Abstract
Background:
Intestinal parasitic infections are among the most common infections and health problems worldwide. Due to the lack of epidemiologic information of such infections, the prevalence of, and the risk factors for, enteric parasites were investigated in residents of Roudehen, Tehran Province, Iran.
Methods:
In this cross-sectional study, 561 triple fecal samples were collected through a two-stage cluster-sampling protocol from Jun to Dec 2014. The samples were examined by formalin-ether concentration, culture, and with molecular methods.
Results:
The prevalence of enteric parasites was 32.7% (95% CI 27.3–38). Blastocystis sp. was the most common intestinal protozoan (28.4%; 95% CI 23.7–33.0). The formalin-ether concentration and culture methods detected Blastocystis sp., Entamoeba coli, Giardia intestinalis, Dientamoeba fragilis, Iodamoeba butschlii, Entamoeba complex cysts or trophozoite, Chilomastix mesnilii, and Enterobius vermicularis. Single-round PCR assay for Entamoeba complex were identified Entamoeba dispar and E. moshkovskii. E. histolytica was not observed in any specimen. Multivariate analysis showed a significant association of parasites with water source and close animal contact. There was no correlation between infections and gender, age, occupation, education, or travel history. Protozoan infections were more common than helminth infections.
Conclusion:
This study revealed a high prevalence of enteric protozoan parasite infection among citizens of Rodehen. As most of the species detected are transmitted through a water-resistant cyst, public and individual education on personal hygiene should be considered to reduce transmission of intestinal parasites in the population.
Keywords: Blastocystis, Entamoeba, Intestinal parasite, Prevalence, Protozoa
Introduction
Intestinal parasite infection remains a health problem, especially in developing countries, in spite of efforts by the WHO and governments to eliminate parasites and prevent and treat parasitic disease. Worldwide, over 3.5 billion people are infected with an intestinal parasite annually, and 4.5 million exhibit clinical symptoms (1). Parasitic infections may be asymptomatic or extend to morbidity and mortality, depending on the nutrition and health status of affected individuals (2, 3).
The prevalence of intestinal parasitic infections has been related to low levels of education, health practices, malnourishment, contaminated food and/or water, climate, population growth, and socioeconomic and health conditions as well as close contact with animals (2–5). Intestinal parasitic diseases are among the most important infectious diseases that have a direct relationship to personal and public hygiene (4, 5).
A dramatic decrease in the prevalence of parasitic infections has been reported in Iran (6), although the occurrence of enteric protozoans is still common and remain a health problem in some parts of the country (4, 7–9). Prior studies reported Giardia intestinalis and Entamoeba coli to be the most common intestinal parasites in Iran (8, 9), but recently Blastocystis sp. has been suggested to be the most prevalent (10, 11).
The goal of this study was to determine the prevalence of, and the risk factors for, intestinal parasites in Roudehen, Tehran Province, Iran.
Materials and Methods
Study Area
Roudehen is a city in Damavand County, Tehran Province; northern Iran located 35 km east of Tehran, in the Alborz Mountains. Roudehan has a mountain temperate climate. It is 1850 m above sea level and has an area of 50 km2 with a population of 21477 in 7393 households (12).
Study design and sampling
The cross-sectional study was conducted from Jun to Dec 2014. The triple faecal samples from 561 residents were collected in a two-stage cluster-sampling scheme. In the first stage, the city was divided into 82 blocks, and 23 blocks were randomly selected. In the second stage, 10 households within each block were randomly selected. All members of selected households were invited to participate in the study. If in the selected household, no one answered to investigators on two separate dates, or if all individuals in the household refused to participate in the study, the next household was invited.
The aim and design of the study were explained to household members. Agreeable individuals and the parents of children under age 15 gave written consent and filled a questionnaire form including sex, age, occupation, education, drinking water source, and history of travel and animal contact. Three samples were collected from each participant with one-day interval.
Laboratory procedures
Stool samples were transferred immediately after collection to the Department of Parasitology and Mycology, Iran University of Medical Sciences, for examination and identification of parasites.
