Abstract
Gout is a disease with elusive treatment options. Reduction of the size of l-alanine crystals as a model crystal for gouty tophi with the use of a monomode solid-state microwave was examined as a possible therapeutic aid. The effect of microwave heating on l-alanine crystals in the presence of gold nanoparticles (Au NPs) in solution and synovial fluid (SF) in a plastic pouch through a synthetic skin patch was investigated. In this regard, three experimental paradigms were employed: Paradigm 1 includes the effect of variable microwave power (5–10 W) and variable heating time (5–60 s) and Au NPs in water (20 nm size, volume of 10 μL) in a plastic pouch (1 × 2 cm2 in size). Paradigm 2 includes the effect of a variable volume of 20 nm Au NPs in a variable volume of SF up to 100 μL in a plastic pouch at a constant microwave power (10 W) for 30 s. Paradigm 3 includes the effect of constant microwave power (10 W) and microwave heating time (30 s), constant volume of Au NPs (100 μL), and variable size of Au NPs (20–200 nm) placed in a plastic pouch through a synthetic skin patch. In these experiments, an average of 60–100% reduction in the size of an l-alanine crystal (initial size = 450 μm) without damage to the synthetic skin or increasing the temperature of the samples beyond the physiological range was reported.
Introduction
The incidence of hyperuricemia (serum urate levels >7.0 mg/dL for men; >5.7 mg/dL for women), the predecessor of gout, is increasing in the US1−3 and at epidemic proportions worldwide.4,5 Genetics, aging population, several disease states (e.g., hypertension, hypothyroidism, insulin resistance, and renal insufficiency), and lifestyle factors (e.g., appalling increases in morbid obesity, a purine-rich diet, extensive use of thiazide diuretics and low-dose aspirin therapy, and increased consumption of alcohol) contribute to the increased prevalence of hyperuricemia and gout.6,7 Gout, caused by a purine metabolism disorder, is an ancient crystal-induced form of inflammatory arthritis.2,5,8 Uric acid, the final product of purine catabolism, is excreted from the body in urine.9 Ninety-eight percentage of uric acid circulates in plasma and synovial fluid (SF) as the mono-deprotonated ionic form, urate3 [monosodium urate monohydrate (MSUM)]. Deposition of needlelike MSUM crystals triggers an inflammatory immune response in the joint,10 and persistent MSUM deposition can develop into chronic tophaceous gout.
Currently, acute gout is managed by nonsteroidal anti-inflammatory drugs (NSAIDs), colchicine and corticosteroids. NSAIDs are known to have numerous side effects.11−13 In chronic gout patients, invasive tophi removal surgery is advised, which can lead to joint infection and/or permanent joint damage or in some cases can lead to amputation.14,15 To date, decisions to use various gout therapies have been based on the efficacies and adverse effect profiles of these drugs and the patient’s ability to tolerate the prescribed doses. With the increased incidence of and the long-term medical implications and economic burden of treatment failure, chronic arthritis, and patients in whom standard gout therapies are prohibited, it is imperative that novel gout treatments to be developed.
The Aslan Research Group has recently introduced and demonstrated a noninvasive, nonpharmaceutical, relatively inexpensive technique called metal-assisted and microwave-accelerated decrystallization (MAMAD),16 which is based on the combined use of gold nanoparticles (Au NPs) and low-power microwave heating and has the potential to be an alternative treatment for gout during and after the formation of tophi in humans. Subsequently, it is important to briefly mention the previous uses of microwaves, synthetic skin, and Au NPs in biomedical applications.
Microwaves are used as therapeutic agents (diathermy, hyperthermia therapy, and radiofrequency lesioning and ablation) to diagnose and treat cancer and in various other diagnostic procedures.17,18 Deep-penetrating lower frequency microwaves (915 MHz and 2.45 GHz) have been used for ablation of tumors in the prostate, liver, and lungs and coagulation of blood in the liver and spleen. Shallow-penetrating higher frequency microwaves (5.8–10 GHz) have been used to ablate cardiac arrhythmias, corneas, damaged spinal nerves, metastatic livers, plantar warts, skin cancers, uterine fibroids, and varicose veins.17−20 Microwave energy is transformed into thermal energy upon exposure of dielectric substances (i.e., weak electricity conductors and strong electrostatic field supporters with induced or inherent dipoles) to certain frequencies of microwave irradiation.21 Whether microwave heating will induce thermal skin damage depends on the microwave exposure time, power, and frequency and the absorption rate, sensitivity, and hydration level of the irradiated tissue.22,23 Microwave heating of the skin, a laminar tissue in which the outer layer (stratum corneum) is considerably less hydrated than the deeper granular tissues, induces continuous polar molecule realignment in an alternating electromagnetic field (i.e., molecular dipole rotation).22 As the current in the electromagnetic field alternates, the molecules continuously reverse direction. The continuous rotation of polar molecules and their collision with the nearby molecules causes kinetic energy to be transformed into thermal energy.24−26 Where normal and damaged skin falls on the dielectric spectrum depends on the degree to which the stratum corneum is hydrated, which in turn affects the depth of penetration of microwaves into the tissue.27 Synovial joints and surrounding soft tissues are more hydrated than the skin and therefore have greater dielectric properties. In patients with chronic tophaceous gout, the dielectric properties of the skin, synovial joints, and tissues necessitate the use of appropriate levels of microwave power to penetrate these tissues and decrystallize joint-embedded crystal aggregates. Therefore, before using microwave heating and Au NPs in gout patients, the extent of microwave heating-induced tissue damage (if any) must be determined using synthetic skin patches to simulate human skin.
Synthetic skin patches used in our experiments to simulate adult human skin were validated for dielectric constant, thermal conductivity, puncture pressure, and so forth under similar physical conditions as the human tissue it was designed to mimic.28 The puncture pressure (2 N/mm2) and the realistic texture and consistency of the synthetic skin are similar to those of the human skin, which has a puncture pressure of 2.5 ± 0.3 N/mm2.29 As determined by SynDaver Labs, there are striking similarities between the synthetic skin used in these experiments and the human skin, resulting in a human skin analog that responds to various stimuli in a manner similar to the actual skin. In these experiments, synthetic skin patches were used to determine the extent to which microwave heating induced tissue damage and whether the l-alanine crystals could be decrystallized in the presence of Au NPs through a synthetic skin patch using our MAMAD technique.
