Abstract
Objectives
We examined somatostatin receptor type 2A (SSTR2A) expression in primary and metastatic small intestine neuroendocrine tumors (SI-NETs).
Methods
A representative formalin-fixed paraffin-embedded tumor section from each liver metastasis and available primary tumor were stained for SSTR2A expression. SSTR2A expression was evaluated by the Her/neu scoring system and the scoring system proposed by Volante et al.
Results
A total of 156 liver metastases were from 10 males and 16 females who had ≥2 resected liver lesions. Based on the Her/neu scoring system, moderate-strong SSTR2A labeling was observed in 121 of 156 (78%) liver tumors. Fifteen of 26 (58%) subjects had all liver tumors with moderate-strong SSTR2A labeling, whereas 11 (42%) had ≥1 liver tumors with negative-weak labeling. Eleven of 16 (69%) primaries stained showed heterogeneous SSTR2A expression. Their liver metastases showed only negative-weak labeling in 1, only moderate-strong labeling in 5, and both negative-weak and moderate-strong labeling in 5 of the 11 cases. Using the Volante scoring system, 0 (0%), 2 (1%), 38 (24%) and 116 (74%) of 156 liver lesions were scored 0, 1, 2 and 3, respectively. No statistically significant association was observed between SSTR2A expression and Ki67 index (p=0.56). Fifteen of 18 (83%) metastatic tumors with Ki67 index>20% had moderate-strong SSTR2A labeling. Most of the liver tumors with weak SSTR2A expression or an IHC score of 2 were detected by OctreoScan.
Conclusions
SSTR2A expression in liver metastases of SI-NETs can be variable even in the same patients, which cannot be predicted by its expression in primaries. SSTR2A expression is not associated with Ki67 index.
Keywords: SSTR2A, heterogeneity, liver metastasis, small intestine, neuroendocrine tumor
INTRODUCTION
Somatostatin receptors (SSTRs) are G-protein-coupled receptors that inhibit adenylate cyclase and cyclic adenosine monophosphate production upon ligand binding, consequently regulating cell cycle, cell proliferation and hormone secretion[1]. Five subtypes, including SSTR1, SSTR2A, SSTR3, SSTR4, and SSTR5, have been characterized in the human, all of which have been detected in gastroenteropancreatic neuroendocrine neoplasms[2–5]. Among them, SSTR2A is the most predominantly expressed SSTR in these tumors, including small intestine neuroendocrine tumors (NETs)[5–8].
Most patients with small intestine NET present with an advanced or incurable disease at initial diagnosis[9, 10]. However, small intestine NETs are usually well-differentiated with a prolonged disease course. Medical management (other than surgical resection) is frequently needed in these patients. However, there are limited therapeutic options. Typical cytotoxic chemotherapy is ineffective in treating this disease. SSTR-based therapies have been shown to decrease NET hormone production, improve symptoms and slow tumor growth in patients with unresectable small bowel NETS[11–16].
SSTR2A shows a high affinity for somatostatin analogues (SSAs), such as octreotide and lanreotide[17–19]. SSAs are standard diagnostic and treatment tools for small intestine NETs. For example, 111In-DTPA-Octreoscan and the newly developed, more sensitive 68Ga-based PET tracers are used to detect SSTR2A expressing NETs[20–24]. SSAs have been routinely used to control carcinoid syndrome caused by excess serotonin production by metastatic NETs[16]. In addition, peptide receptor radionuclide therapy (PRRT) using radiolabeled SSAs is a promising treatment option for metastasized or unresectable NETs[22, 25, 26].
Although most well-differentiated NETs strongly express SSTR2A, poorly differentiated neuroendocrine carcinomas frequently display reduced SSTR2A expression. In addition, like other malignancies, small intestine NETs exhibit tumor heterogeneity. In this study, we hoped to determine how often metastatic NETs differed from one-another and from the primary tumor in regards to SSTR2A expression. We also wished to determine whether metastases with higher proliferative rates (as measured by Ki67) were less likely to express SSTR2A.