Parasite examination
Pea-sized pieces of fecal samples were submitted to formalin-ether concentration technique to identify ova, cysts or oocysts of parasites. The remaining sample was emulsified in PBS and passed through a two-layer gauze filter to remove larger particulates, and the suspension was centrifuged at 1000 × g for 5 min. The sediments were separated into separate portions for culture and molecular study. One hundred mg of the sediment was resuspended in 200 μl 2% polyvinylpolypyrrolidone in PBS and stored at −80 °C until submitted to DNA extraction and 500 μl of which was cultured in a biphasic medium, horse serum slant overlaid with 5 ml of Ringer’s solution, 200 μl of rice starch (5 mg/ml) and penicillin-streptomycin incubated at 35.5 °C and examined three times at 48 h intervals (13). Samples were considered positive if helminth eggs, larvae, or cysts, and/or trophozoites of protozoans were detected by at least one of the two techniques.
Molecular examination
All samples containing Entamoeba trophozoites or cysts were submitted to molecular study. A single-round PCR assay (14) was performed for discrimination of Entamoeba species (Entamoeba dispar, Entamoeba moshkovskii, Entamoeba histolytica). DNA was extracted from 200 μl of frozen sediment of stool samples using QIAamp DNA minikit (Qiagen, Hilden, Germany) according to manufacturer’s instruction. PCR of Entamoeba complex was performed using the forward primer EntaF and reverse primers EhR, EdR, and EmR, specific for E. histolytica, E. dispar, and E. moshkovskii, respectively (14). The PCR reaction was designed to amplify a 166 bp PCR product with E. histolytica DNA, a 752-bp PCR product with E. dispar DNA, and a 580 bp product with E. moshkovskii DNA of a small-subunit rRNA gene (14). In the PCR reaction, a 25 μl PCR reaction mixture consisted of 2 μl of template DNA, 0.25 μM of each the forward primer EntaF and reverse primers EhR, EdR, and EmR primers, 12.5 μl Taq DNA polymerase mastermix (Ampliqon, Denmark), and 6.5 μl H2O. PCR was carried out with the following amplification conditions: 1 cycle at 94 °C for 3 min followed by 30 cycles at 94 °C for 60 sec, 58 °C for 60 sec, and 72 °C for 60 sec, and a final extension of 72 °C for 7 minutes. Sterile distilled water was included as a negative control and the mix of genomic DNA of E. histolytica reference strain HM-1, IMSS Clone 6 (ATCC® Number, 50527 ™), E. dispar reference strain SAW 760 (ATCC® Number, PRA260™), and E. moshkovskii reference strain Laredo (ATCC® Number, 30042 ™) were used, as positive control, to validate the results of multiplex PCR. The PCR products were detected on ethidium bromide stained 1.5% agarose gels.
Data management and analysis
The sampling weights were calculated by multiplication of three weighting components: 1) inverse sampling weight, 2) inverse of nonresponse for each block, and 3) post-stratification weights calculated by dividing census-derived population weights by sample weights for each of 18 age/sex groups (Table 1).
Table 1:
Socio-demographic characteristics of residents of Roudehen, Tehran Province, Iran from Jun to Dec 2014
| Characteristics | n | Un-weighted % | Weighted % |
|---|---|---|---|
| Sex | |||
| Female | 324 | 57.8 | 49.7 |
| Male | 237 | 42.3 | 50.3 |
| Age group | |||
| 0–10 | 111 | 19.8 | 15.6 |
| 11–20 | 72 | 12.8 | 14.1 |
| 21–30 | 84 | 15.0 | 21.6 |
| 31–40 | 105 | 18.7 | 20.0 |
| 41–50 | 82 | 14.6 | 13.5 |
| 51–60 | 62 | 11.1 | 8.1 |
| 60< | 45 | 8.0 | 7.1 |
| Occupation | |||
| Housewife | 191 | 34.1 | 27.5 |