Au NPs, which have a myriad of biomedical applications, have unique physical, chemical, and electronic properties that enable multimodal, site-specific therapeutic delivery, and their nanometer size confers the ability to permeate cellular membranes and interact with biomolecules.30,31 The MAMAD technique is based on the combined use of Au NPs that are dispersed within an aqueous solution and low-power microwave heating, where a microwave-induced temperature gradient is created between the cooler Au NPs and the warmer microwave-heated solution. Decrystallization of target crystals is based on the microwave heating-induced temperature gradient and the time, frequency, and power of microwave heating. The unique feature of the combined use of Au NPs in solution and microwave heating for the potential treatment of gout is the absorption and reflection of electromagnetic energy and the subsequent conversion to kinetic energy when Au NPs are exposed to microwave heating. The microwave heating-induced increase in the kinetic energy of Au NPs causes them to collide with the adjacent target crystals in solution, effectively turning the Au NPs into “nanobullets,” which shatter and eventually decrease the surface area of the target crystals.32
The purpose of this study was to investigate the effect of microwave heating (using a medically approved microwave source) of target crystals (i.e., l-alanine as a model crystal for tophi) in the presence of Au NPs in a plastic pouch (a model joint capsule) through a synthetic skin patch (a model for human skin) using three different experimental paradigms. A monomode, solid-state microwave source operating at 8 GHz and a variable power up to 20 W and an applicator that focuses the microwave energy to a ∼5 mm circular area were employed. First, we used a plastic pouch with two different microwave powers and various microwave time intervals in the presence of a constant size and volume of Au NPs (paradigm 1). Second, we employed a plastic pouch with microwave power at 10 W and a 30 s time interval and a mixture of different volumes of Au NPs and SF (paradigm 2). Finally, we utilized a plastic pouch under a synthetic skin patch with microwave power at 10 W and a 30 s time interval and 30 μL volume of different sizes of Au NPs (paradigm 3). In these experiments, we sought to determine whether an 8 GHz microwave applicator (at 5 or 10 W) could decrystallize a large (up to ∼450 μm in size and weighing ∼0.10 mg) l-alanine crystal in a plastic pouch containing Au NPs with or without SF and with or without a synthetic skin patch. These experiments using simulated human skin over l-alanine crystals are important for future applications of our MAMAD technique for the treatment of patients with tophaceousgout. To determine whether the change in the temperature after microwave heating of the plastic pouch and synthetic skin patch would exceed the physiological range and/or cause tissue damage, we assessed the microwave heating-induced temperature changes via an infrared thermometer and the synthetic skin damage qualitatively and quantitatively via real-time temperature measurements and optical microscopy.
Results and Discussion
In this study, three different experimental paradigms were used to decrystallize l-alanine crystals via the MAMAD technique, as shown in Figure 1. In experimental paradigm 1, a plastic pouch with two different microwave powers (5 or 10 W), various microwave time intervals (5, 10, 20, 30, 40, or 60 s), and Au NPs of size 20 nm and volume 10 μL were used. Experimental paradigm 2 employed a plastic pouch with a constant microwave power (10 W) and time interval (30 s) and varying volumes of Au NPs (10, 50, or 100 μL) and SF (10, 50, or 100 μL). Experimental paradigm 3 utilized a plastic pouch and synthetic skin patch with a constant microwave power (10 W) and time interval (30 s) and a constant Au NP volume (30 μL) with varying sizes of Au NPs (20, 200, or 200 nm). In addition, control experiments related to all experimental paradigms in this study were carried out under identical conditions (except microwave heating) at room temperature. Because the long-term goal of this research project is to determine whether the MAMAD technique using low-power microwave-accelerated Au NPs can be used to decrystallize MSUM deposits in patients suffering from gout, the experiments detailed in this paper were undertaken. We sought to determine to what extent, if any, low-power microwave heating would induce surface area changes in the synthetic skin patches (i.e., cause tissue damage). In addition, it is important to determine whether the temperature increases after microwave heating were within the physiological range (36–38 °C).
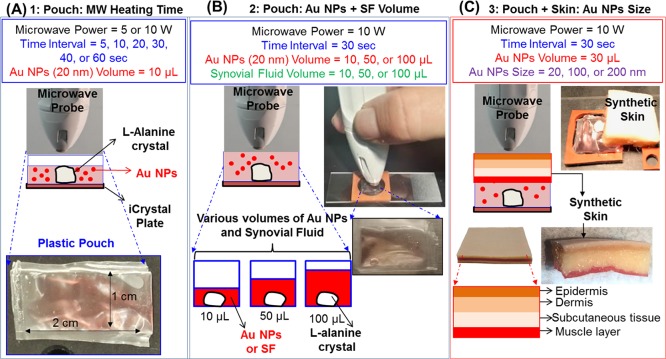
Figure 1.
Schematic representation of the three paradigms used in the decrystallization of l-alanine crystals on iCrystal plates via the MAMAD technique. (A) Paradigm 1: within a plastic pouch (no synthetic skin patch), the microwave power (5 or 10 W) and time intervals (5, 10, 20, 30, 40, or 60 s) were changed, the size (20 nm) and volume (10 μL) of Au NPs were kept constant, and no SF was used. (B) Paradigm 2: within a plastic pouch (no synthetic skin patch), the microwave power (10 W), time interval (30 s), and Au NP size (20 nm) were kept constant, and the volumes (10, 50, or 100 μL) of Au NPs and SF (10, 50, or 100 μL) were changed (C) Paradigm 3: within a plastic pouch through the synthetic skin patch, the microwave power (10 W), time interval (30 s), and volume (30 μL) of Au NPs were kept constant, and the size (20, 100, or 200 nm) of Au NPs was changed.