MATERIALS AND METHODS
Patient Selection
Patients who underwent partial hepatectomy or wedge resection for liver metastases of small intestine NETs between 2003 and 2013 were identified by reviewing the pathology department archives at Vanderbilt University Medical Center. Included in the study were patients who had two or more liver metastases that were resected at our institution. Twenty-six cases were identified with blocks available for immunohistochemical (IHC) studies, of which, 16 had primary tumor blocks. All liver tumors included in the study had Ki67 index and tumor size available, which have been reported previously[27]. All study patients had well differentiated small bowel NETs. Clinical histories, including preoperative octreotide treatment and imaging studies, were obtained from electronic medical records. This study was approved by the Vanderbilt Institutional Review Board.
SSTR2A Labeling and Analysis
A 4-μm representative formalin-fixed paraffin-embedded tumor section from each liver tumor and available primary tumors was used for IHC-labeling for SSTR2A (Biotrend, Schwabhausen, Germany). The IHC stains were reviewed by two pathologists (MC and CS) without knowledge of the Ki67 or clinical course of the patient. Membranous SSTR2A expression was scored using the Her2/neu-scoring system as negative (0+), weak (1+), moderate (2+) and strong (3+) as shown in Table 1[28, 29] or using the scoring system proposed by Volante et al as shown in Table 2 [30]. Liver metastases were separated into 2 groups based on SSTR2A expression assessed using the Her2/neu-scoring system: group 1 with negative-weak and group 2 with moderate-strong expression. Cytoplasmic SSTR2A expression was also evaluated in the liver lesions.
Table 1.
Scoring system for Membranous SSTR2A expression by immunohistochemistry
| Interpretation | Description |
|---|---|
| Strong | Strong membranous reactivity (3+) in more than 10% of tumor cells |
| Moderate | Weak to Moderate membranous reactivity (2+) in more than 10% of tumor cells |
| Weak | Faint/barely perceptible membranous reactivity (1+) in more than 10% of tumor cells |
| Negative | No staining or staining (0+) in 10% or less of tumor cells |
Table 2.
Somatostatin Receptor 2A Immunohistochemistry Scoring System Proposed by Volante et al.
| SCORE | STAINING |
|---|---|
| 0 | Absence of any immunoreactivity |
| 1 | Pure cytoplasmic immunoreactivity (focal or diffuse) |
| 2 | Membranous reactivity in <50% of tumor cells |
| 3 | Membranous reactivity in >50% of tumor cells |
Statistical Analysis
Linear mixed-effects models were used to assess the associations between SSTR2A expression and Ki67 index, and between SSTR2A expression and tumor size, accounting for within-patient correlation due to multiple measurements per patient. In all models, logarithmically transformed Ki67 index and tumor size were used, and patients’ age and sex were included as covariates. Above statistical analyses were conducted using R version 3.3 statistical software[31]. Student’s t test and Fisher’s exact test were used to compare membranous and cytoplasmic SSTR2A expression between octreotide-treated and untreated groups, respectively. All statistical tests were two-sided, and a p-value < 0.05 was considered statistically significant.
RESULTS
General Clinical and Pathologic Features
The 26 patients included 10 males and 16 females, with a median age of 60 years, ranging from 20 to 75 years (Table 3). Ki67 index was available for 19 primary tumors from 19 patients including 16 in-house cases with primary tumor blocks available for Ki67 immunohistochemistry and 3 referral cases with Ki67 stain submitted for review. Based on the World Health Organization (WHO) 2010 classification of the digestive neuroendocrine neoplasms[32], the 19 cases included 16 grade 1 (Ki67≤2%) and 3 grade 2 tumors (Ki67 ranging from 3 to 8%). A total of 156 liver lesions were resected from the 26 patients, 18 (12%) of which were grade 3 with the Ki67 index ranging from 21% to 31%. Overall, 8 (31%) cases had one or more liver metastases that were grade 3 (Table 3). The mean tumor number per patient was six, ranging from 2 to 12. Mean tumor size was 1.3 cm, ranging from 0.1 to 5.6 cm (Table 3).
