Abstract

Inhalable, noncombustible cannabis products are playing a central role in the expansion of the medical and recreational use of cannabis. In particular, the practice of “dabbing” with butane hash oil has emerged with great popularity in states that have legalized cannabis. Despite their growing popularity, the degradation product profiles of these new products have not been extensively investigated. The study herein focuses on the chemistry of myrcene and other common terpenes found in cannabis extracts. Methacrolein, benzene, and several other products of concern to human health were formed under the conditions that simulated real-world dabbing. The terpene degradation products observed are consistent with those reported in the atmospheric chemistry literature.
Introduction
Terpenes and terpenoids are present in such a wide diversity of environments (nature, food, cosmetics, pharmaceuticals, and drugs) that their consequences for inhalation toxicology cannot be ignored. Additionally, their inclusion in flavored electronic cigarettes1 and ubiquitous presence in inhalable cannabis products are of particular concern. The medicinal and psychoactive effects of cannabis have been proposed to be enhanced by terpenes, a phenomenon known as the “entourage effect”,2 and these relatively unsubstantiated assertions of benefits have led the cannabis industry to place a heavy emphasis on these aroma compounds.
Terpenoid degradation in the context of cannabis has not been extensively studied;3,4 however, it has attracted attention in the context of atmospheric chemistry.5,6 For instance, the reactions of terpenoids with O3 and NOx are well-known, but they are not directly applicable to e-cigarettes or inhalable cannabis products. However, these and other studies of pyrolysis and combustion of terpenoids should serve as a starting point toward understanding the reaction pathways in consumer vaporization devices. Despite the growing popularity of flavored e-cigarettes and terpene-enriched cannabis extracts, the chemical profiles of their terpene degradation products have not been evaluated in detail.
Of very recent concern is the practice of dabbing, which has emerged as a dangerous and rapidly growing trend in cannabis consumption. It consists of inhaling the vapors produced by placing a small amount of cannabis extract (a “dab”) on a small heated surface (the “nail”), which is connected to a water pipe.7 Its delivery of harmfully large amounts cannabinoids8,9 represents a potential danger to consumers, but little is known about the toxicants the process may produce.
The principal extract used in dabbing is butane hash oil (BHO). BHO is a resinous, nonpolar extract of the cannabis made using butane as a solvent.10 BHO has active ingredient (tetrahydrocannabinol (THC) or cannabidiol) contents ranging between 50 and 90%,8,11 with terpene content ranging from 0.1 to 34% (unpublished). Myrcene is unequivocally the most abundant terpene in cannabis, followed by limonene, linalool, pinene, caryophyllene, and humulene; however, the plant can contain up to 68 additional terpenic compounds in trace amounts.12 Additionally, some consumers increase the terpenoid content by dipping BHO in a vial of terpenes prior to use (“terp dipping”).13
BHO is made by passing butane over cannabis buds and leaves, and subsequently “purging” the butane from the product under vacuum at room temperature or in an oven. Different nuances in its processing can lead to slightly different consistencies, which take on terms such as shatter, budder, crumble, pull-and-snap, wax, and so on. In all of its forms, the extract is a sticky, resinous substance similar to the oleo-resins of other plants.14 Because the process does not involve heating the extract to the point that delta-9-tetrahydrocannabinolic acid (THCA, the native form of this substance found in the plant) decarboxylates (unpublished) into the active THC, BHO is not orally active and must be vaporized for the users to achieve its effects.15
BHO production started out as a dangerous “backyard-chemist” style operation that is famous for causing numerous explosions and house fires. Through the course of legalization, the production has steadily gained sophistication. The most modern, legal extraction laboratories live up to the OSHA standards with full ventilation and butane recovery. Modern techniques also include steps to “de-wax” the product by dissolving the crude BHO in isopropyl alcohol and chilling in a freezer, and, finally, filtering off the precipitated waxes in a process known as winterization. Many subtleties in its production exist, but many remain secretive due to the highly competitive nature of the cannabis marketplace and the general inability of extract producers to file patents due to the drug’s legal status at the federal level.