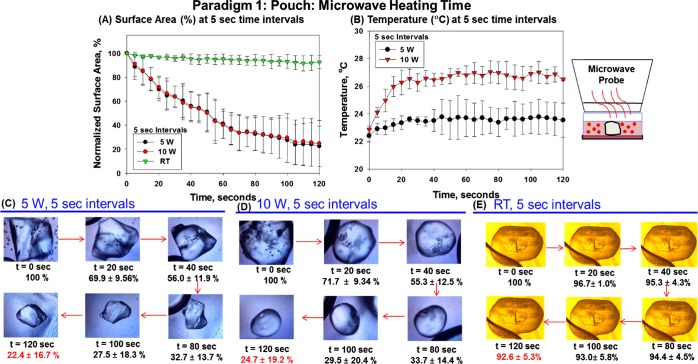
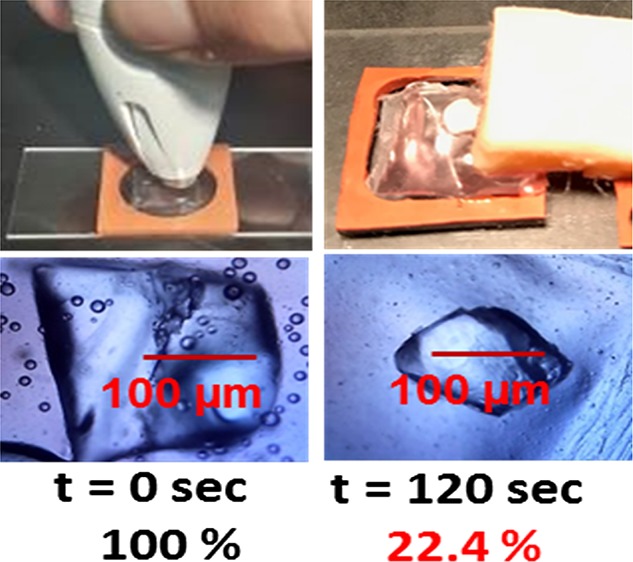
Paradigm 1 was used to determine the change in the surface area of l-alanine crystals and the final temperature of the plastic pouch after 120 s (at 5 s time intervals) of microwave heating at 5 or 10 W. Figure 2 shows the graphs of the percentage change in the surface area of l-alanine crystals in a plastic pouch (A) and the final temperature of the pouch (B) after 120 s (at 5 s time intervals) of microwave heating at 5 or 10 W. Optical images of l-alanine crystals during microwave heating show a decrease in the l-alanine crystal surface area from 100% at t = 0 s to 22.4 ± 16.7% at t = 120 s at 5 W (C), a decrease in the surface area from 100% at t = 0 s to 24.7 ± 19.2% at t = 120 s at 10 W (D), and a decrease in the surface area from 100% at t = 0 s to 92.6 ± 5.3% at t = 120 s at room temperature. At 5 and 10 W, there were no significant changes in the temperature measured at 120 s of microwave heating compared to 0 s. Because microwave heating at 10 W caused no significant plastic pouch temperature increases, we opted to use microwave heating at 10 W for the reminder of our experiments to ensure sufficient microwave power to penetrate the synthetic skin patches. These data suggest that low-power microwave-induced temperature increases were within the physiological range. It is important to note that similar results were obtained with respect to the percentage change in the crystal surface area and initial versus final pouch temperatures with microwave heating when 10, 20, 30, 40, and 60 s intervals of microwave heating at 5 or 10 W were examined (Figures S1–S5, Supporting Information).
Figure 2.
Paradigm 1: pouch: microwave heating time. (A) Percentage change in the surface area (%) of l-alanine crystals in a plastic pouch for 120 s (at 5 s time intervals) of microwave heating at 5 or 10 W. (B) Temperature of the plastic pouch for 120 s (at 5 s time intervals) of microwave heating at 5 or 10 W. Optical images of l-alanine crystals during microwave heating at 0, 20, 40, 80, 100, and 120 s (C) at 5 W, (D) at 10 W, and (E) at room temperature.
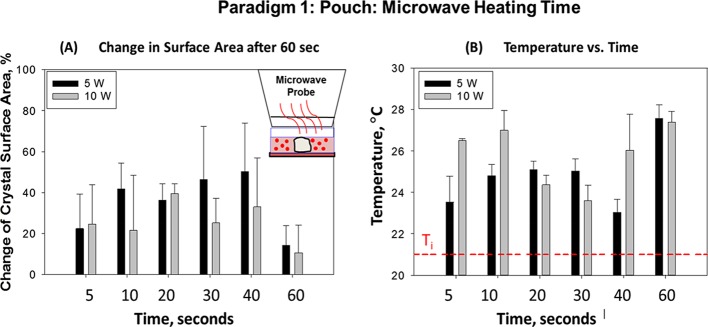
Figure 3 shows the bar graph summary of data related to the time-dependent change in the surface area of l-alanine crystals and temperature collected from three different paradigm 1 experimental trials. The percentage change in the surface area of l-alanine crystals in a plastic pouch after microwave heating at different time intervals (5, 10, 20, 30, 40, and 60 s) (A) and the final temperature of the plastic pouch after 60 s of microwave heating at 5 or 10 W (B) are shown. There was no significant change in the surface area of the l-alanine crystals after 60 s of microwave heating at 5 or 10 W. At no time point studied did the microwave heating-induced increase in the pouch temperature exceed the physiological range. The final temperature of the pouch after 60 s of microwave heating was not significantly different (∼6–7 °C) from the initial temperature.
Figure 3.
Paradigm 1: pouch: microwave heating time: summary of data collected from three different experimental trials. (A) Percentage change in the surface area (%) of l-alanine crystals in a plastic pouch after microwave heating at different time intervals (5, 10, 20, 30, 40, and 60 s) at 5 or 10 W. (B) Temperature of the plastic pouch after 120 s of microwave heating at 5 or 10 W. Ti = initial temperature of the plastic pouch before the commencement of microwave heating. Data are presented as the mean ± standard deviation of the three different experiments.
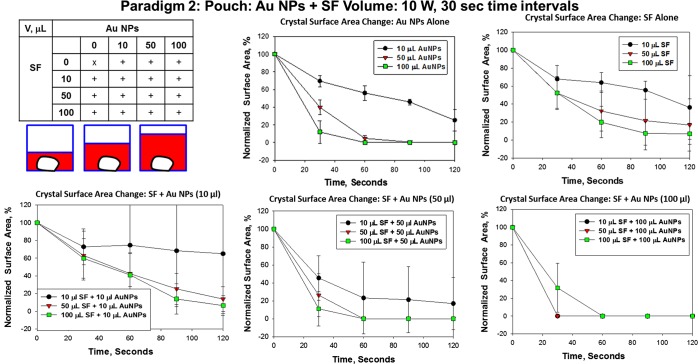
Figure 4 shows the summary graphs of data collected from the three different paradigm 2 experimental trials used to determine the most effective volumes of Au NPs and SF to decrystallize l-alanine crystals in a plastic pouch during 120 s (at 30 s time intervals) of microwave heating at 10 W. In the absence of SF, the smallest volume (10 μL) of Au NPs resulted in only a decrease of 30% in the crystal size. The 30 s time point was chosen as the minimum microwave heating time required to induce the surface area and pouch temperature changes. The following comparisons were made at the 30 s time point: In the presence of Au NPs at the medium volume (50 μL), the crystal size was reduced by 60%. The largest volume (100 μL) of Au NPs resulted in a 90% decrease in the crystal size. In the absence of Au NPs, SF (10 μL) resulted in a reduction of the crystal size by only 30%, and both the 50 and 100 μL volumes decreased the crystal size by 50% as compared to Au NPs (100 μL) in the absence of SF, which decreased the crystal size by 90%. A mixture of 10 μL of SF and 10 μL of Au NPs resulted in only a 30% reduction in the crystal size. In the presence of the smallest volume (10 μL) of Au NPs, both the 50 and 100 μL volumes of SF resulted in a decrease in the crystal size by 50%. A mixture of 10 μL of SF and 50 μL of Au NPs resulted in a 50% reduction in the crystal size, whereas 50 μL of SF mixed with 50 μL of Au NPs decreased the crystal size by 70%. SF (100 μL) in the presence of Au NPs (50 μL) resulted in a 90% reduction in the crystal size. SF (10 or 50 μL) in the presence of Au NPs (100 μL) resulted in a 100% reduction in the crystal size, whereas SF (100 μL) in the presence of Au NPs (100 μL) resulted in a 70% reduction of the crystal size, which is not statistically significant. These data demonstrate that the presence of Au NPs with or without SF resulted in a decrease in the l-alanine crystal size. The control samples showed insignificant changes in the absence of microwave heating when compared to experimental samples. The average reduction in the surface area of an l-alanine crystal in 10, 50, and 100 μL of Au NPs at room temperature was 6.6, 17.5, and 26.3%, respectively, and l-alanine crystal submerged in 10, 50, and 100 μL of SF only had a reduction of 0.3, 4.4, and 6.7%, respectively. In the initial mixture of Au NPs (10 μL) and SF (10, 50, and 100 μL), the surface area reduction of the l-alanine crystal was 19.7, 13.2 and 12.7%, and in the second mixture of Au NPs (50 μL) and SF (10, 50, and 100 μL), the surface area reduction of the l-alanine crystal was 17.1, 19.4, and 23.5%, and in the final mixture of Au NPs (100 μL) and SF (10, 50, and 100 μL), the average reduction in the surface area of l-alanine crystal was 9.5, 24.5, and 17.7%, respectively. The final temperature of the plastic pouch containing various mixtures of Au NPs (10, 50, and 100 μL) and SF (10, 50, and 100 μL) during 120 s (at 30 s time intervals) of microwave heating at 10 W was similar to the initial pouch temperature. (Figures S6 and S9, Supporting Information). There were no significant changes in the temperature measured after 120 s (at 30 s time intervals) of microwave heating at 10 W compared to 0 s in any of the mixtures of Au NPs and SF tested. Control experiments were performed at room temperature.