Table 3.
Demographics, Tumor Grade, Liver Tumor Number, Tumor Size, and Membranous SSTR2A Expression
| Case No. | Age (yrs) | Sex | Primary tumor | No. of Liver Tumors Resected | Size (cm) | Liver Tumor | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ki67 | Grade | SSTR2A | Ki67 range (%) | Grade | SSTR2A | ||||||||
| 0 | 1+ | 2+ | 3+ | ||||||||||
| 1 | 58 | M | NA | NA | NA | 5 | 0.3–3.2 | 0.2–20.0 | G1,G2 | X | X | ||
| 2 | 61 | F | NA | NA | NA | 11 | 1.2–5.5 | 2.0–14.3 | G1,G2 | X | X | X | |
| 3 | 59 | F | 0.6 | G1 | 2+ | 4 | 0.2–2.0 | 0.2–3.9 | G1,G2 | X | X | ||
| 4 | 67 | F | 1.3 | G1 | NA | 3 | 0.5–3.4 | 1.0–2.7 | G1 | X | |||
| 5 | 60 | F | 2.4 | G1 | 2+ | 3 | 0.1–1.0 | 2.0–10.0 | G1,G2 | X | X | ||
| 6 | 60 | M | 2.7 | G1 | 3+ | 3 | 0.8–5.0 | 0.7–2.0 | G1 | X | |||
| 7 | 58 | M | 1.3 | G1 | 2+ | 5 | 0.5–3.3 | 0.1–12.5 | G1,G2 | X | X | ||
| 8 | 61 | F | NA | NA | NA | 12 | 0.2–2.6 | 0.3–21.0 | G1,G2,G3 | X | X | X | |
| 9 | 61 | F | 0.8 | G1 | 2+ | 3 | 0.4–0.8 | 0.4–2.5 | G1 | X | |||
| 10 | 71 | M | 1.6 | G1 | 2+ | 7 | 0.1–0.4 | 14.9–22.4 | G2,G3 | X | X | ||
| 11 | 75 | M | 1.2 | G1 | 2+ | 12 | 0.4–2.5 | 0.1–26.0 | G1,G2,G3 | X | X | X | |
| 12 | 44 | M | NA | NA | NA | 7 | 0.3–1.7 | 0.8–25.4 | G1,G2,G3 | X | |||
| 13 | 62 | F | 0.6 | G1 | 2+ | 5 | 0.3–1.3 | 0.1–7.2 | G1,G2 | X | |||
| 14 | 63 | M | 4.0 | G2 | 2+ | 5 | 0.4–3.1 | 2.5–28.8 | G1, G2,G3 | X | X | ||
| 15 | 61 | M | 7.6 | G2 | 1+ | 3 | 0.9–1.4 | 7.1–12.2 | G2 | X | |||
| 16 | 49 | F | 1.5 | G1 | 0 | 2 | 2.0–2.5 | 2.5–3.2 | G1, G2 | X | |||
| 17 | 55 | F | 0.1 | G1 | 1+ | 3 | 1.0–2.0 | 0.1 | G1 | X | |||
| 18 | 71 | M | 2.0 | G1 | NA | 7 | 0.5–1.7 | 0.1–22.2 | G1,G2,G3 | X | |||
| 19 | 60 | F | 3.0 | G2 | NA | 3 | 0.6–3.2 | 15.0–31.0 | G2,G3 | X | |||
