Abstract
Background
The Clinical Outcomes in Surgical Therapy trial demonstrated that laparoscopic colectomy (LC) was equivalent to open colectomy (OC) for 30 day mortality, time to recurrence, and overall survival in colon cancer (CC) patients. Current utilization of LC for CC is not well known.
Study Design
Surgical data were reviewed for all patients randomized onto a national phase III clinical trial for adjuvant therapy in stage 3 CC [North Central Cancer Treatment Group (NCCTG) trial N0147]. Colon resections were grouped as open (traditional laparotomy) or laparoscopic, including: laparoscopic, laparoscopic assisted, hand assisted, and laparoscopic converted to OC. Statistical methods included non-parametric methods, categorical analysis, and logistic regression modeling.
Results
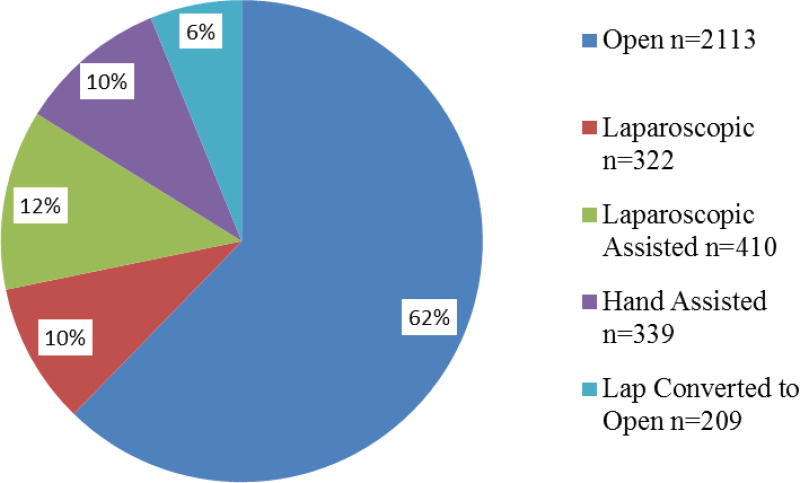
3,393 evaluable patients were accrued between 2004–2009, including 53% males, median age 58, 86% white, and 70% with a BMI >25. 2113(62%) underwent OC. 1280(38%) were initiated as laparoscopic procedures, of which 25%(322) were laparoscopic, 32%(410) laparoscopic assisted, 26%(339) hand assisted, and 16%(209) LC converted to OC. Significant predictors of LC (vs OC) in multivariate models were T-stage (T1 or T2 vs T3 or T4, p=0.0286), and absence of perforation, bowel obstruction, or adherence to surrounding organs (p<0.01 each). Increasing rates of LC were observed over time, with LC eclipsing OC in 2009 (p<0.0001). Surgical efficacy, measured by lymph node retrieval, was similar with the mean number of lymph nodes retrieved higher in the LC group (20.6 vs 19.5 nodes, p=0.0006).
Conclusions
This study demonstrated a steadily increasing utilization of LC for the surgical treatment of colon cancer between 2004–2009, with LC preferred by study completion. Surgical efficacy was similar in stage 3 CC patients.
