Abstract
Background:
The aim of this study was the seroepidemiological survey for detecting the status of human fasciolosis in Lorestan Province, western Iran.
Methods:
This cross-sectional study was conducted in 2015–16. Based on statistical estimations, 1256 serum samples were collected from different parts of Lorestan Province, western Iran, and stored at −20 °C until use. The collected serum samples were analyzed at Tehran University of Medical Sciences, Tehran, Iran using indirect ELISA method.
Results:
Anti-Fasciola antibodies were detected in 16 (1.3%) individuals. Regarding the seropositivity to fasciolosis, no significant differences were found between age groups, sex, level of education and occupation; however significant differences were observed regarding location, consuming local freshwater plants and water resources (P<0.02.)
Conclusion:
Local freshwater plants and unfiltered water resources were probably the main sources of the infection. Health education by local health centers to elevate awareness of people, and providing facilities for safer drinking water, especially in rural areas may help decrease the risk of fasciolosis infection in this region.
Keywords: Fasciolosis, Seroepidemiology, Freshwater plants, Iran
Introduction
One of the leading zoonotic helminthic diseases is fasciolosis caused by Fasciola hepatica and F. gigantica. It is listed by WHO among the neglected tropical diseases (1). Furthermore, 17 contaminated million individuals are estimated to be infected and around 180 million individuals living in endemic regions are assumed to be at risk of the diseases (2–4). Human and other mammalian are considered as definitive hosts for fasciolosis infected by eating aquatic plants or by drinking water contaminated with metacercariae (5).
Although fasciolosis is generally considered as a notable veterinary problem, human fasciolosis has recently been regarded as a main health issue in numerous countries (6, 7). According to WHO report, Iran has been placed among six countries recognized to have a serious concern with fasciolosis (8). Before 1989, human fasciolosis was pronounced sporadically in Iran (9–12). Fasciolosis has led to two important epidemics in Iran in 1989 and 1999, respectively, which have been the biggest epidemics of fasciolosis in the history (13). In the recent years, new foci of the disease have been observed in Iran such as Kohgyluyeh va Boyerahmad and Kermanshah provinces (14, 15).
Several techniques including serological and parasitological methods are used for the diagnosis of fasciolosis. Parasitological methods (detection of parasite ova in stool or biliary aspirates) have the highest specificity, but some factors such as low rate of parasite eggs, transient infection, acute and obstructive infections reduce the sensitivity of these methods. Serological tests are usually used for recognition of anti-Fasciola antibodies in serum samples in acute phase and in ectopic fasciolosis. These methods are appropriate for diagnosis of chronic fasciolosis by identifying specific antigens in stool samples and antibodies in the serum as well (16). Therefore, serological methods such as ELISA are commonly used for the diagnosis of human fasciolosis in Iran (17, 18).
Because of the specific climatic condition of Iran and occurrence of new foci for fasciolosis (14, 15) the neighboring of Lorestan Province with these new foci, as well as the reports by a previous study revealing Fasciola parasite cases from Pirabad village of Doroud city in Lorestan (19), we decided to investigate the seroprevalence of human fasciolosis using indirect ELISA throughout the Lorestan Province, Iran.
Materials and Methods
Serum collection
Lorestan Province is located in western Iran (Fig.1). The population of this province, which has ten major cities, is 1,716,527. Overall, 1256 serum samples were collected from the people of Lorestan Province by random stratification recommended by statistician in 2015–16 (Table 1).
Fig. 1:
Location of Lorestan Province in Iran
Table 1:
Sample size in different areas of Lorestan Province
| Aligudarz | Borujerd | Khorramabad | Delfan | Dorud | Kuhdasht | Azna | Pol-e-Dokhtar | Selseleh | Dowreh | |
|---|---|---|---|---|---|---|---|---|---|---|
| Urban | 51 | 140 | 202 | 36 | 60 | 70 | 30 | 43 | 20 | 2 |
| Rural | 29 | 110 | 146 | 54 | 40 | 100 | 20 | 50 | 30 | 23 |
| Total | 80 | 250 | 348 | 90 | 100 | 170 | 50 | 93 | 50 | 25 |
The samples were collected in accordance with the population of each city. Based on a random sampling, people were asked to present in health centers for collecting sera. Each patient filled a questionnaire including information on food diet, vegetable consumption, travelling to northern Iran, clinical symptoms etc.
