267-7_28. [DOI] [PubMed] [Google Scholar]
- 8.Khalifa B. H. A., Moissenet D., Vu Thien H., Khedher M. Virulence factors in Pseudomonas aeruginosa: mechanisms and modes of regulation. Annual Biology Clinic (Paris) 2011;69:393–403. doi: 10.1684/abc.2011.0589. [DOI] [PubMed] [Google Scholar]
- 9.Fratini F., Cilia G., Mancini S., Felicioli A. Royal Jelly: An ancient remedy with remarkable antibacterial properties. Microbiological Research. 2016;192:130–141. doi: 10.1016/j.micres.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Townsend G. F., Morgan J. F., Hazlett B. Activity of 10-hydroxydecenoic acid from royal jelly against experimental leukæmia and ascitic tumours. Nature. 1959;183(4670):1270–1271. doi: 10.1038/1831270a0. [DOI] [PubMed] [Google Scholar]
- 11.Fujiwara S S., Imai J., Fujiwara M., Yaeshima T., Kuwashima T., Kobayashi K. A potent antibacterial protein in royal jelly. The Journal of Biological Chemistry. 1990;265:11333–11337. [PubMed] [Google Scholar]
- 12.Yuksel S., Akyol S. The consumption of propolis and royal jelly in preventing upper respiratory tract infections and as dietary supplementation in children. Journal of Intercultural Ethnopharmacology. 2016;5(3):308–311. doi: 10.5455/jice.20160331064836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi K., Nakanishi T., Yumoto H., Adachi T., Matsuo T. CCL20 production is induced in human dental pulp upon stimulation by Streptococcus mutans and proinflammatory cytokines. Oral Microbiology and Immunology. 2008;23(4):320–327. doi: 10.1111/j.1399-302x.2008.00431.x. [DOI] [PubMed] [Google Scholar]
- 14.Boukraa L. Additive activity of royal jelly and honey against Pseudomonas aeruginosa. Alternative Medicine Review. 2008;13(4):330–333. [PubMed] [Google Scholar]
- 15.Brudzynski K., Sjaarda C., Lannigan R. MRJP1-containing glycoproteins isolated from honey, a novel antibacterial drug candidate with broad spectrum activity against multi-drug resistant clinical isolates. Frontiers in Microbiology. 2015;6, article no. 711 doi: 10.3389/fmicb.2015.00711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fontana R., Mendes M. A., de Souza B. M., et al. Jelleines: a family of antimicrobial peptides from the Royal Jelly of honeybees (Apis mellifera) Peptides. 2004;25(6):919–928. doi: 10.1016/j.peptides.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Lerrer B., Zinger-Yosovich K. D., Avrahami B., Gilboa-Garber N. Honey and royal jelly, like human milk, abrogate lectin-dependent infection-preceding Pseudomonas aeruginosa adhesion. ISME Journal. 2007;1(2):149–155. doi: 10.1038/ismej.2007.20. [DOI] [PubMed] [Google Scholar]
- 18.Gilboa-Garber N. Pseudomonas aeruginosa lectins. Methods in Enzymology. 1982;83(C):378–385. doi: 10.1016/0076-6879(82)83034-6. [DOI] [PubMed] [Google Scholar]
- 19.Glick J., Garber N. The intracellular localization of Pseudomonas aeruginosa lectins. Journal of General Microbiology. 1983;129:3085–3090. doi: 10.1099/00221287-129-10-3085. [DOI] [PubMed] [Google Scholar]
- 20.Tielker D., Hacker S., Loris R., et al. Pseudomonas aeruginosa lectin LecB is located in the outer membrane and is involved in biofilm formation. Microbiology. 2005;151(5):1313–1323. doi: 10.1099/mic.0.27701-0. [DOI] [PubMed] [Google Scholar]
- 21.Chemani C., Imberty A., De Bentzmann S., et al. Role of LecA and LecB lectins in Pseudomonas aeruginosa-induced lung injury and effect of carbohydrate ligands. Infection and Immunity. 2009;77(5):2065–2075. doi: 10.1128/IAI.01204-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bucior I., Pielage J. F., Engel J. N. Pseudomonas aeruginosa Pili and Flagella mediate distinct binding and signaling events at the apical and basolateral surface of airway epithelium. PLoS Pathogens. 2012;8(4) doi: 10.1371/journal.ppat.1002616.e1002616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grishin V., Krivozubov M. S., Karyagina A. S., Gintsburg A. L. Pseudomonas aeruginosa lectins as targets for novel antibacterials. Acta Naturae. 2015;7(2):29–41. [PMC free article] [PubMed] [Google Scholar]
- 24.Rachmaninov O., Zinger-Yosovich K. D., Gilboa-Garber N. Preventing Pseudomonas aeruginosa and Chromobacterium violaceum infections by anti-adhesion-active components of edible seeds. Nutrition Journal. 2012;11(1, article no. 10) doi: 10.1186/1475-2891-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siryaporn A., Kuchma S. L., O'Toole G. A., Gitai Z., Ausubel F. M. Surface attachment induces Pseudomonas aeruginosa virulence. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(47):16860–16865. doi: 10.1073/pnas.1415712111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi N., Unten S., Kakura H., et al. Diverse biological activities of healthy foods. In Vitro. 2001;15(1):17–23. [PubMed] [Google Scholar]
- 27.Yanagita M., Kojima Y., Mori K., Yamada S., Murakami S. Osteoinductive and anti-inflammatory effect of royal jelly on periodontal ligament cells. Biomedical Research. 2011;32(4):285–291. doi: 10.2220/biomedres.32.285. [DOI] [PubMed] [Google Scholar]
- 28.Baggiolini M., Dewald B., Maser B. Interleukin-8 and related chemotactic cytokines; CXC and CC chemokines. Advances in Immunology. 1994;55:97–179. [PubMed] [Google Scholar]
- 29.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392(6676):565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell G. B., Albright B. N., Caswell J. L. Effect of interleukin-8 and granulocyte colony-stimulating factor on priming and activation of bovine neutrophils. Infection and Immunity. 2003;71(4):1643–1649. doi: 10.1128/IAI.71.4.1643-1649.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stellari F., Bergamini G., Ruscitti F., et al. In vivo monitoring of lung inflammation in CFTR-deficient mice. Journal of Translational Medicine. 2016;14(1, article no. 226) doi: 10.1186/s12967-016-0976-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koyama S., Sato E., Nomura H., et al. The potential off various lipopolysaccharides to release IL-8 and G-CSF. American Journal of Physiology. Lung Cellular and Molecular. 2000;278:658–666. doi: 10.1152/ajplung.2000.278.4.L658. [DOI] [PubMed] [Google Scholar]
- 33.Koeppen K., Hampton T. H., Jarek M., et al. A Novel Mechanism of Host-Pathogen Interaction through sRNA in Bacterial Outer Membrane Vesicles. PLoS Pathogens. 2016;12(6) doi: 10.1371/journal.ppat.1005672.e1005672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hieshima K., Imai T., Opdenakker G., et al. Molecular cloning of a novel human CC chemokine liver and activation- regulated chemokine (LARC) expressed in liver. Chemotactic activity for lymphocytes and gene localization on chromosome 2. The Journal of Biological Chemistry. 1997;272(9):5846–5853. doi: 10.1074/jbc.272.9.5846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
No inhibitory effect of royal jelly on lasR gene expression in P. aeruginosa PAO1 and TUH-54. Data represent the means ± SDs of 3 independent experiments.