Introduction
Laparoscopic techniques for colon resection were first reported in 19911,2 and have demonstrated the advantages commonly attributed to laparoscopic surgery including less pain, shorter recovery and quicker return to baseline function. After initial reports demonstrated the feasibility of laparoscopic colectomy (LC) for colon cancer (CC), reports of port site recurrences questioned the safety and oncologic efficacy of LC for CC.3–6 Subsequently, several studies were published addressing the safety and efficacy of laparoscopic surgery for colon cancer.7–9 The seminal trial in the United States was the Clinical Outcomes in Surgery Trial (COST)7, which was reported in 2004. This prospective randomized trial evaluating laparoscopic colectomy for colon cancer performed by credentialed surgeons demonstrated similar rates of overall recurrence, wound recurrence, overall survival, reoperation, 30 day mortality, hospital readmission and complications. Benefits for the laparoscopic surgery group were shown in the perioperative recovery period with shorter hospital stay and reduced use of narcotics. After the safety and efficacy of LC for colon cancer was established in prospective randomized clinical trials, the procedure was gradually adopted by the surgical community. The rates of acceptance and utilization of this procedure are not well known and have been questioned.10
The North Central Cancer Treatment Group (NCCTG) N0147 trial was a large multi-institutional prospective randomized clinical trial designed to evaluate the efficacy of different chemotherapy regimens used in adjuvant therapy for stage 3 colon cancer.11 This trial was sponsored by the NCCTG but was available to other cooperative study groups through the intergroup mechanism. The trial was open to a diverse range of hospitals including community hospitals, university affiliated hospitals and university medical centers. Only patients with pathologically proven stage 3 colon cancer after surgical resection were eligible for this trial. Surgical data was collected prospectively prior to randomization and was a mandatory part of the eligibility criteria for entry into the trial. Although the NCCTG N0147 trial was not specifically designed to evaluate surgical methods, the time period in which it was conducted and the complete and prospective nature of the surgical data collection made it an ideal vehicle to study the utilization and efficacy of LC in the era immediately following the dissemination of the results of the COST study. In addition an analysis of the factors associated with attempted LC was possible with the data collected for enrollment in this trial. The goals of this study were to evaluate the use and efficacy of LC in the time period after the COST trial and to assess factors associated with the type of procedure attempted. This report describes the utilization, efficacy and factors associated with LC for colon cancer using the surgical data for over 3300 stage 3 colon cancer patients entered into the NCCTG N0147 trial.
Methods
The NCCTG N0147 trial was conducted from February 10, 2004 to November 25, 2009 and included patients with histologically proven stage 3 (any T, N1 or N2, M0) colon cancer after complete surgical resection. Tumors were required to be at least 12 cm from the anal verge and an en bloc resection was required for patients with locally advanced tumors. Other eligibility criteria included age ≥18, ≥1 pathologically confirmed involved lymph node, Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, and adequate hematologic, hepatic and renal function. No prior chemotherapy, immunotherapy, or radiotherapy for colon cancer was allowed. Investigational review board approval was required at all of the participating centers and all participants were provided written informed consent.
Multiple chemotherapy regimens were compared, including combinations of oxaliplatin, irinotecan, 5-fluorouracil (5FU), leucovorin (LV), and cetuximab for the 3397 patients enrolled in this trial. Due to reported findings from other studies on adjuvant chemotherapy regimens for stage 3 colorectal cancer which were revealed during the course of the N0147 trial, some of the chemotherapy arms in the N0147 study were adjusted or deleted. The final analysis on the efficacy of the adjuvant chemotherapy regimens from this study reported on 2686 patients who received the FOLFOX ± cetuximab regimen.11 Because the surgical resection of the stage 3 colon cancers was completed on all enrolled patients prior to randomization to the different chemotherapy regimens, there was no effect of these regimens on the surgical or demographic data collected in this study. Therefore the surgical data on all 3397 patients initially involved in this trial were used for this analysis. The surgical procedure performed was entirely at the discretion of the operating surgeon and had no effect on a patient’s entry into the trial. The documentation necessary for entry into the trial included a copy of the surgeon’s dictated operative note as well as the final pathology report from the resected surgical specimen. The operative and pathology reports were reviewed by a member of the surgery committee of the NCCTG who was knowledgeable in laparoscopic surgery. The reviewing surgeon assigned each operative procedure to a category of laparoscopic or open colectomy as defined in Table 1. For this analysis all minimally invasive procedures, including laparoscopic, laparoscopic assisted, hand assisted and laparoscopic converted to open were grouped together under the category of laparoscopic colectomy. The laparoscopic converted to open cases were included in the laparoscopic category because the surgical procedure was initiated laparoscopically. Therefore the LC group includes all laparoscopic cases both attempted (laparoscopic converted to open category) and completed (laparoscopic, laparoscopic assisted and hand assisted).