Figure 4.
Paradigm 2: pouch: Au NPs + SF volume (10 W, 30 s time intervals). Summary of data collected from three different paradigm 2 experiments. Percentage change in the surface area (%) of l-alanine crystals in a plastic pouch during 120 s (at 30 s time intervals) of microwave heating at 10 W. Data are presented as the mean ± standard deviation of the three different experiments. Table shows the 15 experimental groups used in this study: 1–3 Au NPs (10, 50, or 100 μL) alone, 4–6 SF (10, 50, or 100 μL) alone, and 7–15 SF (10, 50, or 100 μL) + Au NPs (10, 50, or 100 μL).
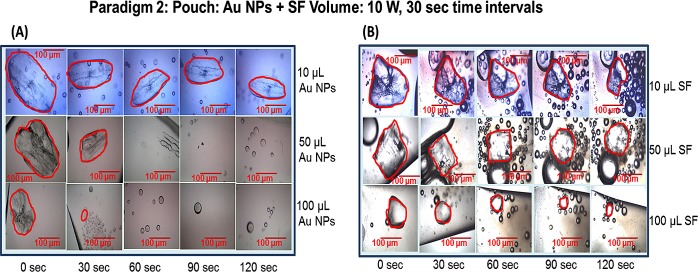
Paradigm 2 was used to determine the effect of different volumes of Au NPs alone or SF alone to decrystallize (i.e., decrease the surface area) l-alanine crystals in a plastic pouch. Figure 5, panel A shows the optical images of the observed change in the surface area of l-alanine crystals in a plastic pouch containing different volumes of Au NPs (10, 50, and 100 μL; no SF) during 120 s (at 30 s time intervals) of microwave heating at 10 W. Panel B shows the optical images of l-alanine crystals in a plastic pouch containing different volumes of SF (10, 50, and 100 μL) with no Au NPs during 120 s (at 30 s time intervals) of microwave heating at 10 W. These data demonstrate that the 100 μL volume of Au NPs resulted in the dramatic decrease in the crystal size at the 30 s time and disappearance of the crystal by the 60 s time point. On the other hand, the highest volume of SF did not totally decrystallize the l-alanine crystal by the 120 s time point.
Figure 5.
Paradigm 2: pouch: Au NPs + SF volume (10 W, 30 s time intervals). Optical images of the observed change in the surface area of l-alanine crystals in a plastic pouch containing different volumes of Au NPs (10, 50, and 100 μL; no SF) (A) or different volumes of SF (10, 50, and 100 μL; no Au NPs) (B) during 120 s (at 30 s time intervals) of microwave heating at 10 W.
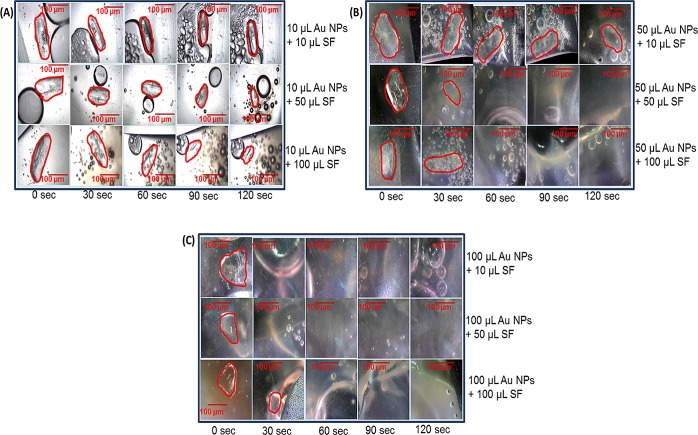
In addition, paradigm 2 was used to determine the effect of different volumes of Au NPs mixed with SF on the size of l-alanine crystals after 120 s (at 30 s time intervals) of microwave heating at 10 W. Figure 6, panels A–C show the optical images of the observed change in the surface area of l-alanine crystals in a plastic pouch containing different volumes of Au NPs (10, 50, and 100 μL) mixed with different volumes of SF (10, 50, and 100 μL) during 120 s (at 30 s time intervals) of microwave heating at 10 W. These results demonstrate that the highest volume of Au NPs (100 μL) in the presence of SF (10, 50, and 100 μL) resulted in a greater reduction in the crystal than the two lower volumes of Au NPs (10 and 50 μL).
Figure 6.
Paradigm 2: pouch: Au NPs + SF volume (10 W, 30 s time intervals). Summary of data collected from three different paradigm 2 experiments. Percentage change in the surface area (%) of l-alanine crystals in a plastic pouch during 120 s (at 30 s time intervals) of microwave heating at 10 W. Data are presented as the mean ± standard deviation of the three different experiments. Table shows the 15 experimental groups used in this study: 1–3 (A) 10 μL of Au NPs (10, 50, or 100 μL of SF), 4–6 (B) 50 μL of Au NPs (10, 50, or 100 μL of SF), and 7–15 (C) 100 μL of Au NPs (10, 50, or 100 μL of SF).