In addition to butane extraction, supercritical CO2 extraction has gained traction due to the fact that is does not leave any trace of hydrocarbon solvents in the end product.16 The cannabis extract made by this method, colloquially known as CO2 oil, has a lesser viscosity than BHO, a property that allows it to be used in vaporizer pens on its own with no cutting agents. The lesser viscosity is due to the fact that the supercritical extraction process requires the product to be first decarboxylated (heating in an oven at 100+ °C),17 leaving an extract consisting of all THC (an oil at room temperature) and no THCA (a solid at room temperature). CO2 oil is generally more expensive than BHO and mostly present on the market in prefilled vaporizer cartridges and not commonly as a standalone extract for dabbing. Because this extraction method does not leave residual hydrocarbons, it has been named, along with alcohol extracts, as the only allowable medical extracts to be sold under the medical cannabis regulations in New York,18 Minnesota, Ohio, and Pennsylvania.
According to a recent survey,11 the main reasons for using dabs are that less material is needed to get the desired effect and a “cleaner high.” Consumers consider dabbing to be a form of vaporization, and, therefore, view it as easier on the lungs than smoking.19 However, little information exists on the prevalence of dabbing. From 213 BHO extraction laboratories in the 17 states raided in 2014, 2015 saw a steep increase in the number of laboratories raided to 337 in 26 states.20 An analysis of the Twitter content related to dabs found a greater popularity in the states that have legalized recreational and/or medical cannabis.21
Different types of nails, the surface on which vaporization occurs, exist on the market. Use of an electrically controlled nail (“e-nail”) allows temperature control; but, more commonly, users heat the nail (made of titanium, ceramic, or quartz) with a crème brulee torch22 and have no temperature control. A minority of dabbers use lower temperatures to preserve flavor, whereas a majority use higher temperatures to assure complete vaporization with no wasted material. E-nail users posting online cite a preferred temperature around 710 °F (378 °C), but cite a range from 340–482 °C.23−25 Raber et al. reported a dabbing temperature of 300 °C, but this was only an (low) estimate. The boiling point of THC has recently been predicted to be ca. 417 °C,26 but vaporization can occur at temperatures lower than this by the use of a “carb cap” that reduces pressure on its surface during inhalation.27
This study is an initial effort toward assessing the safety of dabbing cannabis extracts. Due to the fact that these consist of a complex mixture, we have begun our focus on terpenoids, the component we predict to be the most thermally labile. To study dabbing, we carefully recreated the inhalation topography and temperatures employed by users. The study described herein is the first to investigate the degradation products from dabbing and is focused on the terpene fraction of the extracts used by consumers.
Results and Discussion
Sample Generation and Product Identification
We investigated the dabbing temperature ranges (TRs, Figure 1) inclusive of and beyond the ranges of those reported by the users. The vapor collection and analysis methods were based on those by Jensen et al.28 using an impinger filled with NMR solvent for vapor collection. In the dabbing simulation experiments herein, the vapor generated from the heated ceramic nail connected to a water pipe passed through a cold trap followed by the impinger. The impinger was, in turn, connected to a smoking machine that generated the airflow. Degradation products from myrcene, limonene, linalool, and Fire OG cannabis terpenes, a commercially available mix specifically fabricated for terp dipping, were monitored.11 The presence of methacrolein (MC) and benzene in vapor NMR samples was confirmed by spiking with authentic samples (Supporting Information). Their levels were quantified by NMR using an internal standard.
Figure 1.
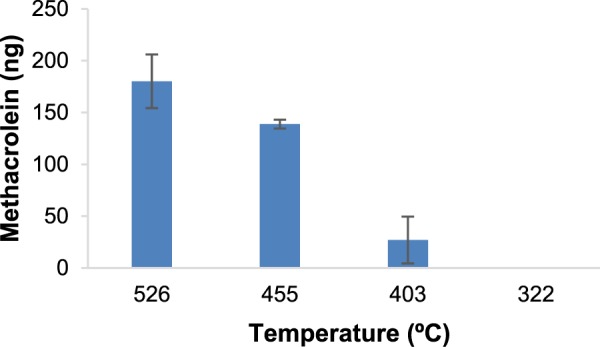
MC (ng) generated in a 40 mg dab using myrcene as a model terpene assuming a 5.9% concentration of terpenes in BHO. Temperature values represent the Tm for each TR. Error bars are determined at the 95% confidence level using the standard deviation of the three replicates taken at each TR. At the lowest TR, MC was not detected by NMR.