| Student | 138 | 24.6 | 25.1 |
| Employed | 132 | 23.5 | 30.6 |
| Children | 61 | 10.9 | 9.1 |
| Retired | 24 | 4.3 | 4.5 |
| Unemployed | 15 | 2.7 | 3.2 |
| Education | |||
| Illiterate | 31 | 5.5 | 4.0 |
| First elementary school | 133 | 23.7 | 20.7 |
| Second elementary school | 80 | 14.3 | 12.4 |
| High school | 145 | 25.9 | 29.2 |
| University | 111 | 19.8 | 24.6 |
| Children under 6-yr | 61 | 10.9 | 9.1 |
| Water Source | |||
| Tap Water | |||
| Yes | 464 | 82.7 | 83.2 |
| No | 97 | 17.3 | 16.8 |
| Spring water | |||
| Yes | 58 | 10.3 | 11.1 |
| No | 503 | 89.7 | 88.9 |
| Filtered water | |||
| Yes | 50 | 8.9 | 8.8 |
| No | 511 | 91.1 | 91.2 |
| Travel history | |||
| Yes | 338 | 60.3 | 59.7 |
| No | 223 | 39.8 | 40.4 |
| Animal contact | |||
| Yes | 72 | 12.8 | 13.3 |
| No | 489 | 87.2 | 86.7 |
For descriptive analysis, frequency and percentage rates were used to describe characteristics of the studied population, including the prevalence of intestinal parasites. All variables were included in multiple logistic regression analyses to identify the adjusted odds ratios (aOR) of risk factors for intestinal parasite infection. Stata v.10.0 (StataCorp LP, Texas, USA) was used to conduct statistical procedures.
Ethical considerations and treatment
The study protocol was reviewed and approved by the Ethics Committee of Iran University of Medical Sciences (IUMS) with the code number: IR.IUMS.REC 93-04-30-25381, following the revised Helsinki Declaration of 2008. Each participant was asked to sign an informed consent. For children under 15 yr, one parent was required to agree and sign the consent form. At the end of the study, a laboratory report was provided to each participant, and, if indicated, they were referred for medical care.
Results
Socio-demographic characteristics of the study participants
Two-hundred-thirty of 906 approached households agreed to participate in the study, for a response rate of approximately 25.4%. Of the 832 individuals in these households, 561 (67.4%) completed participation in the investigation. Over six months, triple fecal samples from 561 subjects, including 324 (50.3%) females and 237 (49.7%) males from four months to 90 yr of age (mean 31 yr) were obtained (Table 1).
Prevalence of intestinal parasites
At least one species of intestinal parasite was found in 32.7% (95% CI 27.3–38.0) of residents [31.4% (95% CI 25.7–37.0) of females and 33.9% (95% CI 24.7–43.9) of males] (Table 2). Blastocystis sp. (28.4%; 95% CI 23.7–33.0) was the most common intestinal parasite observed.
Table 2:
Prevalence of intestinal parasites in residents of Roudehen, Tehran, Iran from Jun to Dec 2014
| Parasite | Male (n = 237) | Female (n = 324) | Total (n = 561) | 95% CIa |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Blastocystis sp. | 64 (27.5) | 95 (29.4) | 159 (28.4) | 23.7–33.0 |
| Entamoeba coli | 13 (6.3) | 15 (5.2) | 28 (5.8) | 3.5–8.0 |
| Giardia intestinalis | 5 (2.0) | 2 (0.4) | 7 (1.2) | 0.4–2.0 |
| Entamoeba dispar | 0 (0) | 2 (0.4) | 2 (0.4) | 0–1.1 |
| Entamoeba moshkovskii | 0 (0) | 1 (0.2) | 1 (0.2) | 0–0.4 |
| Chilomastix mesnili | 1 (0.4) | 1 (0.2) | 2 (0.3) | 0–0.7 |
| Iodamoeba butschlii | 2 (0.7) | 2 (0.6) | 4 (0.7) | 0–1.5 |
| Dientamoeba fragilis | 3 (1.3) | 3 (0.9) | 6 (1.1) | 0.2–2.0 |
| Enterobius vermicularis | 1 (0.2) | 0 (0) | 1 (0.2) | 0–0.4 |
| Total | 78 (33.9) | 102 (31.4) | 180 (32.7) | 27.3–38.0 |
CI: confidence interval
Of 180 (32.7%) infected individuals, 159 (14.2%) were infected with a single species, 20 (1.4%) with two species, and four (0.5%) with three species.