| 20 | 70 | F | NA | NA | NA | 7 | 0.5–3.8 | 11.2–31.0 | G2,G3 | X | X | X | |
| 21 | 72 | F | NA | NA | NA | 12 | 0.1–2.6 | 0.1–0.9 | G1 | X | X | ||
| 22 | 59 | F | 1.6 | G1 | 2+ | 5 | 0.8–5.4 | 0.1–1.6 | G1 | X | X | ||
| 23 | 54 | F | NA | NA | NA | 9 | 0.7–3.5 | 0.1–1.1 | G1 | X | |||
| 24 | 20 | F | 2.4 | G1 | 3+ | 9 | 0.3–1.8 | 0.1–1.4 | G1 | X | X | ||
| 25 | 65 | F | 0.1 | G1 | 1+ | 3 | 0.5–5.6 | 0.1–1.5 | G1 | X | X | ||
| 26 | 55 | M | 0.1 | G1 | 3+ | 5 | 0.8–1.9 | 0.1–2.8 | G1 | X | X | ||
Tumor Heterogeneity in SSTR2A Expression among Liver Metastases
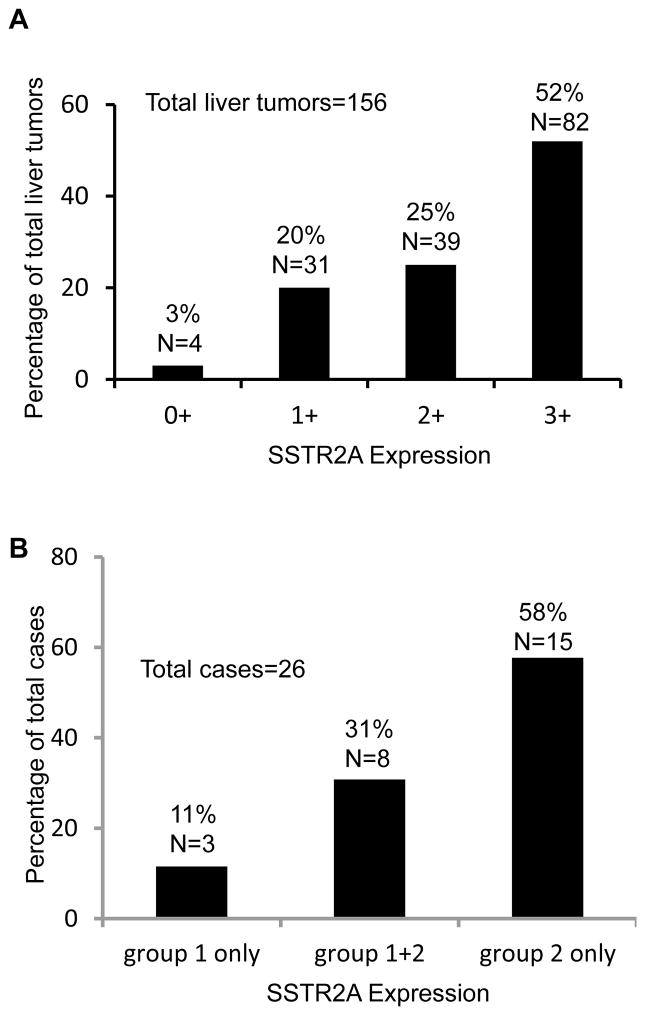
Negative, weak, moderate, and strong membranous SSTR2A expressions evaluated using the Her2/neu-scoring system were seen in 4 (3%), 31 (20%), 39 (25%), and 82 (52%) of 156 liver tumors, respectively (Figure 1A). Based on the membranous SSTR2A expression, liver tumors were classified as either group 1 (negative-weak) or group 2 (moderate-strong) expression. Fifteen (58%) of 26 patients had group 2 tumors only, whereas 3 (11%) had group 1 tumors only. The remaining 8 (31%) patients had both group 1 and group 2 tumors (Figure 1B, Table 3). Therefore, intertumoral heterogeneity of SSTR2A expression in liver metastases was present in nearly a third of patients (Figure 2). Of note, the 3 cases with group 1 tumors only contained either WHO grade 1 or grade 2 liver tumors. Overall 42% (11/26) patients had one or more tumors that had negative-weak SSTR2A expression on the plasma membrane.
Figure 1.
Membranous expression of somatostatin receptor type 2A (SSTR2A) in liver metastases from small intestinal neuroendocrine tumors (NETs). A. Distribution of 156 liver metastases with different SSTR2A expression. B. Distribution of cases with group 1 (0–1+) only liver tumors, group 2 (2–3+) only tumors, and both.