Subjects were informed about the objectives and procedures of the study. They signed a written informed consent. For children this form was taken from parents or their legal guardians. The study was conducted in accordance with the Health Insurance Portability and Accountability Act (HIPAA) guidelines and all procedures were approved by the Ethics Committee of Tehran University of Medical Sciences, Tehran, Iran.
After blood sampling, sera were obtained and stored at −20 °C until use. The collected serum samples were analyzed using ELISA method (17). Finally, absorbance was measured by an ELISA reader at 490 nm.
Antigen preparation
Fasciola infected livers from Tehran slaughterhouses were transferred to the laboratory of Helminthic Diseases, School of Public Health, Tehran University of Medical Sciences, Iran. Parasites were isolated from infected livers and washed for 6 times with normal saline. Afterwards, they were homogenized in 0.045 mM PBS with electrical homogenizer. The supernatant was kept in refrigerator for later usage (17).
ELISA test
ELISA test was conducted based on previous study with some modifications (17). One hundred microliters of somatic antigen (10 mg/ml) was added to wells of plates and incubated overnight at 37 °C and then 200 microliters of BSA 2% was dispensed to plates. Wells of plates were washed with PBS/Tween 20 for three times. One hundred microliters of a serum samples (diluted1:250) was added to wells coated with antigen and incubated at 37 °C for 30 min. Sera of a Fasciola-infected patient and a healthy individual were tested in parallel, as positive and negative controls, respectively. Plates were washed 5 times with PBS/Tween 20. Peroxidase conjugated goat anti-human IgG (diluted 1:10000) was added to wells and incubated at 37 °C for 30 min. After final washing step with PBS/Tween 20, 100 microliters of OPD (o-phenylenediamine dihydrochloride) was added to the wells and reaction was stopped with adding 50 microliters of stopper solution (12.5% H2SO). OD was measured at 490 nm with ELISA reader. Cut-off was calculated as X±3 SD.
Statistical analysis
Statistical analysis was done using SPSS version 22 (Chicago, IL, USA). Chi square test was used for data analyzing. Cut-off was calculated as Mean±3 SD.
Results
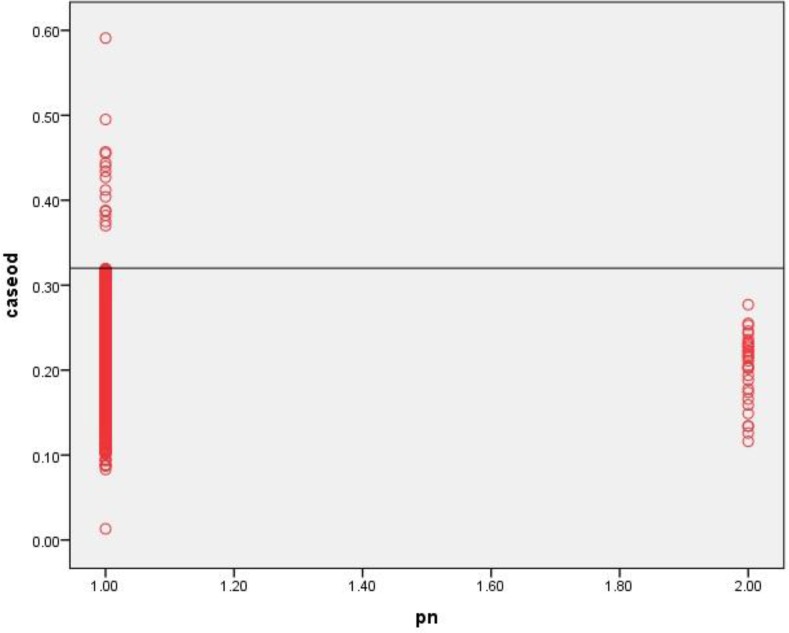
Cut-off for ELISA was calculated as 0.32. Out of 1256 examined cases, 577 (46%) were male and 679 (54%) were female. Overall, 16 persons (1.3%) were serologically positive for fasciolosis. Seropositivity to fasciolosis among the female and male subjects were 1.5% and 1%, respectively, which was not statistically significant (chi square = 0.47, P-value = 0.49). Fig. 2 demonstrates the distribution of OD absorbance in subjects and healthy control cases. Table 2 reveals seropositivity rate of fasciolosis in different locations of Lorestan Province and the highest rate was seen in Borujerd district. Table 3 shows the seropositivity in different age groups. The highest rate of seropositivity was seen in ≥ 60 yr old individuals.