Table 1.
Definitions of Surgical Categories Used in N0147 Patients
| Procedure type | Definition |
|---|---|
| Open colectomy | Procedure done through a standard laparotomy incision with no laparoscopic or hand assistance. |
| Laparoscopic colectomy | Procedure done completely laparoscopic with intracorporeal anastomosis. |
| Laparoscopic assisted colectomy | Procedure in which the colon was mobilized laparoscopically (with or without vascular dissection and ligation) but the anastomosis was done extracorporeally. |
| Hand assisted colectomy | Procedure in which a hand port is used in conjunction with laparoscopic mobilization, anastomosis done intracorporeally or extracorporeally. |
| Laparoscopic colectomy converted to open colectomy | Procedure begun with intent to perform laparoscopically but converted to open for any reason. |
Statistical analysis was performed using frequency tables and categorical methods (Chi-square, Fisher’s Exact) were used to describe the distributions of covariates. Non-parametric methods were used (eg, Wilxocon test, Kruskal-Wallis test), when appropriate. Univariate and multivariate Logistic regression models were used to explore the associations between covariates and the outcome of having a laparoscopic (vs open) colectomy. Covariates with multiple levels (eg, BMI, tumor location) were redefined into smaller and more clinically relevant classifications for modeling purposes (eg, eliminating categories with extremely low frequencies). All p-values reported are 2-sided and values <0.05 are considered statistically significant. Analyses have not been adjusted for multiple comparisons.
Results
A total of 3,397 patients underwent colectomy for colon cancer in this trial, of which surgical data was complete for 3393 (99.9%), who form the basis for this analysis. Demographic data for these patients is listed in Table 2. 52.5% of patients were male, the average age was 57.6 (range 19 – 86), 85.9% were white and the majority (70.1%) were overweight or obese with a BMI > 25. There were no statistically significant differences between the patients who had LC or OC for these demographic characteristics. The majority of patients were insured with private or government sponsored insurance.
Table 2.
Patient Characteristics by Procedure Type
| Characteristic | Laparoscopic (n=1,280) |
Open (n=2,113) |
Total (n=3,393) |
p Value |
|---|---|---|---|---|
|
| ||||
| Age, y | 0.2756 | |||