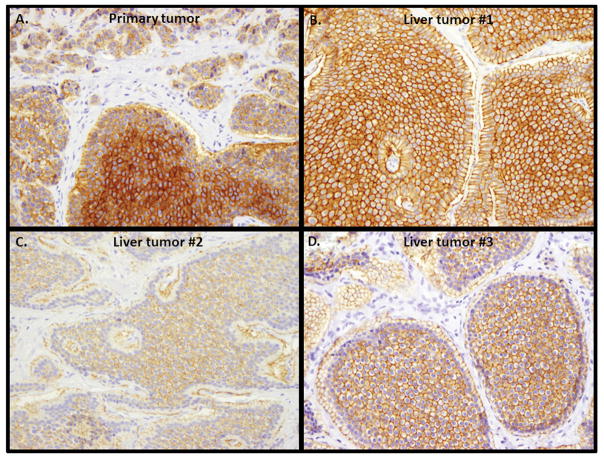
Figure 2.
Variable somatostatin receptor type 2A (SSTR2A) expression among primary tumor and its metastatic liver lesions. A. primary tumor with weak, moderate and strong SSTR2A membranous expression (original magnification 200X); B. A liver lesion from the same patient showing strong SSTR2A membranous expression (original magnification 200X); C. A second liver lesion from the same patient showing weak SSTR2A membranous expression (original magnification 200X); D. A third liver lesion from the same patient showing moderate SSTR2A expression (original magnification 200X).
Using the scoring system proposed by Volante et al[30], SSTR2A IHC scores of 2 and 3 were detected in 38 (24%) and 116 (74%) of 156 liver lesions, respectively. Only 2 of 156 (1%) liver metastases had cytoplasmic only SSTR2A expression (score 1), and none showed complete negative labeling. In the Volante scoring system, SSTR2A IHC scores of 2 and 3 are considered as positive[30]. All 26 cases had at least one liver metastasis with positive SSTR2A expression, 9 of which (41%) had an IHC score of 3 in all of their liver lesions.
Comparison of SSTR2A Expression between Primary Tumors and Their Liver Metastases (Table 4)
Table 4.
Comparison of Membranous SSTR2A Expression between Primary Tumors and Their Liver Metastases
| Liver tumor | ||||
|---|---|---|---|---|
| Primary tumor | Group 1 (0–1+) only | Group 2 (2–3+) only | Group 1 & 2 | Total cases |
| 0–1+ in >90% tumor cells | 2 | 1 | 0 | 3 |
| 0–1+ in >10% tumor cells and 2–3+ in >10% cells | 1 | 5 | 5 | 11 |
| 2–3+ in >90% tumor cells | 0 | 2 | 0 | 2 |
| Total cases | 3 | 8 | 5 | 16 |
Sixteen patients had a primary small bowel tumor available for SSTR2A labeling. While 12 (67%) had moderate-strong, 4 (33%) had negative-weak SSTR2A expression by the Her2/neu-scoring system (Table 3). Careful examination of SSTR2A expression found that 11 of 16 (69%) primaries showed prominent heterogeneity in SSTR2A expression (Table 4); the same tumors contained >10% of the tumor cells with negative-weak expression and >10% with moderate-strong expression (Figure 2A). Liver metastases arising from these primaries were either group 1 only (1/11, 10%), group 2 only (5/11, 45%), or both (5/11, 45%, Figure 2B–D).
Three of 16 (19%) primaries had negative to weak expression of SSTR2A in more than 90% of tumor cells. Among the 3 cases, 2 had negative-weak and 1 had moderate SSTR2A expression in all liver tumors. An additional 2 (12%) primaries displayed moderate-strong SSTR2A expression in more than 90% of tumor cells. All liver lesions from the 2 cases also showed moderate-strong SSTR2A expression.
Based on the scoring system proposed by Volante et al.[30], SSTR2A IHC scores for 16 primaries were either 2 (5/16, 31%) or 3 (11/16, 69%). The 11 primaries with an IHC score of 3 gave rise to liver metastases with a score ranging from 1 to 3; however, most (44/67, 66%) of their liver metastases had a score of 3. Five cases with a primary showing an IHC score of 2 had liver metastases that were either scored 2 or 3.