Fig. 2:
Analysis of sera from subjects and normal controls from, Lorestan Province, Iran using IgG-ELISA. Serum samples gained from subjects (1256 cases, Lanes 1), and normal controls (30, Lanes 2) *pn= patient number
Table 2:
Seropositivity rate of fasciolosis in different locations of Lorestan Province in 2016
| Location | Aligudarz | Borujerd | Khorramabad | Delfan | Dorud | Kuhdasht | Azna | Pol-e-Dokhtar | Selseleh | Dowreh |
|---|---|---|---|---|---|---|---|---|---|---|
| Seropositivity Rate | 0 | 4% | 0 | 3.3% | 2% | 0.5% | 0 | 0 | 0 | 0 |
Table 3:
Seropositivity rate of fasciolosis in different age groups of Lorestan Province in 2016
| Age Groups (yr) | Total No. | Seropositivity Cases No. (%) |
|---|---|---|
| ≤9 | 3 | 0 (0.0) |
| 10–19 | 85 | 1(1.2) |
| 20–29 | 398 | 4 (1) |
| 30–39 | 295 | 2 (0.7) |
| 40–49 | 189 | 4 (2.1) |
| 50–59 | 149 | 2 (1.3) |
| ≥60 | 137 | 3 (2.2) |
| Total | 1256 | 16 (1.3) |
Of sixteen positive cases, seven cases were illiterate, six preliminary educated and three secondary educated. The relation between seropositivity and education level was not statistically significant (P-value = 0.08).
Out of sixteen positive cases, ten were housekeepers, one student, two farmers and three self-employed. The relation between seropositivity and occupation was not statistically significant (P-value = 0.17).
The prevalence of human fasciolosis in rural and urban areas was 1.9% and 0.4% respectively. Significant relationship between location and fasciolosis infection was observed (chi square = 5.4, P-value = 0.02).
The relation between fasciolosis infection and water resource was statistically significant (P-value 0.001). The highest prevalence rate of fasciolosis (1.8%), was among people who used spring water. Table 4 shows distribution of positive cases of fasciolosis based on the water resources.
Table 4:
Distribution of positive cases of fasciolosis based on the water resources
| Water resources | Total number | Percentage of positive cases |
|---|---|---|
| Tap water (filtered) | 1106 | 0.7 |
| Spring water (unfiltered) | 57 | 8.1 |
| Well water (unfiltered) | 47 | 2.1 |
| Spring water (filtered) | 19 | 5.3 |
| well water (filtered) | 27 | 3.7 |
| Total | 1256 | 1.3 |
Thirteen of seropositive cases used to eat raw vegetables. Significant relationship was observed between eating raw vegetables and fasciolosis infection (chi square = 18.68, P-value < 0.01).