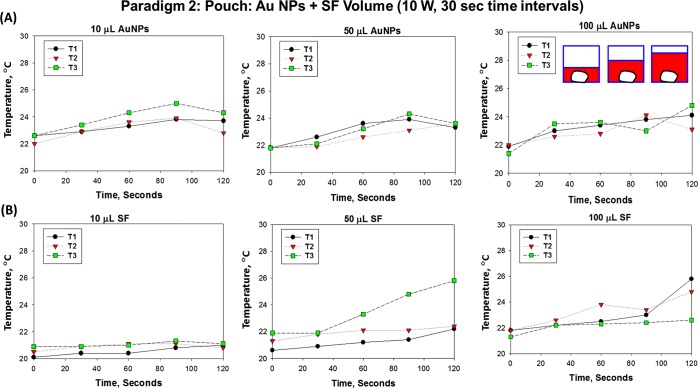
Paradigm 2 was also used to determine the effect of different volumes of Au NPs and SF on the plastic pouch final temperature after 120 s (at 30 s time intervals) of microwave heating at 10 W. Figure 7 shows the results of three different trials using paradigm 2 to determine the change in the temperature of the plastic pouch containing Au NPs only (A, no SF) and SF only (B, no Au NPs) for 120 s (at 30 s time intervals) of microwave heating at 10 W. There was no significant change in the plastic pouch final temperature after 120 s (at 30 s time intervals) of microwave heating at 10 W with 10, 50, or 100 μL of Au NPs or SF. In the presence of Au NPs or SF (10, 50, or 100 μL), there were no significant differences between the initial and final pouch temperatures after microwave heating at 10 W. After microwave heating at 10 W for 120 s (at 30 s time intervals), the modest increases in the temperature (up to 4 °C) would not denature proteins, and therefore, the MAMAD technique could be used in patients with gout.
Figure 7.
Paradigm 2: pouch: Au NPs + SF volume (10 W, 30 s time intervals). Change in the temperature (°C) of the plastic pouch containing (A) Au NPs only (no SF) and (B) SF only (no Au NPs) for 120 s (every 30 s) of microwave heating at 10 W. T1, T2, and T3 indicate three different experiments, trials 1, 2, and 3, respectively.
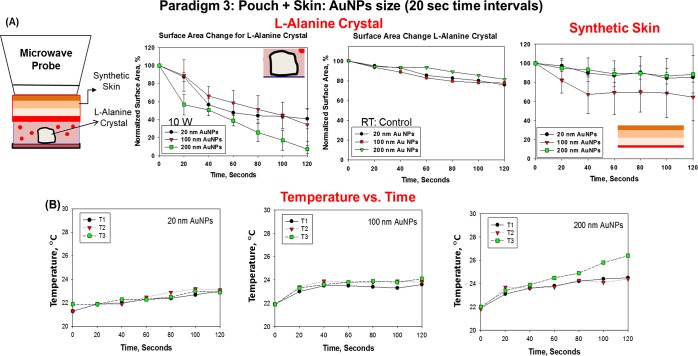
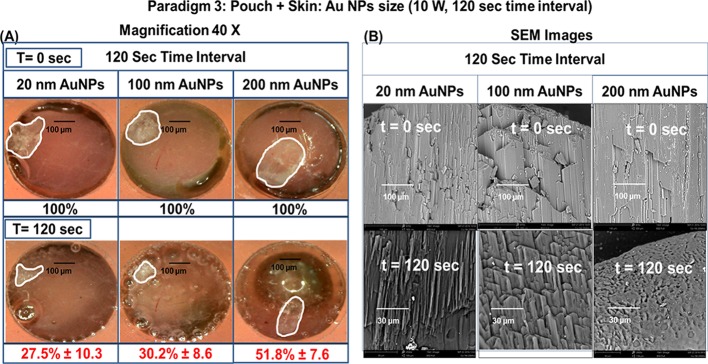
Paradigm 3 was used to determine whether an l-alanine crystal in a plastic pouch could be decrystallized under a synthetic skin patch, during exposure to 120 s (at 20 s time intervals) of microwave heating at 10 W. Figure 8 shows the summary of data collected from three different paradigm 3 experimental trials used to determine the percentage change in the surface area of an l-alanine crystal in a plastic pouch through a synthetic skin patch and the change in the synthetic skin surface area during exposure to 120 s (at 20 s time intervals) of microwave heating at 10 W. In addition, the final temperature of the pouch after 120 s (at 20 s time intervals) of microwave heating at 10 W was determined. At 120 s, in the presence of Au NPs (20 nm), there was a 50% reduction in the size of the l-alanine crystal. There was a 65% reduction in the crystal size in the presence of 100 nm Au NPs and a decrease in the crystal size of 95% with 200 nm of Au NPs. Moreover, Au NPs (20 and 200 nm) produced only a 10% reduction in the surface area of the synthetic skin patch after 120 s of microwave heating (at 30 s time intervals) at 10 W, whereas Au NPs resulted in a 30% reduction of the crystal. There was no significant change in the final temperature of the plastic pouch after 120 s (at 30 s time intervals) of microwave heating at 10 W. We also examined the change in the crystal surface area under the synthetic skin patch and the change in the synthetic skin surface area during exposure to 120 s (at 40 or 60 s time intervals) of microwave heating at 10 W and showed that there were no significant changes in the final temperature compared to the initial temperature of the plastic pouch (Figures S8–S10, Supporting Information).
Figure 8.
Paradigm 3: pouch + synthetic skin patch: Au NPs size (10 W, 20 s time intervals). Summary of data collected from three different experiments. (A) Percentage change in the surface area (%) of l-alanine crystals in a plastic pouch through the synthetic skin patch and a schematic representation of the microwave probe, the synthetic skin patch covering the plastic pouch containing the Au NP solution and one l-alanine crystal. (B) Percentage change in the surface are (%) of the synthetic skin during 120 s (at 20 s time intervals) of microwave heating at 10 W and room temperature. Data are presented as the mean ± standard deviation of the three different experiments. The sizes of Au NPs used in these trials are 20, 100, and 200 nm.