In addition to the NMR method, the dabbing vapor was collected using an adsorption/thermal desorption (ATD) cartridge and analyzed using an automated adsorption/thermal desorption–gas chromatography–mass spectrometry (ATD–GC–MS) method similar to that in Pankow et al.29 Additional product structures (Scheme 1) were assigned by the GC–MS analysis. Other minor products that have been previously described in the literature30 were also tentatively identified in the chromatographs (Supporting Information). Air blanks were collected and analyzed using each of the NMR and the ATD–GC–MS methods.
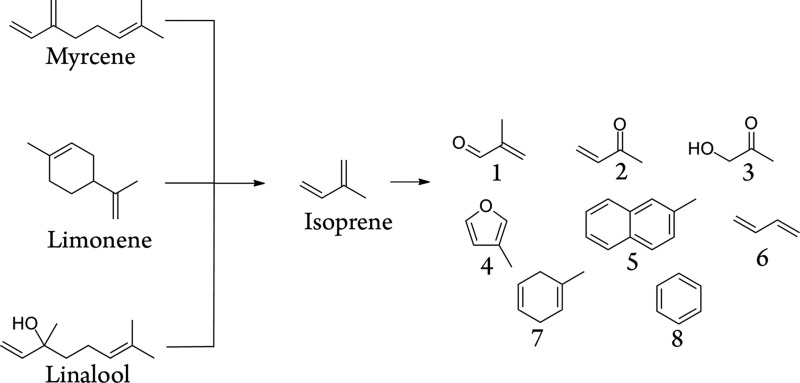
Scheme 1. Terpene Degradation Products Identified via the GC–MS Analysis.
1, Methacrolein; 2, methyl vinyl ketone; 3, hydroxyacetone; 4, 3-methylfuran; 5, 2-methylnapthalene; 6, 1,3-butadiene; 7, 1-methylcyclohexa-1,4-diene; 8, benzene. These and other related products were produced from pure samples of each of limonene, linalool, and myrcene.
Temperatures in dabbing experiments were carefully monitored for consistency using a thermographic camera. As the first drop in terpene touched the nail, an initial temperature (Ti) was recorded. Once a 10 s draw concluded, a final temperature (Tf) was recorded (the nail cooled between 50 and 30 °C during the draw due to convection). A median temperature (Tm) was calculated and averaged for each replicate to afford a representative Tm for each TR.
The 1H NMR spectra from the dabbing samples displayed peaks characteristic of a range of organic acid, aldehyde, and aromatic products. The two products appearing in high abundance in the spectra were the toxins benzene and MC (Scheme 1, Table 1). MC is a well-known degradation product of isoprene,5,31,32 which is itself a known degradation product of myrcene33 and other terpenes.34 Benzene, alkyl benzenes, and polycyclic aromatic hydrocarbons are known to form during terpene thermolysis. For example, benzene has been observed as a degradation product in the synthesis of myrcene by the pyrolysis of β-pinene,35 and it is also a product of solanesol pyrolysis.34 Benzene has also been detected in cannabis smoke.36
Table 1. Methacrolein (MC) and Benzene Levels Produced per mg Terpene Starting Material When Vaporized at the Highest Temperature Range Investigated, ca. 550 °C (Ti) −500 °C (Tf) Using Single Replicate Experiments.
| MC (ng/mg terpene) | benzene (ng/mg terpene) | |
|---|---|---|
| “Fire OG” | 127 | 10 |
| limonene | 261 | 63 |
| linalool | 103 | ND |
| myrcene | 81 | 60 |
Product Quantification
Given the wide diversity of the terpenes present in BHO, the relatively high abundance of myrcene and the similarity of the products from each of the terpenes studied (Table 1 and Scheme 1), we focused on myrcene as a model terpene in evaluating the effect of temperature on the yields of MC and benzene. Assuming 40 mg as an average size dab,22 each dab contains 2.36 mg of terpenes, which is based on an average concentration of terpenes of 5.9% in BHO (unpublished data). The amount of MC obtained per dab based on these calculations is displayed in Figure 1.