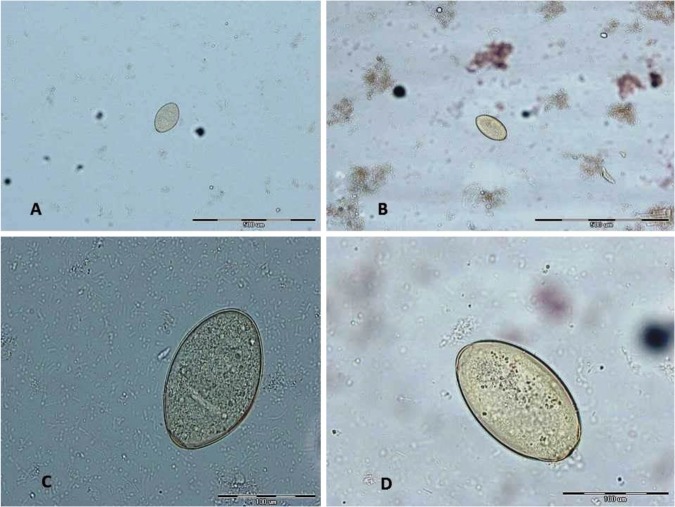
For all seropositive cases, stool examination was performed 3 times and parasite eggs were observed in four patients (Fig. 3).
Fig. 3:
Ova of Fasciola in stool examination. A, B: With ×100 magnifications C, D with ×400 magnifications (These pictures were originally captured in this study.)
Patients diagnosed as positive in this study, were referred to a physician for appropriate treatment. The treatment was a single oral dose of 10 mg of triclabendazole (Egaten) per kilogram of body weight (10 mg/kg). The results were assessed 4 weeks after treatment by stool exam.
Discussion
In this study, the seroprevalence of human fasciolosis was detected as 1.3% in Lorestan Province, which according to WHO epidemiological classification, is considered “hypoendemic” (6). This study was conducted following the constant studies previously directed in Iran to define the state of human fasciolosis (15, 20). Since six seropositive persons were diagnosed with fasciolosis in one of the villages of Lorestan Province in a previous study (19), the current study was designed to cover the whole province.
While some previous studies have reported the seroprevalence as gender-specific (4, 20–25), no significant difference between genders was observed in our study. This result was in arrangement with several similar studies (13–15, 26–33).
In outbreaks in Kermanshah and Guilan, the highest infection prevalence was observed in 10–19 yr and 10–29 yr respectively (14, 34, 35); while in non-epidemic circumstances, the highest prevalence of infected cases was seen in older age groups (40–59 yr) (28). In this study, highest positive rate was seen in age group of ≥60 yr old. Iran is typically considered as one of the areas where the pattern of fasciolosis infection in adults is more than children (2). This result may be due to non-epidemic causes in this region; older adults are involved in cleaning and washing vegetables for eating and preparing salads and local foods, agricultural activities.
One of the important factors associated with human fasciolosis is dietary habits (36–38). In the current study, statistically significant difference was observed between the prevalence of infection and eating raw vegetables. There are a number of wild aquatic and semi aquatic plants related with human fasciolosis in Iran (13, 15, 37). As noted already, in this province, all seropositive individuals ate Nasturtium officinalis (locally name Balmak) which is a popular water plant commonly consumed by local residents. (19). In this area, this plant is considered hypoglycemic which can help treat diabetes or prevent it. In the present study, out of 1256 people, 414 (32.96%) consumed Balmak and among 16 seropositive cases, 13 had a history of eating this vegetable and there was a significant correlation between the eating of Balmak and seropositivity of fasciolosis (chi square = 18.68, P-value < 0.01). In the epidemic occurred in 1988 in Guilan Province, 91% of infected individuals had consumed a local plant called ‘’Khalvash” (Mentha piperita) (39). However, some studies did not find a significant relationship between consuming raw vegetables and seropositivity of fasciolosis (28, 40, 41).
In this study, statistically significant relationship between water resources and human fasciolosis was observed. The highest rate of the infection was seen in individuals who had used unfiltered spring water (8.1%) and unfiltered well water (2.1%) respectively.