| Mean (SD) | 57.3 (10.9) | 57.8 (11.2) | 57.6 (11.1) | |
| Median | 58.0 | 58.0 | 58.0 | |
| Range | 19.0 – 86.0 | 19.0 – 85.0 | 19.0 – 86 | |
|
| ||||
| Sex, male, n (%) | 655 (51.2) | 1127 (53.3) | 1782 (52.5) | 0.2210 |
|
| ||||
| Race, n (%) | 0.2959 | |||
| White | 1082 (84.5) | 1832 (86.7) | 2914 (85.9) | |
| Black or African American | 97 (7.6) | 143 (6.8) | 240 (7.1) | |
| Native Hawaiian or Pacific Islander | 4 (0.3) | 12 (0.6) | 16 (0.5) | |
| Asian | 66 (5.2) | 83 (3.9) | 149 (4.4) | |
| American Indian or Alaska Native | 7 (0.5) | 9 (0.4) | 16 (0.5) | |
| Not reported: patient refused or not available | 18 (1.4) | 20 (0.9) | 38 (1.1) | |
| Unknown: patient unsure | 6 (0.5) | 14 (0.7) | 20 (0.6) | |
|
| ||||
| Body Mass Index, kg/m2 | 0.8541 | |||
| Missing, n | 6 | 7 | 13 | |
| BMI<20, underweight, n (%) | 49 (3.8) | 91 (4.3) | 140 (4.1) | |
| 20<=BMI<25, normal, n (%) | 322 (25.3) | 547 (26.0) | 869 (25.7) | |
| 25<=BMI<30, overweight, n (%) | 470 (36.9) | 740 (35.1) | 1210 (35.8) | |
| 30<=BMI<35, obese, n (%) | 254 (19.9) | 427 (20.3) | 681 (20.1) | |
| 35<=BMI, obese, n (%) | 179 (14.1) | 301 (14.3) | 480 (14.2) | |
|
| ||||
| Health insurance | 0.0015 | |||
| Missing, n | 1 | 1 | 2 | |
| Private or Medicare/private, n (%) | 957 (74.8) | 1446 (68.5) | 2403 (70.9) | |
| Medicaid or Medicare/Medicaid, n (%) | 44 (3.4) | 79 (3.7) | 123 (3.6) | |
| Medicare, n (%) | 98 (7.7) | 184 (8.7) | 282 (8.3) | |
| No means or self pay (no insurance), n (%) | 43 (3.4) | 101 (4.8) | 144 (4.2) | |
| Military/veteran/other, n (%) | 137 (10.7) | 302 (14.3) | 439 (12.9) | |
Table 3 shows the characteristics of the resected pathology specimens for the patients in this study. Most (73.3%) tumors were T3 with a majority (59.1%) having between 1 and 3 LNs involved. The number of LNs removed was significantly higher in the LC group (p = 0.0006). The two most common tumor locations were the right colon (41.2%) and sigmoid colon (39.3%) with a statistically significant difference between LC and OC for tumors in the cecum (p=0.0428), transverse colon (p=0.0003) and descending colon (p=0.0040).
Table 3.
Tumor Characteristics by Procedure Type
| Characteristic | Laparoscopic (n = 1,280) |
Open (n=2,113) |
Total (n=3,393) |
p Value |
|---|---|---|---|---|
|
| ||||
| T Stage | <0.0001 | |||
| Missing, n | 0 | 1 | 1 | |
| T1 or T2, n (%) | 243 (19.1) | 265 (12.5) | 508 (15.0) | |
| T3, n (%) | 908 (70.9) | 1580 (74.8) | 2488 (73.3) | |