Because dabbing topography has not been previously investigated, we chose an inhalation volume of 338 mL and a 10 s duration to assure a more complete collection of vapor. The concentrations of MC in ppb per dab in this regime are 185 ± 11 ppb at Tm = 526 °C, 157 ± 2 ppb at Tm = 455 °C, 131 ± 9 ppb at Tm = 403 °C, and undetectable at Tm = 322 °C.
Benzene was not detected below the highest TR. Using the same rationale as above for MC emission, one dab of BHO delivers 17 ng of benzene. Represented as a concentration in the draw volume, this value is 15 ± 1.8 ppb.
Degradant Toxicology
MC’s property as a noxious irritant is unsurprising due to its structural similarity to acrolein, a powerful pulmonary irritant37 and an air pollutant of great concern. Ambient concentrations of MC outside of Stockholm were determined to be 0.06 ppb, whereas those at different urban locations in Stockholm were 0.11, 0.13, 0.19, and 0.71 ppb.38 MC’s effect on the respiratory tract in mice has shown it to be a potent irritant, indicating its threshold limit value should not exceed 0.3 ppm.39 Nøjgaard et al. reported changes in the blink frequency during eye exposure to MC at a concentration of 100 ppb and proposed a LOEL of 286 ppb.40 These conflicting reports indicate that the safe levels of MC are yet to be determined.
Unlike MC, the toxicology of benzene has been thoroughly evaluated. Although benzene is a ubiquitous pollutant, the concentrations of benzene found in the dabbing terpenes at the highest TR are far greater than those found in ambient air. The average concentration of benzene, a potent carcinogen, in U.S. air, measured over 137 different sites is 0.313 ppb (313 ppt),3,41 and is correspondingly the “largest single known cancer-risk air toxic (sic).”42
Degradant Formation Mechanism
We propose that the formation of MC and benzene occurs via isoprene as an intermediate (Scheme 1). The GC–MS spectra of limonene, linalool, and myrcene all displayed significant peaks tentatively assigned to isoprene, which suggests that these terpenes, the major terpenes in BHO, break down to their isoprene monomers before further degradation.
Studies of the atmospheric chemistry of isoprene have shown that it reacts with hydroxyl radicals and O2 to form not only MC and HCHO but also methyl vinyl ketone and 3-methylfuran. The GC–MS analysis of each pure terpene studied afforded a tentative identification with a high match quality of MC, methyl vinyl ketone, and 3-methylfuran, as well as 1,3-butadiene and several cyclic and acyclic dienes, polyenes, and aromatics (Scheme 1 and Supporting Information).
Limitations
The main limitation of this study is the fact that the concentrations of MC and benzene determined are likely underestimated. One reason may be the relatively large draw volume used. In addition, the temperature-dependent concentration values were extrapolated from myrcene, which afforded the lowest yield of degradation products of all of the terpenes investigated. Another factor potentially contributing to the underestimation of yields is transfer inefficiency resulting in the potential losses of terpenes and their products. For example, the average myrcene recovery (8.7 ± 0.7 mg) was low compared to the amount delivered onto the nail (59.6 mg). Although this low yield of terpenes in the NMR sample was initially attributed to their limited solubility in DMSO-d6, dabbing experiments using CDCl3 also had low yield by NMR. This may not be due entirely to degradation. Transfer inefficiency in dabbing has been previously described.22
Conclusions
Given the widespread legalization of cannabis in the United States, it is imperative to study the full toxicology of its consumption to guide future policy. The results of these studies clearly indicate that dabbing, although considered a form of vaporization, may in fact deliver significant amounts of toxic degradation products. The difficulty users find in controlling the nail temperature put users at risk of exposing themselves to not only methacrolein but also benzene. Additionally, the heavy focus on terpenes as additives seen as of late in the cannabis industry is of great concern due to the oxidative liability of these compounds when heated. This research also has significant implications for flavored e-cigarette products due to the extensive use of terpenes as flavorings. Future research will also be directed toward assessing the contribution of terpenoids to the existing toxicant formation in e-cigarettes. Additionally, the methods discussed herein will also be used to further study the degradation of cannabis extracts used in dabbing and cannabis e-cigarettes.