In our study, the highest numbers of infection cases (14 out of 16) were seen in people who lived in rural areas. Fasciolosis mainly is a rural disease and rural people with certain occupations such as farmers and ranchers are at greater risk for infection, because of the closer contact with environmental factors like animal reservoirs, intermediate hosts, the consumption of aquatic plants and drinking unfiltered water (22, 42). Nevertheless Ashrafi et al. found the highest number of human cases in urban areas in Guilan Province and stated that it might be due to the vicinity of rural areas to the cities (28).
This study was the first comprehensive one, which covered all urban and rural areas of Lorestan Province. Limitations of the study might be stated as high costs, which prevented the study team from more sampling.
Conclusion
Due to high consumption of freshwater plants among seropositive people, it seems that local health centers may play an important role in educating people, especially about the risks of eating raw or uncooked freshwater plants. Local media should also alert people about aquatic plants and encourage public to cultivate freshwater plants in protected areas and fence them off from livestock in order to disrupt the parasite life cycle. Regarding the poor water filtration especially in rural areas and its relation to the prevalence of fasciolosis, it is encouraged that relevant organizations provide facilities for safer water supplies.
Acknowledgements
This study was part of a PhD thesis, financially supported by the Tehran University of Medical Sciences. The authors would like to thank all the people who participated and helped especially Dr. Mohammad Zeinali (Center for Disease Control and Prevention, Ministry of Health).
Footnotes
Conflict of Interests
The authors declare there is no conflict of interests.
References
- 1.Rokni MB, Lotfy WM, Ashrafi K, et al. Fasciolosis in the MENA region. Neglected Tropical Diseases-Middle East and North Africa. Springer; 2014:59–90. [Google Scholar]
- 2.Mas-Coma S. Epidemiology of fascioliasis in human endemic areas. J Helminthol. 2005; 79(3):207–16. [DOI] [PubMed] [Google Scholar]
- 3.Mas-Coma S, Valero MA, Bargues MD. Fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Adv Parasitol. 2009;69:41–146. [DOI] [PubMed] [Google Scholar]
- 4.González LC, Esteban JG, Bargues MD, et al. Hyperendemic human fascioliasis in Andean valleys: An altitudinal transect analysis in children of Cajamarca Province, Peru. Acta Trop. 2011;120(1–2):119–29. [DOI] [PubMed] [Google Scholar]
- 5.Sripa B, Kaewkes S, Intapan PM, et al. Food-borne trematodiases in Southeast Asia: Epidemiology, pathology, clinical manifestation and control. Adv Parasitol. 2010;72:305–50. [DOI] [PubMed] [Google Scholar]
- 6.Mas-Coma MS, Esteban JG, Bargues MD. Epidemiology of human fascioliasis: A review and proposed new classification. Bull World Health Organ. 1999;77(4):340–6. [PMC free article] [PubMed] [Google Scholar]
- 7.Mas-Coma S. Human fascioliasis: Epidemiological patterns in human endemic areas of South America, Africa and Asia. Southeast Asian J Trop Med Public Health. 2004;35:1–11. [Google Scholar]
- 8.Daumerie D, Savioli L. Working to overcome the global impact of neglected tropical diseases: First who report on neglected tropical diseases. World Health Organization; 2010. [Google Scholar]
- 9.Dowlati Y, Dowlati A, Seyyedi B. Cutaneous fascioliasis. Medical Journal of The Islamic Republic of Iran (MJIRI). 1987;1:62–65. [Google Scholar]