Association between SSTR2A Expression and Ki67 Index or Tumor Size
Ki67 proliferative index and tumor size were compared between liver tumors with negative-weak SSTR2A expression and those with moderate-strong expression. No statistically significant differences were observed in Ki67 index (p=0.56) or tumor size (p=0.14), while adjusting for patients’ age and gender. In addition, there were no significant differences in Ki67 (p=0.56) or tumor size (p=0.14) between liver tumors scored 1–2 and those scored 3 by the Volante scoring system.
High-grade neuroendocrine neoplasms were hypothesized to be associated with loss of SSTR2A expression on the cell membrane. We aimed to examine whether there was decreased SSTR2A expression in well-differentiated NETs with Ki67 index >20% (WHO grade 3). SSTR2A expression was compared between liver tumors with Ki67 ≤20% and those with Ki67>20%. No difference in SSTR2A expression between these 2 groups were found (p=0.56); moderate-strong SSTR2A expression was detected in 15 of 18 (83%) liver tumors with Ki67>20% and in 104 of 138 (75%) liver tumors with Ki67≤20%.
Correlation of SSTR2A expression with SSTR-based imaging and treatment
Twenty one cases received pre-operative OctreoScan (n=20) and/or 68Ga-DOTA-TATE PET-CT imaging studies (n=2). Most of the liver lesions with weak SSTR2A expression by the Her2/neu-scoring system showed activity on OctreoScan. However, some liver tumors with a small tumor size (<1.0 cm) were negative on OctreoScan whether they strongly or weakly expressed SSTR2A. In addition, one 1.7 cm primary tumor with weak SSTR2A expression was not detected by OctreoScan. On the other hand, all liver lesions in the 2 patients who had 68Ga-DOTA-TATE PET-CT imaging studies showed activity even they were less than 1 cm and weakly expressed SSTR2A.
Similar results were obtained when the SSTR-based imaging studies were correlated with SSTR2A expression evaluated using the Volante scoring system. Octreoscan detected most of the large liver lesions whether they had an IHC score of 2 or 3, whereas most of the small lesions (<1.0 cm) were not detected by Octreoscan even when the tumor had an IHC score of 3. Interestingly the OctreoScan-negative, 1.7 cm primary tumor was scored as “weak SSTR2A expresstion” by the Her2/neu-scoring system, but had a score of 3 by the Volante scoring system.
Thirteen of 26 (50%) patients were treated with octreotide before surgical resection of the liver lesions. There was no difference in membranous SSTR2A expression between octreotide-treated and untreated tumors (p=0.6). Cytoplasmic expression of SSTR2A was also not statistically different between the 2 groups (30 of 94 versus 12 of 62, p=0.1).
DISSCUSSION
In this study, we systemically analyzed SSTR2A expression in primary small intestine NETs and their liver metastases. Small intestine NETs are generally considered to uniformly express SSTR2A. We found that SSTR2A expression was heterogeneous in most small intestine NETs and that there was even intertumoral heterogeneity of SSTR2A expression in the liver metastases from the same patient. In addition, SSTR2A expression status of primary tumors did not predict SSTR2A expression in liver metastases. Furthermore, no significant association was observed between SSTR2A expression and Ki67 proliferative index as the majority of tumors with Ki67 index>20% had moderate-strong expression of SSTR2A by the Her2/neu scoring system.