In Figure 9, real-color, high-resolution pictures show the percentage change in the surface area of an l-alanine crystal in a plastic pouch through a synthetic skin patch before (0 s) and after 120 s (at 20 s time intervals) of microwave heating at 10 W (paradigm 3). Using 20 nm Au NPs, there was a decrease in the l-alanine crystal surface area from 100% at t = 0 s to 27.5 ± 10.3% at t = 20 s. At the 20 s time point, the use of 100 nm Au NPs resulted in a decreased crystal surface area of 100% at t = 0 s to 30.2 ± 8.6% at t = 20 s, and 200 nm Au NPs resulted in a decrease from 100% at t = 0 s to 51.8 ± 7.6% at t = 20 s (A). The table in panel B in Figure 9 shows the percentage change in the surface area of l-alanine crystals in plastic pouches, containing various sizes of Au NPs (20, 100, or 200 nm), under a synthetic skin patch after microwave heating from 0 to 120 s (at 20 s time intervals) at 10 W. In the presence of 20 nm Au NPs, the crystal surface area was reduced from 100% at t = 0 s to 40.8 ± 11.0% at t = 20 s, 49.4 ± 2.5% at t = 40 s, 44.6 ± 7.3% at t = 60 s, and 27.5 ± 10.3% at t = 120 s. In the presence of 100 nm Au NPs, the crystal surface area is decreased from 100% at t = 0 s to 34.4 ± 18.2% at t = 20 s, 47.1 ± 29.2% at t = 40 s, 27.8 ± 20.1% at t = 60 s, and 30.2 ± 8.6% at t = 120 s. Au NPs at 200 nm resulted in a decrease in the crystal surface area from 100% at t = 0 s to 7.16 ± 4.6% at t = 20 s, 30.9 ± 15.1% at t = 40 s, 25.6 ± 9.9% at t = 60 s, and 51.8 ± 7.6% at t = 120 s. Real-color, high-resolution pictures show no difference in the percentage change in the crystal surface area under a synthetic skin patch before (0 s) and after 120 s (at 40 and 60 s time intervals) of microwave heating at 10 W (Figure S11, Supporting Information). In addition, scanning electron microscopy (SEM) images of an l-alanine crystal in a plastic pouch through a synthetic skin patch before (0 s) and after (120 s) microwave heating for 120 s at 10 W in the presence of Au NPs (20, 100, or 200 nm) were examined (Figure S11, Supporting Information).
Figure 9.
Paradigm 3: pouch + skin: Au NPs size (10 W, 20 s time intervals; intermittent exposure). (A) Real-color, high-resolution pictures of l-alanine crystals in a plastic pouch through the synthetic skin patch before and after microwave heating for 20 s at 10 W. (B) Schematic representation of the microwave probe, the four synthetic skin layers (epidermis, dermis, subcutaneous, and muscle) covering the plastic pouch containing the Au NP solution and one l-alanine crystal. The table in panel B shows the percentage change in the surface area of l-alanine crystals in plastic pouches, containing various sizes of Au NPs (20, 100, or 200 nm), under the synthetic skin patch after microwave heating for 0, 20, 40, 60, or 120 s at 10 W.
In Figure 10, real-color, high-resolution pictures show the percentage change in the surface area of an l-alanine crystal in a plastic pouch through a synthetic skin patch before (0 s) and after 120 s of microwave heating at 10 W (paradigm 3). At the 120 s time point, using 20 nm Au NPs, there was a decrease in the l-alanine crystal surface area from 100% at t = 0 s to 27.5 ± 10.3% at 120 s. The use of 100 nm Au NPs resulted in a decreased crystal surface area from 100% at 0 s to 30.2 ± 8.6% at 120 s, and 200 nm Au NPs resulted in a decrease from 100% at 0 s to 51.8 ± 7.6% at 120 s (A). Panel B shows the SEM micrographs of the decrystallization of l-alanine crystals in plastic pouches through synthetic skin patches before and after microwave heating for 120 s at 10 W.
Figure 10.
Paradigm 3: pouch + skin: Au NPs size (10 W, 120 s at 20 s time intervals) (A) Real-color pictures of the synthetic skin and l-alanine before and after microwave heating after 120 s (at 20 s time intervals) at 10 W. (B) SEM images of l-alanine crystals in a plastic pouch through the synthetic skin patch before and after microwave heating for 120 s (every 20 s) at 10 W. Data are presented as the mean ± standard deviation of the three different experiments. The sizes of the Au NPs used in this trial are 20, 100, and 200 nm.
In these experiments, we sought to determine whether the change in the temperature after microwave heating of the plastic pouch under the synthetic skin patch would exceed the physiological range or cause tissue damage. In conclusion, these results demonstrate qualitatively and quantitatively via real-time temperature measurements and optical microscopy that an l-alanine crystal in a plastic pouch can be decrystallized through a synthetic skin patch using microwave heating in the presence of Au NPs without damage to the skin or exceeding physiological temperatures. These results bring us much closer to the possibility of using the MAMAD technique for the decrystallization of gout-induced crystal deposits through the mammalian skin.
Experimental Methods
Materials and Instrumentation
Synthetic skin containing epidermis, dermis, subcutaneous tissue, and muscle was purchased from SynDaver Labs (Tampa, FL, USA). Bovine synovial fluid was purchased from Lampire Biological Laboratories (Pipersville, PA, USA). Au NPs (7.2 × 1011 particles/mL; optical density = 1.0; size: 20 nm, diameters: 100 nm, 200 nm; catalog number 741965) were purchased from Sigma-Aldrich (Milwaukee, WI, USA). l-Alanine powder was purchased from Sigma-Aldrich (Milwaukee, WI, USA). All aqueous solutions were prepared using deionized water that was obtained using a Millipore Direct-Q 3 UV apparatus maintained at 18.2 MΩ·cm resistivity at 25 °C, which indicates low levels of anionic contamination (EMD Millipore, Billerica, MA, USA). Synthetic skin was stored in antialgae solution purchased from The Clorox Company (Lawrenceville, GA, USA).
A compact medical microwave (ISYS800, 20 W) with an 8 GHz solid-state microwave generator was obtained from Emblation Microwave (Inglewood, Alloa, Scotland, UK). A digital microscope camera UM12 5 MP USB was obtained from ViTiny (Taylors, SC, USA), and a Nikon SMZ800 digital stereomicroscope was purchased from Nikon Instruments Inc. (Melville, NY, USA). An infrared thermometer (IR1000 12:1) was purchased from Klein Tools (Lincolnshire, IL. USA). A universal measurement instrument 4 with a precision of 0.025% and a dynamic range of 15 000:1 was purchased from FISO Technologies (Quebec, Canada). An incubator (model 10–140) with a photo-optic lamp was purchased from Osram (Munich, Germany). A Phenom XL scanning electron microscope (resolution = ≤800 nm; light optical magnification = 3–16×) was purchased from Phenom World (Eindhoven, Netherlands). A Fourier transform infrared spectrometer of acquired spectral range KBr 6300–350 cm–1 and ZnSe 5100–600 cm–1 was purchased from Agilent Technologies (Santa Clara, CA, USA). The X-ray powder diffraction instrument was purchased from Rigaku MiniFlex (The Woodlands, TX, USA). A tabletop Impulse 4″ sealer (model H#458) was obtained from Uline Inc. (Pleasant Prairie, WI, USA). Polyurethane plastic pouches were made from 2-mil poly tubing purchased from Uline (Pleasant Prairie, WI, USA). A 4.5 mm sterile biopsy punch was purchased from Acuderm Inc. (Lauderdale, FL, USA).