- 10.Hanjani A, Nikakhtar B, Arfaa F, et al. A case of infection with Fasciola hepatica with allergic manifestations. Acta Med Iranica. 1971:149–151. [Google Scholar]
- 11.Farid H. Human infection with Fasciola hepatica and Dicrocoelium dendriticum in Isfahan area, central Iran. J Parasitol. 1971;57. [Google Scholar]
- 12.Neshat H, Eslami A. The importance and incidence of food born helminth infections in man, in Iran. Iran J Public Health. 1976;5:56–61. [Google Scholar]
- 13.Moghaddam AS, Massoud J, Mahmoodi M, et al. Human and animal fascioliasis in Mazandaran Province, northern Iran. Parasitol Res. 2004; 94(1):61–9. [DOI] [PubMed] [Google Scholar]
- 14.Hatami H, Asmar M, Masoud J, et al. The first epidemic and new-emerging human fascioliasis in Kermanshah (western Iran) and a ten-year follow up, 1998–2008. Int J Prev Med. 3(4):266–72. [PMC free article] [PubMed] [Google Scholar]
- 15.Sarkari B, Ghobakhloo N, Moshfea AA, et al. Seroprevalence of human fasciolosis in a new-emerging focus of fasciolosis in Yasuj district, southwest of Iran. Iran J Parasitol. 2012; 7(2): 15–20. [PMC free article] [PubMed] [Google Scholar]
- 16.Ashrafi K. The status of human and animal fascioliasis in Iran: A narrative review article. Iran J Parasitol. 2015; 10(3): 306–328. [PMC free article] [PubMed] [Google Scholar]
- 17.Rokni MB, Massoud J, O’Neill SM, et al. Diagnosis of human fasciolosis in the gilan province of northern Iran: Application of cathepsin l-ELISA. Diagn Microbiol Infect Dis. 2002;44:175–179. [DOI] [PubMed] [Google Scholar]
- 18.Rahimi MT, Ashrafi K, Koosha S, et al. Evaluation of fast-elisa versus standard-ELISA to diagnose human fasciolosis. Arch Iran Med. 2011; 14(1):18–21. [PubMed] [Google Scholar]
- 19.Kheirandish Farnaz, Kayed Mohammad Hassan, Behrouz Ezatpour, et al. Seroprevalence of human fasciolosis in Pirabad, Lorestan Province, western. Iran J Parasitol. 2016;11(1): 24–29. [PMC free article] [PubMed] [Google Scholar]
- 20.Rokni MB. The present status of human helminthic diseases in Iran. Ann Trop Med Parasitol. 2008; 102(4):283–95. [DOI] [PubMed] [Google Scholar]
- 21.Esteban JG, Flores A, Angles R, et al. High endemicity of human fascioliasis between Lake Titicaca and La Paz Valley, Bolivia. Trans R Soc Trop Med Hyg. 1999; 93(2):151–6. [DOI] [PubMed] [Google Scholar]
- 22.Esteban J-g, Gonzalez C, Curtale F, et al. Hyperendemic fascioliasis associated with schistosomiasis in villages in the Nile Delta of Egypt. Am J Trop Med Hyg. 2003; 69(4):429–37. [PubMed] [Google Scholar]
- 23.Ashrafi K, Saadat F, O‟neill S. The Endemicity of Human Fascioliasis in Guilan Province, Northern Iran: the Baseline for Implementation of Control Strategies. Iran J Public Health. 2015; 44(4): 501–511. [PMC free article] [PubMed] [Google Scholar]
- 24.Periago MV, Valero MA, El Sayed M, et al. First phenotypic description of Fasciola hepatica/Fasciola gigantica intermediate forms from the human endemic area of the Nile Delta, Egypt. Infect Genet Evol. 2008; 8(1):51–8. [DOI] [PubMed] [Google Scholar]
- 25.Saberinasab M, Mohebali M, Molawi G, et al. Seroprevalence of human fascioliasis using indirect ELISA in Isfahan district, central Iran in 2013. Iran J Parasitol. 2014; 9(4):461–5. [PMC free article] [PubMed] [Google Scholar]