The formalin-ether concentration detected 115 (20.5%) Blastocystis sp., 25 (5.3%) Entamoeba coli, seven (1.2%) Giardia intestinalis, four (0.7%) Iodamoeba butschlii, one (0.2%) Chilomastix mesnili, one (0.2%) four-nuclei Entamoeba cysts, and one (0.2%) Enterobius vermicularis.
Culture revealed Blastocystis sp. in 115 (20.3%), E. coli in 13 (2.5%), Dientamoeba fragilis in six (1.1%), I. butschlii in two (0.3%), Entamoeba complex trophozoite in two (0.4%), and C. mesnili in two (0.3%) of the population (Table 2).
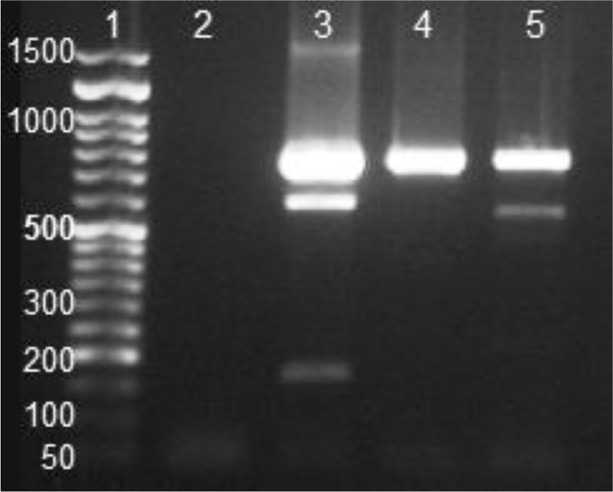
Entamoeba complex positive samples were identified by single-round PCR assay in two samples: one was E. dispar, and the other sample showed mixed infection of E. dispar and E. moshkovskii. Entamoeba histolytica was not observed in any specimen (Fig. 1).
Fig. 1:
Single-Round PCR amplification of DNA extracted from fecal of Entamoeba complex positive samples. Lane 1, 50 bp ladder (Cat NO. PR901633); Lane 2, negative control; Lane 3, positive control: a mixture of standard DNA of E. histolytica (166 bp), E. dispar (752 bp), and E. moshkovskii (580 bp); lane 4–5 two cyst positive samples; line 4, 752 bp amplification showed E. dispar-positive infection; line 5, 580 and 752 bp amplified products showed co-infection of E. moshkovskii and E. dispar.
Intestinal parasite and possible risk factors
The results of unadjusted and adjusted logistic regression analyses of the socio-demographic correlates of intestinal parasitic infections among residents of Roudehen are presented in Table 3.
Table 3:
Univariate and multivariate analysis of intestinal parasitic infections and potential risk factors in residents of Roudehen, Tehran Province, Iran from Jun to Dec 2014
| Risk factor | Prevalence % (95 % CIa) | ORb (95 % CI) | aORc (95 % CI) |
|---|---|---|---|
| Sex | |||
| Female | 31.4 (25.7–37.0) | 1 | 1 |
| Male | 33.9 (24.7–43.9) | 1.1 (0.7–1.8) | 1.4 (0.7–2.5) |
| Age groups | |||
| 0–10 | 15.2 (9.3–21.3) | 1 | 1 |
| 11–20 | 32.2 (20.7–43.7) | 2.7 (1.3–5.4) | 1.7 (0.6–4.4) |
| 21–30 | 34.6 (23.6–45.7) | 3 (1.7–5.2) | 2.3 (0.8–6.4) |
| 31–40 | 40.5 (30.2–50.8) | 3.8 (2.1–6.9) | 2.7 (0.7–10.0) |
| 41–50 | 31.4 (22.9–40.0) | 2.6 (1.7–4) | 1.5 (0.5–4.6) |
| 51–60 | 44.7 (34.7–54.8) | 4.5 (2.6–7.9) | 2.6 (0.9–6.8) |
| 60< | 32.0 (21.8–42.3) | 2.6 (1.3–5.3) | 1.3 (0.5–3.2) |
| Occupation | |||
| Employed | 36.1(26.4–45.7) | 1 | 1 |
| Unemployed | 14.2 (–2.0–30.1) | 0.3 (0.1–1.1) | 0.2 (0.1–1) |
| Housewife | 38.9 (31.3–46.5) | 1.1 (0.6–2.02) | 1.4 (0.6–3.2) |
| Retired | 40.5 (21.4–59.6) | 1.2 (0.5–2.8) | 1.2 (0.3–4.7) |
| Student/Soldier | 31.3 (20.3–42.2) | 0.8 (0.5–1.5) | 1.0 (0.4–2.7) |
| Children | 9.1 (2.6–15.4) | 0.2 (0.1–0.4) | 0.3 (0.0–2.0) |
| Education | |||
| Illiterate | 35.7 (20.3–51.1) | 1 | 1 |
| First elementary school | 33.1 (22.6–43.6) | 1.0 (0.4–2.3) | 1.0 (0.5–2.0) |
| Second elementary school | 42.9 (28.8–56.9) | 1.5 (0.6–4.2) | 1.2 (0.4–3.8) |