The iCrystal plates [silicon isolators and poly(methyl methacrylate)] were designed by the Aslan Research Group and produced in-house using silicon isolators purchased from Grace Bio-Labs (Portland, OR USA). Glass slides, coverslips (microslides; thickness: 0.96–1.06 mm), 20 mL scintillation vials, stir bars, and thermometers were purchased from Sigma-Aldrich (St. Louis, MO, USA). Cyanoacrylate (Gorilla Glue) was purchased from Gorilla Glue, Inc. (Cincinnati, OH, USA). ImageJ, image processing, and analysis in Java Software (https://imagej.nih.gov/ij/) and SigmaPlot 12.5 Systat Software, Inc. (San Jose, CA, USA) were used to determine the extent of decrystallization of l-alanine crystals.
Preparation of the Synthetic Skin Patches
A scalpel was used to adjust the thickness of the synthetic skin plate to an anatomical range of 1.5–2 mm, which more closely approximates human skin layers. Cyanoacrylate was used to bond the epidermis, dermis, subcutaneous, and muscle layers together. The bonded synthetic skin was cut into patches (4 mm × 4 mm width; 5 mm depth) via a scalpel. Synthetic skin patches, which were processed under a laminar flow hood, were immersed in antialgae solution (60 mL of antialgae solution in 4500 mL of deionized water and refrigerated at 4 °C), used to prevent contamination and to maintain synthetic skin integrity, and were stored in sealed 20 mL scintillation vials and refrigerated at 4 °C. The bonded synthetic skin was 6 mm thick: 1 mm epidermis, 1 mm dermis, 2 mm subcutaneous fat layer, and 2 mm muscle layer. In humans, the skin thickness at a tophi site ranges from 2–4 mm. The synthetic skin used in these experiments was 2 mm thicker than the human skin tissue at a tophi site. Before each experiment, the synthetic skin patches were warmed to 20 to 22 °C via an incubator. l-Alanine crystals were placed in each iCrystal plate well, and synthetic skin patches were positioned over each crystal in each well. Synthetic skin patches and l-alanine crystals were exposed to 30 μL of Au NPs (20, 100, or 200 nm). Sample wells containing a synthetic skin patch, an l-alanine crystal, and Au NPs were exposed to microwave heating at 5 W every 30 s (up to 120 s) with a 10 s delay to record images via an optical microscope. Each experimental trial was repeated in triplicate. Crystals were observed for percentage change in the surface area, as measured before and after microwave heating, via a scanning electron microscope and quantified using ImageJ software. In these experiments, the temperatures were determined via an infrared thermometer positioned 30 cm above each sample.
Preparation of a Plastic Pouch Containing an l-Alanine Crystal
Polyurethane plastic tubing was cut with a surgical knife into 1 cm × 2 cm rectangles and heat-sealed with a tabletop sealer on three sides. After a large l-alanine crystal (∼450 μm in size and weighing 0.1 mg) was placed in each plastic pouch, the fourth side of each pouch was heat-sealed.
Decrystallization of an l-Alanine Crystal in a Pouch on an iCrystal Plate with Various Microwave Heating Time Intervals Using the MAMAD Technique
An l-alanine crystal was inserted into a 1 × 2 cm plastic pouch made from 2-mil poly tubing. Au NPs (20 nm) were placed in each pouch containing an l-alanine crystal, heat-sealed, and examined under an optical microscope before decrystallization. The plastic pouch containing the l-alanine crystal was placed on an iCrystal plate and exposed to microwave heating at 5 or 10 W for 5, 10, 20, 30, 40, or 60 s intervals with a 10 s delay to measure the pouch temperatures and record the images.
Decrystallization of an l-Alanine Crystal in a Plastic Pouch on an iCrystal Plate with Various Volumes of Au NPs and SF without Synthetic Skin Patches Using the MAMAD Technique
The pouched l-alanine crystal experiments were used to determine the effect of different volumes of Au NPs and SF on crystals in the presence of microwave heating for 30 s at 10 W. Different volumes of 20 nm Au NPs (10, 50, or 100 μL) and bovine SF (10, 50, or 100 μL) were mixed in each plastic pouch containing an l-alanine crystal. The plastic pouch containing the l-alanine crystal was placed in the well of an iCrystal plate and exposed to microwave heating at 10 W for 30 s with a 10 s delay to measure pouch temperatures and record images. l-Alanine crystals were observed for change in the surface area via ImageJ. Experimental trials were repeated in triplicate.
Decrystallization of an l-Alanine Crystal in a Pouch on an iCrystal Plate with Various Sizes of Au NPs under Synthetic Skin Patches Using the MAMAD Technique
The synthetic skin patches (∼4.5 mm diameter) were sectioned out via a biopsy punch, placed in an antialgae solution, and cooled to 2 °C. A plastic pouch containing an l-alanine crystal and 30 μL of Au NPs (20, 100, or 200 nm) was placed in the well of an iCrystal plate and covered with a synthetic skin patch and coverslip before exposure to microwaves. The synthetic skin patch over a plastic pouch containing an l-alanine crystal and Au NPs was exposed to microwave heating at 10 W for 120 s (at 30 s time intervals) with a 10 s delay to record images via an optical microscope. Each experimental trial was repeated in triplicate. The crystals were observed for change in the surface area using a scanning electron microscope and quantified via ImageJ. The temperature of each plastic pouch and synthetic skin patch was measured via an infrared thermometer.
Acknowledgments
The research reported in this publication was partially supported by the National Institute of General Medical Sciences of the National Institutes of Health (USA) under Award number UL1GM118973. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b00816.
Additional information related to temperature measurements during microwave heating, and SEM and optical microscope images of l-alanine crystals under experimental conditions (PDF)
Author Present Address
§ Morgan State University, Department of Civil Engineering, 1700 East Cold Spring Lane, Baltimore MD 21251 (K.A.).
The authors declare no competing financial interest.