| T4, n (%) | 129 (10.1) | 267 (12.6) | 396 (11.7) | |
|
| ||||
| Lymph node involvement, n (%) | 0.7185 | |||
| 1 – 3 | 750 (58.6) | 1254 (59.3) | 2004 (59.1) | |
| >=4 | 530 (41.4) | 859 (40.7) | 1389 (40.9) | |
|
| ||||
| No. nodes examined, mean (SD) | 20.6 (11.5) | 19.5 (11.0) | 19.9 (11.2) | 0.0006 |
|
| ||||
| Tumor location, n (%) | ||||
| Cecum | 313 (24.5) | 453 (21.5) | 766 (22.6) | 0.0428 |
| Ascending colon | 247 (19.3) | 382 (18.1) | 629 (18.6) | 0.3807 |
| Hepatic flexure | 72 (5.6) | 111 (5.3) | 183 (5.4) | 0.6452 |
| Transverse colon | 87 (6.8) | 221 (10.5) | 308 (9.1) | 0.0003 |
| Splenic flexure | 47 (3.7) | 104 (4.9) | 151 (4.5) | 0.0863 |
| Descending colon | 63 (4.9) | 157 (7.4) | 220 (6.5) | 0.0040 |
| Sigmoid colon | 510 (39.9) | 822 (39.0) | 1332 (39.3) | 0.5960 |
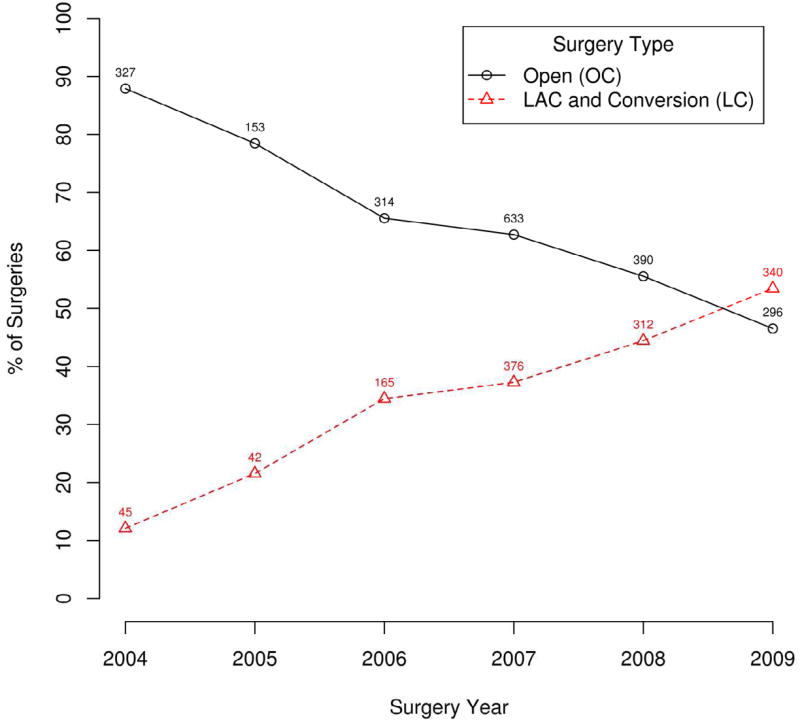
Multivariate analysis of factors associated with LC (Table 4) revealed that patients who had bowel perforation, obstruction or adherence to a surrounding organ were less likely to undergo a laparoscopic procedure. Additionally the year in which the patient was operated on was significantly associated with the type of procedure that was performed. The types of procedures performed are shown in Figure 1. The change in the laparoscopic compared to open procedures over time are shown in Figure 2. The increase in laparoscopic procedures over time was highly statistically significant (p<0.0001) with the laparoscopic approach eclipsing the open approach by the end of the study (2009).
Table 4.
Multivariate Cox Models for Factors Associated with Laparoscopic Colectomy
| Variable | OR(95% CI) | Wald p value |
Overall Wald p value |
|---|---|---|---|
| Adherence | 0.68 (0.54–0.85) | 0.0008 | 0.0008 |
| Bowel obstruction | 0.39 (0.31–0.49) | <0.0001 | 0.0001 |
| Bowel Perforation | 0.59 (0.40–0.88) | 0.0092 | 0.0092 |
| Stage | 0.0279 | ||
| T3 | 0.76 (0.62–0.93) | 0.0874 | |
| T4 | 0.77 (0.57–1.05) | 0.3671 | |
| Payment method | 0.0009 | ||
| Medicare/Medicaid | 1.16 (0.75–1.79) | 0.7024 | |
| Military | 0.93 (0.60–1.44) | 0.0733 | |
| Private | 1.42 (0.96–2.10) | 0.0005 | |
| Surgery year | <0.0001 | ||
| 2005 | 2.04 (1.28–3.25) | 0.0002 | |
| 2006 | 4.22 (2.92–6.12) | 0.0972 | |
| 2007 | 4.53 (3.22–6.38) | 0.0018 | |
| 2008 | 6.23 (4.39–8.84) | <0.0001 | |
| 2009 | 9.16 (6.42–13.06) | <0.0001 |
Figure 1.
Colectomy procedure type, n=3,393.
Figure 2.
Laparoscopic colectomy vs open colectomy, by year.