Methods
Materials
Terpenes included myrcene ≥95%, stabilized, FCC, FG (Sigma-Aldrich); (R)-(+)-limonene analytical standard (Sigma-Aldrich); linalool ≥97%, FCC, FG; and Fire OG terpene mix (Blue River Extracts).
NMR Experiments
Air is drawn at a constant rate using and the Single Cigarette Smoking Machine (SCSM-STEP, CH Technologies) calibrated to pull 338 mL air during a 10 s dab. A HIVE Domeless Element 10 mm ceramic nail (HIVE Ceramics) was attached to a small dab water pipe (Zion Cannabis in Portland, OR). For each separate experiment, the water pipe was filled with 20 mL of fresh 200 ppm solution of NaCl Biological, Certified Crystalline (Fisher Scientific) in HPLC grade water (Honeywell).
Terpene (15 μL) was delivered per dab using a Hamilton 50 μL analytical syringe. Five dabs were done per experiment. The vapor was collected through a cold trap chilled with isopropyl alcohol/dry ice at −77 °C, proceeded by an impinger containing 750 μL of DMSO-d6 + 0.05% v/v tetramethylsilane (99.9%, Cambridge Isotope). After the experiment was concluded, the cold trap was washed with the NMR solvent in the impinger and collected quantitatively using an Eppendorf P1000 pipette in an NMR tube. The water pipe and the cold trap were connected by 5 cm of 1/2 in. outer diameter ACF0027-F Tygon S3 E-3603. The end connected to the water pipe was wrapped in Teflon tape to make it fit snugly. The cold trap and the impinger were connected by 3.5 cm of 1/2 in. outer diameter ACF0027-F Tygon S3 E-3603. The impinger and the SCSM were connected by 5 cm of 3/8 in. outer diameter ACF0017-F Tygon S3 E-3603. The tubing was discarded after every experiment, so sorptive losses were consistent with every experiment.
All of the NMR samples were spiked with 10 μL of a 17.33 mM solution of 2,3,5,6-tetrachloronitrobenzene (TCI Chemicals) in DMSO-d6 using an Eppendorf P10 pipette. This standard solution was made by adding 11.23 mg of 2,3,5,6-tetrachloronitrobenzene to 3 mL of DMSO-d6.
Myrcene dab NMR experiments at each TR (Figure 1) were performed in triplicate. Terpene experiments shown in Table 1 were performed once each. The exact conditions used in recording the NMR spectra are presented in the SI.
ATD–GC–MS
The same water pipe (containing 20 mL 200 ppm solution of NaCl) and the same ceramic nail were connected to an ATD cartridge with 5 cm of 1/2 in. outer diameter ACF0027-F Tygon S3 E-3603 wrapped in the Teflon tape to make a seal and then attached to 5 cm of 3.5 cm of 3/8 in. outer diameter ACF0027-F Tygon S3 E-3603, also wrapped with Teflon tape on the end to assure an air-tight seal. The other end of the ATD cartridge was connected to the SCSM-STEP using 5 cm of 3.5 cm of 3/8 in. outer diameter ACF0027-F Tygon S3 E-3603. The ATD cartridges used contained 100 mg of 35/60 mesh Tenax TA and 200 mg of 60/80 mesh Carbograph 1 TD (Camsco Inc., Houston, TX). The same dabbing topography used in the NMR experiments were used in the ATD cartridge sample collections. This high flow rate exceeds that normally used for these cartridges, but this was allowed due to the fact that these experiments were only used for product identification and not quantification. The conditions used in the ATD cartridge analysis are explained in the SI.
Thermography
The temperature of the nail was acquired real time by the infrared thermography using an FLIR T450sc 2.0 (FLIR Systems). The emissivity of the ceramic nail was determined experimentally to be 0.9 by comparing it to the known value of insulating electrical tape, 0.97. The reflected temperature was determined by the reflector method to be 23 °C. The TRs used in the NMR experiments are shown in Figure 1, and the TR used in the ATD–GC–MS experiment was the second hottest TR with a Tm of 455 °C. All of the temperature data for each experiment are presented in the SI.
