Abstract
Recognition of foreign nucleic acids by the immune system is essential to host protection against many viral and bacterial infections. It relies on the capacity of innate immune sensors to selectively distinguish self‐ and non‐self‐nucleic acids, on the basis of a variety of parameters including base modifications, sequence composition, length or subcellular localisation. In this issue of EMBO Reports, Luecke et al 1 describe that the sensing of cytoplasmic double‐stranded DNA by the cyclic GMP–AMP (cGAMP) synthase (cGAS) is much more sensitive for longer fragments, when low doses of cytoplasmic DNA are used. This finding repositions length as the predominant factor governing the discrimination between self‐ and non‐self‐cytoplasmic DNA.
Subject Categories: Immunology; Microbiology, Virology & Host Pathogen Interaction; Signal Transduction
Cytoplasmic DNA detection by cGAS is involved in the immune response to a growing number of pathogens, including human immunodeficiency virus type 1 (HIV‐1) and Mycobacterium tuberculosis 2, 3, 4. cGAS engagement results in cGAMP synthesis and the rapid activation of stimulator of interferon genes (STING), ultimately leading to type I interferon (IFN) production and the regulation of thousands of genes facilitating pathogen clearance. Safeguarding against sensing of endogenous nuclear DNA, cGAS resides in the cytoplasm of non‐dividing cells, where it should principally encounter pathogenic DNA. Nonetheless, it is now evident that cGAS can also detect cytoplasmic self‐DNA and that a specific machinery aiming at limiting the accumulation of such cytoplasmic DNA operates to protect from aberrant cGAS activation.
Deletion of the exonuclease TREX1 leads to the cytoplasmic accumulation of endogenous ~60‐nt‐long single‐stranded DNAs (ssDNAs), resulting in cGAS‐dependent IFN‐stimulated gene induction and autoimmune pathology 5, 6. Such cytoplasmic ssDNA fragments may arise from DNA end resection during double‐strand break repair 7, and are therefore likely to be generated at low concentrations in steady‐state conditions. The single strandedness of these endogenous cytoplasmic ssDNA fragments already decreases their affinity to cGAS by more than 15‐fold over double‐stranded DNA (dsDNA) 8, but TREX1 clearance of these ssDNAs further limits cGAS activation to self‐ssDNAs. Similarly to ssDNAs, endogenous dsDNAs and DNA:RNA hybrids are degraded by other nucleases such as DNase II and Rnaseh2b, respectively 6, 9, pointing to the fact that various types of self‐DNA molecules can find their way to cGAS, but that this is prevented by several nucleases. Thus, it is the saturation or bypass of such cytoplasmic nucleases by an accumulation of self‐DNA substrates that will cause cGAS engagement in the absence of infection. The regulatory activity of cytoplasmic nucleases can also directly impact on cGAS sensing of non‐self‐DNA. This is illustrated by the finding that HIV‐1 structured ssDNA can activate type I IFN, through cGAS, and that TREX1 degradation of such ssDNA antagonises IFN production during HIV‐1 infection 2, 3. Initial studies demonstrating the involvement of cGAS in detection of HIV‐1, murine leukaemia virus or simian immunodeficiency virus were carried out in TREX1‐deficient cells or SAMHD1‐deficient cells, which both facilitate cGAS detection of accumulating DNA products 2, 10.
Yet, this last point questions the entire relevance of cGAS sensing of pathogenic DNA, which would need to accumulate above a certain threshold of intracellular pathogens to be activated, a stage at which detection of infection would be too late to block. One possibility to resolve this conceptual issue would be that the sensitivity of cGAS for pathogenic DNA is greater than for endogenous cytoplasmic DNA. As already mentioned, the affinity of cGAS for dsDNA is > 15‐fold greater than for ssDNA 8. Consequently, structured ssDNAs such as that generated by HIV‐1 are more immunostimulatory than less structured ssDNAs 3. In addition, longer ssDNAs that are more likely to form such double‐stranded structures are also more immunostimulatory than shorter ssDNAs 3. While these findings support the idea that long viral ssDNAs should be better cGAS ligands than ~60‐nt endogenous ssDNAs constitutively produced, they did not directly address the sensitivity of cGAS for pathogenic DNA.
In this issue of EMBO Reports, Luecke et al discovered that while transfected short dsDNAs (~80–100 bp) were immunostimulatory in a dose‐dependent manner, longer dsDNAs (> 500–1,000 bp) retained the capacity to activate type I IFN production at the lowest concentration studied (0.017 μg/ml), in human cells 1. This immunostimulatory activity of transfected longer dsDNAs was cGAS‐STING‐dependent, but origin (from purified PCR products and plasmid restriction digestions), sequence and IFI16‐independent 1. Critically, in vitro stimulation of recombinant cGAS confirmed the increased specificity of cGAS for long dsDNAs, resulting in enhanced cGAMP synthesis 1. Collectively, the work of Luecke et al (1) establishes that cGAS detection of > 500–1,000 bp dsDNA is much more sensitive than that of 50–100 bp dsDNA, and still visible at very low concentrations (0.017 μg/ml). This key discovery suggests that cGAS can detect very low quantities of cytoplasmic dsDNA molecules, if long enough, while shorter dsDNA products need to accumulate at high concentrations to be detected, which is presumably antagonised by endogenous nucleases. Whether cytoplasmic nucleases are better at degrading shorter DNA is unclear, but it would be expected that TREX1, which degrades DNA from its 3′ end, would be less efficient at degrading longer products. Regardless, since similar masses of DNA were used in these studies, the amount of long DNA molecules actually reaching the cytoplasm was far smaller than that of shorter ones, suggesting that cGAS activation by long dsDNA did not relate to nuclease saturation, and comforts an intrinsically greater sensitivity for longer DNA products indicated by the in vitro assays.