| High school | 35.7 (27.5–44.0) | 1.0 (0.4–2.4) | 0.8 (0.2–2.6) |
| University | 31.7 (20.8–42.6) | 1.0 (0.4–2.4) | 0.6 (0.2–2.1) |
| Children under 6-yr | 9.1 (2.5–15.6) | 0.2 (0.0–0.6) | 1.1 (0.2–7.4) |
| Water Source | |||
| Tap water | |||
| Yes | 30.9 (26.4–35.5) | 0.6 (0.4–1.2) | 0.3 (0.0–0.9) |
| No | 41.1 (25.2–57) | ||
| Spring water | |||
| Yes | 38.1 (17.7–58.6) | 2.1 (0.6–8.3) | 0.7 (0.2–1.9) |
| No | 32 (27.5–36.5) | ||
| Filtered water | |||
| Yes | 29.9 (18.8–40.9) | 1 (0.6–1.6) | 0.3 (0.0–1.2) |
| No | 33 (27.1–38.7) | ||
| Travel history | |||
| Yes | 30.6 (24.1–37.1) | 0.8 (0.5–1.3) | 0.8 (0.4–1.5) |
| No | 35.7 (26.6–44.7) | ||
| Animal contact | |||
| Yes | 45.3 (28.8–61.7) | 1.9 (1–3.6) | 1.8 (1.0–3.2) |
| No | 30.7 (25.9–35.5) |
CI: Confidence interval;
OR: Odds ratio;
aOR: Adjusted odds ratio
Among possible risk factors investigated in this study, drinking water source and close animal contact were found to have a significant relationship with intestinal parasitic infections. The aOR for intestinal parasitic infections was 1.8 (95% CI 1.0–3.2) in animal-contact subjects. City tap water (aOR=0.3, 95% CI 0.0–0.9) intake showed a possible protective effect against intestinal parasitic infections. Intestinal parasitic infections showed no significant correlation with sex, age, occupation, education, or travel history.
Discussion
The prevalence of both pathogenic and nonpathogenic intestinal parasites was high in residents of Roudehen. The drinking water source and close animal contact were the most significant factors in the infection of population. The results of the study showed the existence of several intestinal parasites of public health importance among residents of Roudehen. To our knowledge, this is the first well-designed study reporting the true prevalence of intestinal parasitic infections in the general population. The prevalence of enteric parasites in the residents of Roudehen was 32.7% (95 % CI 27.3–38), similar to a study (32.3%) conducted in apparently healthy rural and semirural inhabitants of western (Lorestan), northwestern (West Azerbaijan), and northeastern (Golestan) Iran (9). However, it is higher than the previous prevalence (19.3%) reported in the general population throughout the country (8) and lower than the prevalence (48.8%) reported in the rural areas of Bandar-Abbas (10). The variations in prevalence of intestinal parasites in these studies could be due mainly to the sensitivity of applied parasitological techniques as well as to diversity in socioeconomic, geographic, sanitary/hygiene, cultural, and educational status, and nutrition of study subjects. In this study, only a single case (0.2%) of helminth infection with E. vermicularis was detected. The high prevalence of protozoan parasites observed compared to helminths is in agreement with previous findings in Iran (6, 10, 11, 15) and other developing and developed countries (2, 16).