Discussion
In the USA, the use of laparoscopic surgery for different applications in general surgery has evolved rapidly since it was first described for cholecystectomy in 1989. Initially, there was skepticism over the use of laparoscopic surgery for bowel resection, especially when malignancy was the reason for resection.3–6 This skepticism was gradually replaced with enthusiasm for laparoscopic colectomy after the safety and efficacy of this procedure was demonstrated by multiple randomized clinical trials.7–9 The N0147 trial was started April 10, 2004 one month prior to the report of the COST trial7 (May 13, 2004) that documented the outcomes and benefits of laparoscopic colectomy for colon cancer. Because the N0147 trial started almost simultaneously with the most important report on the safety and efficacy of laparoscopic colectomy in the USA, we felt that the surgical data from N0147 would be ideal for evaluating the acceptance and utilization of laparoscopic surgery for colon cancer in the initial 5 years after it was shown to be safe and beneficial. In addition, since this trial involved multiple types of institutions across North America, the surgical data should be representative of the surgical techniques used for colon cancer in a broad spectrum of institutions, therefore providing an accurate reflection of the use of LC for colon cancer in North America during that time.
The data from this study indicate a significant change in the use of laparoscopic surgery for colon cancer over this time period. The percentage of colon cancers resected laparoscopically increased steadily each year during the study and by the end of the study the majority of the cancers were resected laparoscopically. As one would expect, the number of cases attempted laparoscopically was significantly lower when there was bowel obstruction, perforation, or adherence to an adjacent organ. This likely represents the surgeon’s judgment that laparoscopic colectomy was not appropriate when these locally advanced tumors were encountered. Additionally the significantly higher rate of open resection of tumors of the transverse and left colon and laparoscopic resection of cecal tumors is consistent with recommendations for laparoscopic colectomy at that time. As surgeons have gained experience in laparoscopic colectomy and the techniques and equipment have improved, tumors in all locations in the colon are now felt to be appropriate for minimally invasive techniques.
While the efficacy of a surgical procedure for malignancy depends on many factors including the intra-operative ability to assess for metastatic disease, complete resection of the tumor with a negative margin and the immediate and long term outcomes of the surgery, many of these factors can be difficult to evaluate in a clinical trial. A surrogate marker for efficacy that has been used is the number of lymph nodes retrieved in the resected specimen. This data gives an indication of the completeness of resection as an incomplete resection of the colonic mesentery would yield a lower number of lymph nodes. Previously published prospective randomized trials comparing LC to OC have demonstrated the number of LNs removed in LC was equivalent to OC. Two prospective randomized trials from Europe (Barcelona, Spain8 and COLOR trial9) demonstrated an equivalent number of LN retrieved from both the laparoscopic and open colectomy arms. Our data demonstrated a statistically significant (p<0.0006) higher number of LNs from specimens of patients who had LC (20.6 vs. 19.5), confirming that LC is equivalent to OC for this measure of surgical efficacy. Although the difference in the number of lymph nodes retrieved was statistically significant, this significance is due to the large sample size and for all practical purposes the difference of one lymph node in the LC specimens is not clinically meaningful.
Rea et al. studied the utilization of laparoscopic colectomy for colon cancer through the National Inpatient Sample database during the time periods 2001 – 2003 and 2005–2007.10 Their study of over 740,000 elective colectomies demonstrated that despite an almost threefold increase (2.3% vs 8.9%) in laparoscopic colectomy for colon cancer between these two time periods the absolute rate of LC for colon cancer was low. The authors state “there is clear lack of adoption of LC in the United States”. Our study refutes this assertion, demonstrating a predominance of LC for stage 3 colon cancer by the 5th year after the COST trial (2009). There may be multiple reasons for this difference but it seems that the Rea study did not collect data for sufficient time after the publication of the COST trial to demonstrate that surgeons had adopted laparoscopic surgery for colon resection in colon cancer. Considering the time for adoption of other new surgical procedures (for example breast conservation surgery for breast cancer) it appears as though the utilization of laparoscopic colectomy for colon cancer has occurred fairly quickly.