Approximately two thirds of primary small intestine NETs displayed intratumoral heterogeneous SSTR2A expression as defined in Table 4. While the primaries with homogenous SSTR2A expression likely give rise to liver metastases with similar homogeneity, those with heterogeneous expression can likewise have liver lesions with heterogeneous SSTR2A expression among the different metastases (intertumoral heterogeneity). Therefore, SSTR2A expression in primaries does not always predict its expression in every liver metastasis. SSTR-based molecular imaging has widely been used to diagnose, stage, and follow up NETs. One would assume that not every metastatic lesion can be detected by such techniques even in patients with a SSTR2A expressing primary NET due to the common presence of SSTR intertumoral heterogeneity of the metastases. However, correlation of the imaging findings and IHC results revealed that OctreoScan was able to detect most of large liver tumors (≥ 1 cm) with weak SSTR2A expression. But small-sized tumors even with strong SSTR2A expression might not be detected by OctreoScan. In this study, we had 2 patients whose tumors were staged by 68GaDOTATATE PET-CT imaging. Several tumors with a very small size (as small as 0.4 cm) and weak expression of SSTR2A were all detected by 68GaDOTATATE PET-CT imaging. Four liver lesions displayed no SSTR2A expression by IHC. Unfortunately, SSTR-based imaging details of these lesions were not available. Nevertheless, our data suggest that sensitive SSTR-based imaging technologies such as 68GaDOTATATE PET-CT imaging are able to detect the vast majority of liver metastases from small bowel NETs.
In patients with small intestine NET, SSA treatment has been shown to control carcinoid syndrome and prolong time to progression[15, 16, 25]. Multiple factors such as tumor burden and receptor expression levels may determine the treatment effectiveness[33]. For example, PRRT with a radiolabelled analogue (e.g. 90Y-DOTATOC or 177Lu-DOTATATE) is a promising treatment option for stage IV NETs. It can deliver radiation doses into tumor cells via internalization through SSTR (mainly SSTR2A), achieving partial and complete objective responses in up to 50% patients[34–36]. It has been believed that response to PRRT is also correlated to SSTR2A expression and PRRT is only effective in treating tumors with high expression of SSTRs on the plasma membrane[22, 35]. Our data showed that although the majority of liver metastases from small intestine NET had moderate-strong membranous SSTR2A expression, approximately a quarter of the liver lesions showed negative-weak expression. SSA therapies may be less effective in treating lesions with lesser SSTR2A expression. Because of intertumoral heterogeneity, these therapies may have variation in efficacy between tumors in the same patients. Multireceptor targeting, especially for nonresponsive tumors, is being explored.
Multiple methods have been proposed to assess SSTR2A expression in NETs, including the Her/neu scoring system and the scoring system proposed by Volante et al[28–30]. The latter was shown to have good correlation with in vivo scintigraphic data. In this study, we used both the Her2/neu-scoring system and the Volante scoring system to evaluate SSTR2A expression in small intestine NETs and their liver metastases. It appeared that both systems had the same limitation in predicting OctreoScan activity in detecting small liver lesions. In addition, both systems identified a small but significant portion of liver metastases with negative to weak SSTR2A expression or with a SSTR2A IHC score of 0–2. This identification is potentially useful in predicting effectiveness of SSA-based treatment as tumors with weaker SSTR2A expression may be less responsive to the treatment.
PRRT has only been used to treat WHO grade 1 and WHO grade 2 tumors, because it has been assumed that there is reduced SSTR expression in higher-grade tumors[37, 38]. Our study showed that all 19 primaries with Ki67 stain were either grade 1 or 2, but more than 10% of the liver metastases were grade 3 (Ki67>20%) based on the 2010 WHO criteria. Korner et al reported some reduction in SSTR2A expression in 7 gastrointestinal neuroendocrine neoplasms with Ki67 index >20%[39]. However, in this study, we demonstrated that, like grade 1 and grade 2 tumors, the majority of grade 3 liver tumors (well-differentiated NETs with Ki67 index >20%) had moderate/strong SSTR2A expression on the plasma membrane. Therefore, PRRT may still be considered in treating well-differentiated NETs with Ki67>20%. Although the current WHO classification defines digestive NETs with Ki67>20% as poorly differentiated neuroendocrine carcinomas, there are some well-differentiated NETs with Ki67 index >20%, usually up to 30% as shown in this study. Grade 3 well-differentiated NETs may be required to be managed similarly to grade 1 or 2 tumors. Therefore, the current WHO classification needs to be modified to more accurately grade NETs.