The prevalence of Entamoeba complex in the residents of Roudehen was (0.4%; 95% CI 0–1.1), similar to a study in Zahedan (0.5%) (7). However, it is lower than found in inhabitants of central, southern, and northern Iran (1.4%; 95% CI 1.2–1.5) (17), Bandar-Abbas (5.8%) (10), and Lorestan, West Azerbaijan, and Golestan (8.4%; 95% CI 6.9–10.3) (9). The pathogenic member of the Entamoeba complex, E. histolytica, was not found in the study population, and a single co-infection of E. moshkovskii with E. dispar was detected. Our study confirmed the previous finding showing extremely low prevalence of E. histolytica in the Iranian population. Haghighi et al. (7) and Solaymani-Mohammadi et al. (9) did not detect E. histolytica, and Hooshyar et al. found only eight E. histolytica infections among 101 successfully cultured Entamoeba-cyst positive isolates, resulting from screening 16592 individuals (18).
The most frequently detected intestinal protozoan was Blastocystis sp. (28.4%; 95% CI 23.7–33.0), similar to other studies in Iran (10, 11, 15) and in the world (19, 20). Epidemiological surveys have shown Blastocystis sp. to be the most prevalent human intestinal parasite worldwide (19, 20). As this parasite is transmitted to humans through the fecal-oral route, the rate of infection should be related to poor hygiene, consumption of contaminated food or water, and close animal contact (19, 21). Although the role of Blastocystis in disease is controversial (21, 22), it is a good criterion for hygiene.
In the present study, the prevalence of Giardia infection was 1.2% (95% CI 0.4–2.0), lower than the previous studies in Iran. The overall prevalence of Giardia has shown a significant declining trend during the past decade from 25.8% (15), 10.9% (8), 10.6% (9), 10.1% (7), 5.4% (11), and 2.5% (23), to 1.2% in this study. Beside of the improvement in general hygiene in the country, these variations are probably associated with the population and area of the diagnostic approaches used.
The true prevalence of D. fragilis in the healthy population is not clear, with literature reporting 0.2%–71% of gastrointestinal patients infected with Dientamoeba (24, 25). We found 1.1% (95% CI 0.2–2.0) of the apparently healthy population to harbor Dientamoeba, within the 0.5%–2.4% range reported in several studies in Iran (9, 26, 27).
In this study, several possible factors associated with intestinal parasites were investigated. Intestinal parasitic infections were more frequent in males at 33.9% (CI: 24.7–43.9) (aOR= 1.40, CI: 0.7–2.5), similar to other studies in Iran (11) and throughout the world (28). It is possible that greater participation by men in outdoor activities increases exposure to infection. The prevalence of parasitic infections in the age group 51–60 yr was 44.7% (95% CI 43.7–54.8) (aOR= 2.6, CI: 0.9–6.8), higher than other groups, similar to results of other studies (4, 16). The most common infections in this age group were Blastocystis sp (42.2%, 95% CI 31.5–52.9). This pattern may suggest that incidence of Blastocystis infection increases with age (28, 29). The prevalence of parasitic infection showed no significant relationship with occupation or education; although the highest prevalence was found in retired people at 40.5% (95% CI 21.4–59.6) (aOR= 1.3, 95% CI 0.3–5.5).
Household sanitation in this urban community was adequate. Few people consumed water from sources other than tap water. Prevalence of intestinal parasites in people who used spring water was 38.1% (95% CI 17.7–58.6), suggesting the possibility of waterborne transmission (aOR= 0.3, 95% CI 0.0–0.9). Close contact with animals was associated with the risk of intestinal parasitic infection of 45.3% (95% CI 28.8–61.7) (aOR= 1.9, 95% CI 1.0–3.2). The most prevalent parasite was Blastocystis. Considering the zoonotic nature of this parasite (19, 21, 22), infection by household animals, chiefly birds, dogs, and cats, seems probable.