Acknowledgments
Dr. Jorge Escobedo provided the guidance and useful discussion in the experiments and during manuscript drafting. Dr. David Peyton provided the guidance and useful discussion in the NMR methods used. Tim Frasca reviewed the manuscript for content and style.
Glossary
ABBREVIATIONS
- MC
methacrolein
- THC
tetrahydrocannabinol
- CBD
cannabidiol
- BHO
butane hash oil
- TR
temperature range
- HCHO
formaldehyde
- TLV
threshold limit value
- LOEL
lowest observed effect level
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b01130.
Experimental conditions, materials, and characterization data for MC and benzene (PDF)
Author Contributions
J.M.-A. performed all of the experiments, collected sample, and wrote the manuscript. R.M.S. supervised the studies and edited the manuscript. W.L. ran the ATD–GC–MS samples, advised on sample collection by this method, and reviewed the manuscript. All of the authors have given approval to the final version of the manuscript.
We thank the NIH and FDA for support of this work via award R01ES025257. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or the FDA.
The authors declare no competing financial interest.
Supplementary Material
References
- Tierney P. A.; Karpinski C. D.; Brown J. E.; Luo W.; Pankow J. F. Flavour chemicals in electronic cigarette fluids. Tob. Control 2016, 25, e10–e15. 10.1136/tobaccocontrol-2014-052175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo E. B. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. 10.1111/j.1476-5381.2011.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Monte D. S.; Tenório J. A. B.; Bastos I. V. G. A.; Mendonça F. d. S.; Neto J. E.; da Silva T. G.; Ramos C. S. Chemical and biological studies of beta-carotene after exposure to Cannabis sativa smoke. Toxicol. Rep. 2016, 3, 516–522. 10.1016/j.toxrep.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal M. S.; Khan M. U. A.; Akbar J.; Shad M. A.; Masih R.; Chaudhary M. T. Isoconversional thermal and pyrolytic GC-MS analysis of street samples of hashish. J. Anal. Appl. Pyrolysis 2016, 122, 175–182. 10.1016/j.jaap.2016.09.026. [DOI] [Google Scholar]
- Atkinson R. Gas Phase Tropospheric Chemistry of Organic Compounds: A Review. Atmos. Environ., Part A 1989, 24, 1–41. 10.1016/0960-1686(90)90438-S. [DOI] [Google Scholar]
- Calogirou A.; Larsen B. R.; Kotzias D. Gas-phase terpene oxidation products: a review. Atmos. Environ. 1999, 33, 1423–1439. 10.1016/S1352-2310(98)00277-5. [DOI] [Google Scholar]
- Stogner J. M.; Miller B. L. The Dabbing Dilemma: A Call for Research on Butane Hash Oil and Other Alternate Forms of Cannabis Use. Subst. Abuse 2015, 36, 393–395. 10.1080/08897077.2015.1071724. [DOI] [PubMed] [Google Scholar]
- Pierre J. M.; Gandal M.; Son M. Cannabis-induced psychosis associated with high potency “wax dabs”. Schizophr. Res. 2016, 172, 211–212. 10.1016/j.schres.2016.01.056. [DOI] [PubMed] [Google Scholar]
- Murray R. M.; Quigley H.; Quattrone D.; Englund A.; Di Forti M. Traditional marijuana, high-potency cannabis and synthetic cannabinoids: increasing risk for psychosis. World Psychiatry 2016, 15, 195–204. 10.1002/wps.20341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stogner J. M.; Miller B. L. Assessing the Dangers of “Dabbing”: Mere Marijuana or Harmful New Trend?. Pediatrics 2015, 136, 1–3. 10.1542/peds.2015-0454. [DOI] [PubMed] [Google Scholar]
- Loflin M.; Earleywine M. A new method of cannabis ingestion: the dangers of dabs?. Addict. Behav. 2014, 39, 1430–1433. 10.1016/j.addbeh.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Ross S. A.; ElSohly M. A. The volatile oil composition of fresh and air-dried buds of Cannabis sativa. J. Nat. Prod. 1996, 59, 49–51. 10.1021/np960004a. [DOI] [PubMed] [Google Scholar]
- danner1620 Terp Dippin’ Kosher Kush Rosin with Blue River Extracts [video file]. https://www.youtube.com/watch?v=YOFkbcMo-so (accessed July 7, 2017).