- 26.Asadian S, Mohebali M, Moudi M, et al. Seroprevalence of human fascioliasis in Meshkin-Shahr District, Ardabil Province, northwestern Iran in 2012. Iran J Parasitol. 2013; 8(4):516–21. [PMC free article] [PubMed] [Google Scholar]
- 27.Abdi J, Naserifar R, Rostami Nejad M, et al. New features of fascioliasis in human and animal infections in Ilam Province, Western Iran. Gastroenterol Hepatol Bed Bench. 2013; 6(3):152–5. [PMC free article] [PubMed] [Google Scholar]
- 28.Ashrafi K, Saadat F, O’Neill S, et al. The endemicity of human fascioliasis in Guilan Province, Northern Iran: The baseline for implementation of control strategies. Iran J Public Health. 2015; 44(4):501–11. [PMC free article] [PubMed] [Google Scholar]
- 29.Esteban JG, Flores A, Aguirre C, et al. Presence of very high prevalence and intensity of infection with Fasciola hepatica among aymara children from the northern Bolivian Altiplano. Acta Trop. 1997; 66(1):1–14. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan M, Kuk S, Kalkan A, et al. Fasciola hepatica seroprevalence in the Elazig Region. Mikrobiyol Bul. 2002; 36(3–4):337–42. [PubMed] [Google Scholar]
- 31.O’Neill SM, Parkinson M, Strauss W, et al. Immunodiagnosis of Fasciola hepatica infection (fascioliasis) in a human population in the Bolivian Altiplano using purified cathepsin l cysteine proteinase. Am J Trop Med Hyg. 1998; 58(4):417–23. [DOI] [PubMed] [Google Scholar]
- 32.Qureshi AW, Tanveer A, Qureshi S, et al. Epidemiology of human fasciolosis in rural areas of Lahore, Pakistan. Punjab Univ J Zool. 2005;20:159–168. [Google Scholar]
- 33.Zumaquero-Ríos JL, Sarracent-Pérez J, Rojas-García R, et al. Fascioliasis and intestinal parasitoses affecting schoolchildren in Atlixco, Puebla State, Mexico: Epidemiology and treatment with nitazoxanide. PLoS Negl Trop Dis. 2013; 7(11):e2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Assmar M, Milaninia A, Amirkhani A, et al. Seroepidemiological investigation of fascioliasis in northern Iran. Medical Journal of The Islamic Republic of Iran (MJIRI). 1991;5:23–27. [Google Scholar]
- 35.Forghan-Parast K, Yadegari D, Asmar M. Clinical epidemiology of human fascioliasis in Gilan. Journal of Gilan University of Medical Sciences. 1993;6:4–11. [Google Scholar]
- 36.Ashrafi K, Valero MA, Massoud J, et al. Plant-borne human contamination by fascioliasis. Am J Trop Med Hyg. 2006; 75(2):295–302. [PubMed] [Google Scholar]
- 37.Mas-Coma S, Bargues MD, Valero MA. Fascioliasis and other plant-borne trematode zoonoses. Int J Parasitol. 2005; 35(11–12):1255–78. [DOI] [PubMed] [Google Scholar]
- 38.Ashrafi K, MA Valero K, Forghan-Parast M, et al. Potential transmission of human fascioliasis through traditional local foods, in northern Iran. Iran J Public Health. 2006;35:57–63. [Google Scholar]
- 39.Salahi MA. Epidemiology of human fascioliasis in Iran. J Arch Military Med. 1(1): 6–12. 2009 [Google Scholar]
- 40.Martínez-Barbabosa I, Gutiérrez-Quiroz M, Romero-Cabello R, et al. Seroepidemiology of fascioliasis in school children in Mexico City. Rev Biomed. 2006;17 [Google Scholar]
- 41.Turhan O, Korkmaz M, Saba R, et al. Seroepidemiology of fascioliasis in the Antalya region and uselessness of eosinophil count as a surrogate marker and portable ultrasonography for epidemiological surveillance. Infez Med. 2006;14:208–212. [PubMed] [Google Scholar]
- 42.Esteban JG, González C, Bargues MD, et al. High fascioliasis infection in children linked to a man-made irrigation zone in Peru. Trop Med Int Health. 2002; 7(4):339–48. [DOI] [PubMed] [Google Scholar]