Given the length of viral and bacterial DNA genomes/products, which is generally greater than hundreds of kilobases, such sensitivity of cGAS to long dsDNA should allow for the rapid detection of infection, before its amplification. As such, assuming that endogenous DNA products are limited to shorter sizes (presumably less than 100 nt) 5, 7, it is the length of pathogenic DNAs that ensures their selective detection by cGAS over that of self‐DNAs. Further studies investigating the activation of cGAS during viral and bacterial infections should help confirm this high sensitivity of cGAS for longer DNAs at early stages of infection, together with its relevance to other types of DNA products including ssDNAs and DNA:RNA hybrids. Finally, the work by Luecke et al (1) underlines the need for a better definition of the antagonistic activities of cytoplasmic DNA nucleases on cGAS sensing of ssDNA, dsDNA and DNA:RNA hybrids in homeostasis and during infections (Fig 1).
Figure 1. Length‐dependent sensing of cytoplasmic DNA by cGAS .
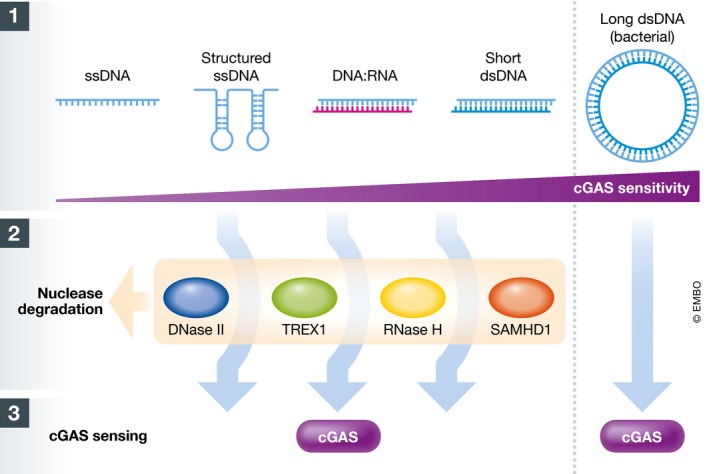
(1) Different types of DNAs are presented, ordered according to the predicted sensitivity of cGAS to these molecules 1, 3, 8 (note: a concurrent analysis of these DNA forms has not been performed to date). Short dsDNAs are ~45–70 bp long 1, with the 45‐bp ISD (interferon stimulatory DNA) being the prototypical sequence used as cGAS ligand (although not representing a physiological DNA produced by the cell). (2) Endogenous nucleases limit the cytoplasmic accumulation of DNAs from (1) (note: the type of endogenous DNA processed by SAMHD1 is unknown) 2, 6, 9, 10. (3) Cytoplasmic DNAs bypassing nuclease degradation bind and activate cGAS. Long dsDNAs (e.g. bacterial genomic DNA) may better avoid nuclease degradation, which may enhance further the sensitivity of cGAS for these DNAs. The combination of cGAS affinity together with resistance to nuclease degradation controls self‐ and non‐self‐cytoplasmic DNA sensing by cGAS.
Acknowledgements
This work was funded in part by the Australian NHMRC (1081167 to M.P.G.), the Australian Research Council (140100594 Future Fellowship to M.P.G.) and the Victorian Government's Operational Infrastructure Support Program.
See also: S Luecke et al (October 2017)
References
- 1. Luecke S, Holleufer A, Christensen MH et al (2017) EMBO Rep 18: 1707–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gao D, Wu J, Wu YT et al (2013) Science 341: 903–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herzner AM, Hagmann CA, Goldeck M et al (2015) Nat Immunol 16: 1025–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Collins AC, Cai H, Li T et al (2015) Cell Host Microbe 17: 820–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang YG, Lindahl T, Barnes DE (2007) Cell 131: 873–886 [DOI] [PubMed] [Google Scholar]
- 6. Gao D, Li T, Li XD et al (2015) Proc Natl Acad Sci USA 112: E5699–E5705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Erdal E, Haider S, Rehwinkel J et al (2017) Genes Dev 31: 353–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kranzusch PJ, Lee AS, Berger JM et al (2013) Cell Rep 3: 1362–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mackenzie KJ, Carroll P, Lettice L et al (2016) EMBO J 35: 831–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maelfait J, Bridgeman A, Benlahrech A et al (2016) Cell Rep 16: 1492–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]