- Stogner J. M.; Miller B. L. The Dabbing Dilemma: A Call for Research on Butane Hash Oil and Other Alternate Forms of Cannabis Use. Subst. Abuse 2015, 36, 393–395. 10.1080/08897077.2015.1071724. [DOI] [PubMed] [Google Scholar]
- ElSohly M. A.Marijuana and the Cannabinoids, 1st ed.; Humana Press: NJ, 2007. [Google Scholar]
- Perrotin-Brunel H.; Perez P. C.; van Roosmalen M. J. E.; van Spronsen J.; Witkamp G. J.; Peters C. J. Solubility of Delta(9)-tetrahydrocannabinol in supercritical carbon dioxide: Experiments and modeling. J. Supercrit. Fluids 2010, 52, 6–10. 10.1016/j.supflu.2009.12.001. [DOI] [Google Scholar]
- Perrotin-Brunel H.; van Roosmalen M. J. E.; van Spronsen J.; Verpoorte R.; Peters C. J.; Witkamp G. J. Supercritical fluid extraction of cannabis: experiments and modelling of the process design. ISASF-Graz 2010, 1–6. [Google Scholar]
- Manufacturing Requirements for Approved Medical Marihuana Products. Official Compilation of Codes, Rules and Regulations of the State of New York, Part 1004, Section 11(b), Title 10, 2017.
- Gieringer D.; St. Laurent J.; Goodrich S. Cannabis Vaporizer Combines Efficient Delivery of THC with Effective Suppression of Pyrolytic Compounds. J. Cannabis Ther. 2004, 4, 7–27. 10.1300/J175v04n01_02. [DOI] [Google Scholar]
- 2015 National Drug Threat Assessment Summary; Report Number DEA-DCT-DIR008-16; US Department of Justice, United States Drug Enforcement Agency; Homeland Security Digital Library, 2015.
- Daniulaityte R.; Nahhas R. W.; Wijeratne S.; Carlson R. G.; Lamy F. R.; Martins S. S.; Boyer E. W.; Smith G. A.; Sheth A. “Time for dabs”: Analyzing Twitter data on marijuana concentrates across the U.S. Drug Alcohol Depend. 2015, 155, 307–311. 10.1016/j.drugalcdep.2015.07.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J. C.; Elzinga S.; Kaplan C. Understanding dabs: contamination concerns of cannabis concentrates and cannabinoid transfer during the act of dabbing. J. Toxicol. Sci. 2015, 40, 797–803. 10.2131/jts.40.797. [DOI] [PubMed] [Google Scholar]
- Burns D. What’s Your Preferred Enail Temp? https://www.reddit.com/r/CannabisExtracts/comments/30mj24/whats_your_preferred_enail_temp/ (accessed July 18, 2017).
- MFletcher I. Is There a Consensus Temp for Low Temps Dabs on an Enail? https://www.reddit.com/r/enail/comments/4gjznx/is_there_a_consensus_temp_for_low_temp_dabs_on_an/ (accessed July 18, 2017).
- Propanekush What Temperature are you Dabbing at? What Type of Nail? https://www.reddit.com/r/CannabisExtracts/comments/3onqt8/what_temperature_are_you_dabbing_at_what_type_of/ (accessed July 18, 2017).
- Tara M.; Lovestead T. J. B. Determination of Cannabinoid Vapor Pressures to Aid in Vapor Phase Detection of Intoxication. Forensic Chem. 2017, 5, 79–85. 10.1016/j.forc.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black S. Why Carb Cap? Elevate Your Dabbing. http://hightimes.com/culture/why-carb-cap-elevate-your-dabbing/ (accessed July 18, 2016).