The study design likely produced results that reflected the city population as a whole. The possible limitation was the low response rate (25.4%). For cultural reasons, collecting feces and passing it to others was difficult for people. However, with statically replacement of sample, a representative sample of the population was collected, and the findings of this study can be generalized to the Roudehen city population. This study was carried out with only urban residents, limiting generalization to the wider Iranian population; thus, further investigation in suburban and rural areas is needed. This study is the first of its type to report the prevalence of intestinal parasitic infections among residents in Roudehen city. We used a combination of conventional techniques (concentration and culture) for detecting enteric parasites and molecular procedure for distinguishing Entamoeba complex parasites. Further molecular studies for identification of D. fragilis are underway since its true prevalence is difficult to reveal by conventional method.
Conclusion
The present study revealed a high prevalence of enteric protozoan parasite infection among citizens of Roudehen in Iran. Protozoan infections were more common than helminth infections. The neglected intestinal parasite Blastocystis was recognized as one of the most significant causes of infection. Consuming tap water had a possible protective effect against intestinal parasites, and contact with animals posed a risk for infection. As most of the detected parasites are transmitted via a water-resistant cyst, public and individual education on personal hygiene should be considered to improve health and prevent transmission of intestinal parasites to people living in this city.
Acknowledgments
Iran University of Medical Sciences and Health Services supported this work by grant number 25381. We are grateful to the residents of the Roudehen for their help in providing samples. We are grateful to Professor C. Graham Clark of the Department of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London, UK for kindly providing DNA of reference strains Entamoeba histolytica, E. dispar and E. moshkovskii. This work represents a part of the MSc dissertation of Nasrin Hemati.
Footnotes
Conflict of Interests
The authors declare that there is no conflict of interest.
References
- 1.Schuster H, Chiodini PL. Parasitic infections of the intestine. Curr Opin Infect Dis. 2001; 14(5):587–91. [DOI] [PubMed] [Google Scholar]
- 2.Calderaro A, Montecchini S, Rossi S, et al. Intestinal parasitoses in a tertiary-care hospital located in a non-endemic setting during 2006–2010. BMC Infect Dis. 2014; 14:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fletcher S, Caprarelli G, Merif J, et al. Epidemiology and geographical distribution of enteric protozoan infections in Sydney, Australia. J Public Health Res. 2014; 3(2):298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daryani A, Sharif M, Nasrolahei M, et al. Epidemiological survey of the prevalence of intestinal parasites among schoolchildren in Sari, northern Iran. Trans R Soc Trop Med Hyg. 2012; 106(8):455–9. [DOI] [PubMed] [Google Scholar]
- 5.Li XX, Chen JX, Wang LX, et al. Prevalence and risk factors of intestinal protozoan and helminth infections among pulmonary tuberculosis patients without HIV infection in a rural county in P. R. China. Acta Trop. 2015; 149:19–26. [DOI] [PubMed] [Google Scholar]
- 6.Rokni MB. The present status of human helminthic diseases in Iran. Ann Trop Med Parasitol. 2008; 102(4):283–95. [DOI] [PubMed] [Google Scholar]
- 7.Haghighi A, Khorashad AS, Nazemalhosseini Mojarad E, Khorashad AS, Nazemalhosseini Mojarad E, Kazemi B, Rostami Nejad M, Rasti S. Frequency of enteric protozoan parasites among patients with gastrointestinal complaints in medical centers of Zahedan, Iran. Trans R Soc Trop Med Hyg. 2009; 103(5):452–4. [DOI] [PubMed] [Google Scholar]
- 8.Sayyari AA, Imanzadeh F, Bagheri Yazdi SA, et al. Prevalence of intestinal parasitic infections in the Islamic Republic of Iran. East Mediterr Health J. 2005; 11(3):377–83. [PubMed] [Google Scholar]