Our study does have limitations which include the limited stage of disease (stage 3 only) which was available for study in this database. This data may not represent the overall utilization of laparoscopic techniques for colon cancer at the participating institutions but it seems reasonable to assume that if its utilization is increasing for stage 3 disease, the same trend would be seen in earlier stages of colon cancer, since tumors which are stage 1 and 2 usually are more amenable to laparoscopic techniques than stage 3 tumors. Further analysis of this data for trends in conversion to open procedures, as well as outcomes by type of surgery is planned.
In summary this study demonstrates a steadily increasing and statistically significant increase in utilization of laparoscopic surgery for colectomy in colon cancer after publication of the COST trial. Laparoscopic surgery may now be the predominant procedure for surgical resection of colon cancer when the appropriate conditions are present.
Acknowledgments
ClinicalTrials.gov number, NCT00079274. This study was conducted as a collaborative trial of the North Central Cancer Treatment Group, Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-35103, CA-35113, CA-35272, CA-114740, CA-32102, CA-14028, CA49957, CA21115, CA31946, CA12027, CA37377 from the National Cancer Institute, Department of Health and Human Services.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Presented at the Western Surgical Association 120th Scientific Session, Colorado Springs, CO, November 2012.
References
- 1.Fowler DL, White SA. Laparoscopy-assisted sigmoid resection. Surg Laparosc Endosc. 1991;1:183–188. [PubMed] [Google Scholar]
- 2.Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy) Surg Laparosc Endosc. 1991;1:144–150. [PubMed] [Google Scholar]
- 3.Berends FJ, Kazemier G, Bonjer HJ, Lange JF. Subcutaneous metastases after laparoscopic colectomy. Lancet. 1994;344:358. doi: 10.1016/s0140-6736(94)91079-0. [DOI] [PubMed] [Google Scholar]
- 4.Reilly WT, Nelson H, Schroeder G, et al. Wound recurrence following conventional treatment of colorectal cancer: a rare but perhaps underestimated problem. Dis Colon Rectum. 1996;39:200–207. doi: 10.1007/BF02068076. [DOI] [PubMed] [Google Scholar]
- 5.Vukasin P, Ortega AE, Greene FL, et al. Wound recurrence following laparoscopic colon cancer resection. Results of the American Society of Colon and Rectal Surgeons Laparoscopic Registry. Dis Colon Rectum. 1996;39:S20–S23. doi: 10.1007/BF02053801. [DOI] [PubMed] [Google Scholar]
- 6.Johnstone PAS, Rohde DC, Swartz SE, et al. Port site recurrences after laparoscopic and thoracoscopic procedures in malignancy. J Clin Oncol. 1996;14:1950–1956. doi: 10.1200/JCO.1996.14.6.1950. [DOI] [PubMed] [Google Scholar]
- 7.The Clinical Outcomes of Surgical Therapy Study Group. A comparison of Laparoscopically Assisted and Open Colectomy for Colon Cancer. N Engl J Med. 2004 May;350:2050–2059. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 8.Lacy AM, Garcia-Valdecasas JC, Delgado S, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomized trial. Lancet. 2002;359:2224–2229. doi: 10.1016/S0140-6736(02)09290-5. [DOI] [PubMed] [Google Scholar]
- 9.Veldecamp R, Kuhry E, Hop WC, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomized trial. Lancet Oncol. 2005;6:477–484. doi: 10.1016/S1470-2045(05)70221-7. [DOI] [PubMed] [Google Scholar]
- 10.Rea JD, Cone MM, Diggs BS, et al. Utilization of laparoscopic colectomy in the United States before and after the Clinical Outcomes of Surgical Therapy study group trial. Ann Surg. 2011;254:282–288. doi: 10.1097/SLA.0b013e3182251aa3. [DOI] [PubMed] [Google Scholar]
- 11.Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplain, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer. JAMA. 2012;307:1383–1393. doi: 10.1001/jama.2012.385. [DOI] [PMC free article] [PubMed] [Google Scholar]