- Jensen R. P.; Strongin R. M.; Peyton D. H. Solvent Chemistry in the Electronic Cigarette Reaction Vessel. Sci. Rep. 2017, 7, 42549 10.1038/srep42549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankow J. F.; Kim K.; McWhirter K. J.; Luo W.; Escobedo J. O.; Strongin R. M.; Duell A. K.; Peyton D. H. Benzene formation in electronic cigarettes. PLoS One 2017, 12, e0173055 10.1371/journal.pone.0173055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw G. W.; Hemingway R. W.; Ingram L. L.; Canady C. S.; McGraw W. B. Thermal degradation of terpenes: Camphene, Delta(3)-carene, limonene, and alpha-terpinene. Environ. Sci. Technol. 1999, 33, 4029–4033. 10.1021/es9810641. [DOI] [Google Scholar]
- Carter W. P. L. Condensed atmospheric photooxidation mechanisms for isoprene. Atmos. Environ. 1996, 30, 4275–4290. 10.1016/1352-2310(96)00088-X. [DOI] [Google Scholar]
- Palen E. J.; Allen D. T.; Pandis S. N.; Paulson S. E.; Seinfeld J. H.; Flagan R. C. Fourier Transform Infrared Analysis of Aerosol Formed in the Photo-Oxidation of Isoprene and [beta]-pinene. Atmos. Environ., Part A 1992, 26, 1239–1251. 10.1016/0960-1686(92)90385-X. [DOI] [Google Scholar]
- Egloff G.; Herrman M.; Levinson B. L.; Dull M. F. Thermal reactions of terpene hydrocarbons. Chem. Rev. 1934, 14, 287–383. 10.1021/cr60049a001. [DOI] [Google Scholar]
- Britt P. F.; Buchanan A. C. III; Owens C. V. Jr. Mechanistic Investigation into the Formation of Polycyclic Aromatic Hydrocarbons from the Pyrolysis of Terpenes. Prepr. Pap.-Am. Chem. Soc., Div. Fuel Chem. 2004, 49, 868–871. [Google Scholar]
- Kolicheski M. B.; Cocco L. C.; Mitchell D. A.; Kaminske M. Synthesis of myrcene by pyrolysis of [beta]-pinene: Analysis of decomposition reactions. J. Anal. Appl. Pyrolysis 2007, 80, 92–100. 10.1016/j.jaap.2007.01.005. [DOI] [Google Scholar]
- Moir D.; Rickert W. S.; Levasseur G.; Larose Y.; Maertens R.; White P.; Desjardins S. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem. Res. Toxicol. 2008, 21, 494–502. 10.1021/tx700275p. [DOI] [PubMed] [Google Scholar]
- Wang H. T.; Hu Y.; Tong D.; Huang J.; Gu L.; Wu X. R.; Chung F. L.; Li G. M.; Tang M. S. Effect of carcinogenic acrolein on DNA repair and mutagenic susceptibility. J. Biol. Chem. 2012, 287, 12379–12386. 10.1074/jbc.M111.329623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson A.; Persson K. A.; Grigoriadis V. Measurements of Some Low Molecular-Weight Oxygenated, Aromatic, and Chlorinated Hydrocarbons in Ambient Air and in Vehicle Emissions. Environ. Int. 1985, 11, 383–392. 10.1016/0160-4120(85)90033-9. [DOI] [Google Scholar]
- Larsen S. T.; Nielsen G. D. Effects of methacrolein on the respiratory tract in mice. Toxicol. Lett. 2000, 114, 197–202. 10.1016/S0378-4274(99)00300-8. [DOI] [PubMed] [Google Scholar]
- Nøjgaard J. K.; Christensen K. B.; Wolkoff P. The effect on human eye blink frequency of exposure to limonene oxidation products and methacrolein. Toxicol. Lett. 2005, 156, 241–251. 10.1016/j.toxlet.2004.11.013. [DOI] [PubMed] [Google Scholar]
- EPA’s Report on the Environment; Ambient Concentrations of Selected Air Toxics. Exhibit 3; US Environmental Protection Agency, 2014. [Google Scholar]
- George B. J.; Schultz B. D.; Palma T.; Vette A. F.; Whitaker D. A.; Williams R. W. An evaluation of EPA’s National-Scale Air Toxics Assessment (NATA): Comparison with benzene measurements in Detroit, Michigan. Atmos. Environ. 2011, 45, 3301–3308. 10.1016/j.atmosenv.2011.03.031. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.