Supplementary Material
References
- Zhu Y.; Pandya B. J.; Choi H. K. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011, 63, 3136–3141. 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- Lawrence R. C.; Felson D. T.; Helmick C. G.; Arnold L. M.; Choi H.; Deyo R. A.; Gabriel S.; Hirsch R.; Hochberg M. C.; Hunder G. G. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis Rheum. 2008, 58, 26–35. 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martillo M. A.; Nazzal L.; Crittenden D. B. The crystallization of monosodium urate. Curr. Rheumatol. Rep. 2014, 16, 400. 10.1007/s11926-013-0400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. U. R.; Díaz-Torné C.; Perez-Ruiz F.; March L. M. Epidemiology of gout: an update. Best Pract. Res., Clin. Rheumatol. 2010, 24, 811–827. 10.1016/j.berh.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Roddy E.; Doherty M. Epidemiology of gout. Arthritis Res. Ther. 2010, 12, 223. 10.1186/ar3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards N. L. Treatment-failure gout: A moving target. Arthritis Rheum. 2008, 58, 2587–2590. 10.1002/art.23803. [DOI] [PubMed] [Google Scholar]
- MacFarlane L. A.; Kim S. C. Gout: a review of nonmodifiable and modifiable risk factors. Rheum. Dis. Clin. North. Am. 2014, 40, 581–604. 10.1016/j.rdc.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuki G.; Simkin P. A. A concise history of gout and hyperuricemia and their treatment. Arthritis Res. Ther. 2006, 8, S1. 10.1186/ar1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortmann R. L.Disorders of purine and pyrimidine metabolism. Harrisons Principles of Internal Medicine, 16th ed.; McGraw-Hill, 2005; Vol. 2, p 2308. [Google Scholar]
- Ghaemi-Oskouie F.; Shi Y. The role of uric acid as an endogenous danger signal in immunity and inflammation. Curr. Rheumatol. Rep. 2011, 13, 160–166. 10.1007/s11926-011-0162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo Clinic . Prednisone and other corticosteroids. http://www.mayoclinic.org/steroids/ART-20045692/.
- Manno M. R.MHS Treatment of Gout. http://www.hopkinsarthritis.org/arthritis-info/gout/gout-treatment/.
- CDC . Gout. http://www.cdc.gov/arthritis/basics/gout.htm.
- Kumar S.; Gow P.. A survey of indications, results and complications of surgery for tophaceous gout. N. Z. Med. J. 2002, 115 (). [PubMed] [Google Scholar]
- Słowińska I.; Słowiński R.; Rutkowska-Sak L. Tophi—surgical treatment. Reumatologia 2016, 54, 267–272. 10.5114/reum.2016.63819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinoffe Y. S. B.; Kioko B. M.; Gordon B. I.; Thompson N. A.; Adebiyi M.; Mauge-Lewis K.; Ogundolie T. O.; Bonyi E.; Mohammed M.; Aslan K. Metal-Assisted and Microwave-Accelerated Decrystallization. Nano Biomed. Eng. 2015, 7, 139–152. 10.5101/nbe.v7i4.p139-152. [DOI] [Google Scholar]
- Wakim K. G.; Herrick J. F.; Martin G. M.; Krusen F. H. Therapeutic Possibilities of Microwaves: Experimental and Clinical Investigation. J. Am. Med. Assoc. 1949, 139, 989–993. 10.1001/jama.1949.02900320019006. [DOI] [PubMed] [Google Scholar]
- Yadava R. L.In RF/microwaves in bio-medical applications. INCEMIC 2003. 8th International Conference on Electromagnetic Interference and Compatibility, 2003; IEEE, 2003; pp 81–85.
- Vrba J.; Lapes M.. Medical applications of microwaves. Proc. SPIE, Microwave and Optical Technology, 2004; pp 392–397.
- de Jong K. P.; Wertenbroek M. W. J. L. A. E. Liver resection combined with local ablation: where are the limits. Dig. Surg. 2011, 28, 127–133. 10.1159/000323823. [DOI] [PubMed] [Google Scholar]
- Anwar J.; Shafique U.; Waheed-uz-Zaman; Rehman R.; Salman M.; Dar A.; Anzano J. M.; Ashraf U.; Ashraf S. Microwave chemistry: Effect of ions on dielectric heating in microwave ovens. Arabian J. Chem. 2015, 8, 100–104. 10.1016/j.arabjc.2011.01.014. [DOI] [Google Scholar]
- Naito S.; Hoshi M.; Yagihara S. Microwave dielectric analysis of human stratum corneum in vivo. Biochim. Biophys. Acta, Gen. Subj. 1998, 1381, 293–304. 10.1016/s0304-4165(98)00041-5. [DOI] [PubMed] [Google Scholar]
- Toker S.; Boone-Kukoyi Z.; Thompson N.; Ajifa H.; Clement T.; Ozturk B.; Aslan K. Microwave Heating of Synthetic Skin Samples for Potential Treatment of Gout Using the Metal-Assisted and Microwave-Accelerated Decrystallization Technique. ACS Omega 2016, 1, 744–754. 10.1021/acsomega.6b00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel S.; Lau R. W.; Gabriel C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys. Med. Biol. 1996, 41, 2251. 10.1088/0031-9155/41/11/002. [DOI] [PubMed] [Google Scholar]
- Grant J. P.; Clarke R. N.; Symm G. T.; Spyrou N. M. In vivo dielectric properties of human skin from 50 MHz to 2.0 GHz. Phys. Med. Biol. 1988, 33, 607. 10.1088/0031-9155/33/5/008. [DOI] [PubMed] [Google Scholar]
- Tamyis N. M.; Ghodgaonkar D. K.; Taib M. N.; Wui W. T.. Dielectric properties of human skin in vivo in the frequency range 20–38 GHz for 42 Healthy volunteers. Proceedings of the 28th URSI General Assembly, 2005. [Google Scholar]
- Gabriel C.Compilation of the Dielectric Properties of Body Tissues at RF and Microwave Frequencies. DTIC Document, 1996. [Google Scholar]
- SynDaver . http://syndaver.com/wp-content/uploads//Documents.
- Shergold O. A.; Fleck N. A. Experimental investigation into the deep penetration of soft solids by sharp and blunt punches, with application to the piercing of skin. J. Biomech. Eng. 2005, 127, 838–848. 10.1115/1.1992528. [DOI] [PubMed] [Google Scholar]
- Dreaden E. C.; Alkilany A. M.; Huang X.; Murphy C. J.; El-Sayed M. A. The golden age: gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. 10.1039/c1cs15237h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreaden E. C.; Austin L. A.; Mackey M. A.; El-Sayed M. A. Size matters: gold nanoparticles in targeted cancer drug delivery. Ther. Delivery 2012, 3, 457–478. 10.4155/tde.12.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioko B.; Ogundolie T.; Adebiyi M.; Ettinoffe Y.; Rhodes C.; Gordon B.; Thompson N.; Mohammed M.; Abel B.; Aslan K. De-crystallization of Uric Acid Crystals in Synovial Fluid Using Gold Colloids and Microwave Heating. Nano Biomed. Eng. 2014, 6, 104–110. 10.5101/nbe.v6i4.p104-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.