- 9.Solaymani-Mohammadi S, Rezaian M, Babaei Z, et al. Comparison of a stool antigen detection kit and PCR for diagnosis of Entamoeba histolytica and Entamoeba dispar infections in asymptomatic cyst passers in Iran. J Clin Microbiol. 2006; 44(6):2258–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuzehkanani AB, Rezaei S, Babaei Z, et al. Enteric protozoan parasites in rural areas of Bandar-Abbas, southern Iran: comparison of past and present situation. Iran J Public Health. 2011; 40(1):80–5. [PMC free article] [PubMed] [Google Scholar]
- 11.Niyyati M, Rezaeian M, Zahabion F, et al. A survey on intestinal parasitic infections in patients referred to a hospital in Tehran. Pak J Med Sci. 2009; 25(1):87–90. [Google Scholar]
- 12.Census Census of the Islamic Republic of Iran, Statistical Centre of Iran 2011. [cited; Available from: http://www.amar.org.ir/
- 13.Clark CG, Diamond LS. Methods for cultivation of luminal parasitic protists of clinical importance. Clin Microbiol Rev. 2002; 15(3):329–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamzah Z, Petmitr S, Mungthin M, et al. Differential detection of Entamoeba histolytica, Entamoeba dispar, and Entamoeba moshkovskii by a single-round PCR assay. J Clin Microbiol. 2006; 44(9):3196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arani AS, Alaghehbandan R, Akhlaghi L, et al. Prevalence of intestinal parasites in a population in south of Tehran, Iran. Rev Inst Med Trop Sao Paulo. 2008; 50(3):145–9. [DOI] [PubMed] [Google Scholar]
- 16.Boonjaraspinyo S, Boonmars T, Kaewsamut B, et al. A cross-sectional study on intestinal parasitic infections in rural communities, northeast Thailand. Korean J Parasitol. 2013; 51(6):727–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hooshyar H, Rezaian M, Mahmoodi M, et al. A field study of the distribution of Entamoeba histolytica /dispar cyst passers in northern, central, and southern Iran. Iran J Publ Health. 2004; 33(2):28–32. [Google Scholar]
- 18.Hooshyar H, Rezaian M, Kazemi B, et al. The distribution of Entamoeba histolytica and Entamoeba dispar in northern, central, and southern Iran. Parasitol Res. 2004; 94(2):96–100. [DOI] [PubMed] [Google Scholar]
- 19.Tan KS. New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin Microbiol Rev. 2008; 21(4):639–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wawrzyniak I, Poirier P, Viscogliosi E, et al. Blastocystis, an unrecognized parasite: an overview of pathogenesis and diagnosis. Ther Adv Infect Dis. 2013; 1(5):167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark CG, van der Giezen M, Alfellani MA, et al. Recent developments in Blastocystis research. In: Rollinson D, editor. Advances in Parasitology: Academic Press; 2013. p. 1–32. [DOI] [PubMed] [Google Scholar]
- 22.Poirier P, Wawrzyniak I, Vivarès CP, et al. New insights into Blastocystis spp.: A potential link with irritable bowel syndrome. PLoS Pathog. 2012; 8(3):e1002545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akhlaghi L, Shamseddin J, Meamar AR, et al. Frequency of intestinal parasites in Tehran. Iran J Parasitol. 2009; 4(2):44–7. [Google Scholar]
- 24.Röser D, Simonsen J, Nielsen HV, et al. Dientamoeba fragilis in Denmark: epidemiological experience derived from four years of routine real-time PCR. Eur J Clin Microbiol Infect Dis. 2013; 32(10):1303–10. [DOI] [PubMed] [Google Scholar]
- 25.Stark D, Barratt J, Chan D, et al. Dientamoeba fragilis, the neglected trichomonad of the human bowel. Clin Microbiol Rev. 2016; 29(3):553–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghazanchaei A, Shargh S, Shabani M, et al. Detection of Dientamoeba fragilis in patients referred to Chaloos Medical Care Centers by nested – polymerase chain reaction (PCR) method. Afr J Biotechnol. 2012; 11(17):4079–82. [Google Scholar]
- 27.Sarafraz S, Farajnia S, Jamali J, et al. Detection of Dientamoeba fragilis among diarrheal patients referred to Tabriz health care centers by nested PCR. Trop Biomed. 2013; 30(1):113–8. [PubMed] [Google Scholar]
- 28.Amin O. Epidemiology of Blastocystis hominis in the United States. Res J Parasitol. 2006; 1(1):1–10. [Google Scholar]
- 29.Engsbro AL, Stensvold CR, Vedel Nielsen H, et al. Prevalence, incidence, and risk factors of intestinal parasites in Danish primary care patients with irritable bowel syndrome. Scand J Infect Dis. 2014; 46(3):204–9. [DOI] [PubMed] [Google Scholar]