Upon binding to SSA, SSTR2A undergoes internalization, trafficking and recycling of the receptor back to the cell membrane. Will SSA treatment affect distribution or expression of SSTR2A? Thirteen of 26 patients were treated with octreotide before surgery. Membranous SSTR2A expression was not different between octreotide-treated and untreated liver lesions. Treated lesions tended to have a higher frequency of cytoplasmic expression compared to untreated lesions; however, statistically the difference was not significant.
In conclusion, intratumoral and intertumoral heterogeneity of SSTR2A expression occurs in small intestine NETs and their liver metastases, which can produce differences in treatment response to SSTR-based therapy among different tumors. In addition, most metastatic NETs with Ki67>20% have significant SSTR2A expression. Well differentiated NETs with high Ki67 index should not preclude patients from SSTR-based therapies.
Acknowledgments
Funding: This work was partially supported by NIH/NIDDK DK058404-11 (ZZ and TK), NIH/NCI P50CA095103 (CS), and NCI CA 096625 (EL).
Footnotes
Compliance with Ethical Standards: The study was reviewed and approved by the Vanderbilt Institutional Review Board. Consent was not required for this study.
Conflict of Interest: None
References
- 1.Patel YC, Greenwood MT, Panetta R, Demchyshyn L, Niznik H, Srikant CB. The somatostatin receptor family. Life Sci. 1995;57:1249–1265. doi: 10.1016/0024-3205(95)02082-t. [DOI] [PubMed] [Google Scholar]
- 2.Jais P, Terris B, Ruszniewski P, LeRomancer M, Reyl-Desmars F, Vissuzaine C, Cadiot G, Mignon M, Lewin MJ. Somatostatin receptor subtype gene expression in human endocrine gastroentero-pancreatic tumours. Eur J Clin Invest. 1997;27:639–644. doi: 10.1046/j.1365-2362.1997.1740719.x. [DOI] [PubMed] [Google Scholar]
- 3.Papotti M, Bongiovanni M, Volante M, Allia E, Landolfi S, Helboe L, Schindler M, Cole SL, Bussolati G. Expression of somatostatin receptor types 1–5 in 81 cases of gastrointestinal and pancreatic endocrine tumors. A correlative immunohistochemical and reverse-transcriptase polymerase chain reaction analysis. Virchows Arch. 2002;440:461–475. doi: 10.1007/s00428-002-0609-x. [DOI] [PubMed] [Google Scholar]
- 4.Kubota A, Yamada Y, Kagimoto S, Shimatsu A, Imamura M, Tsuda K, Imura H, Seino S, Seino Y. Identification of somatostatin receptor subtypes and an implication for the efficacy of somatostatin analogue SMS 201-995 in treatment of human endocrine tumors. J Clin Invest. 1994;93:1321–1325. doi: 10.1172/JCI117090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reubi JC, Schaer JC, Waser B, Mengod G. Expression and localization of somatostatin receptor SSTR1, SSTR2, and SSTR3 messenger RNAs in primary human tumors using in situ hybridization. Cancer Res. 1994;54:3455–3459. [PubMed] [Google Scholar]
- 6.Kimura N, Pilichowska M, Date F, Kimura I, Schindler M. Immunohistochemical expression of somatostatin type 2A receptor in neuroendocrine tumors. Clin Cancer Res. 1999;5:3483–3487. [PubMed] [Google Scholar]
- 7.Reubi JC, Waser B. Concomitant expression of several peptide receptors in neuroendocrine tumours: molecular basis for in vivo multireceptor tumour targeting. Eur J Nucl Med Mol Imaging. 2003;30:781–793. doi: 10.1007/s00259-003-1184-3. [DOI] [PubMed] [Google Scholar]
- 8.Kagimoto S, Yamada Y, Kubota A, Someya Y, Ihara Y, Yasuda K, Kozasa T, Imura H, Seino S, Seino Y. Human somatostatin receptor, SSTR2, is coupled to adenylyl cyclase in the presence of Gi alpha 1 protein. Biochem Biophys Res Commun. 1994;202:1188–1195. doi: 10.1006/bbrc.1994.2054. [DOI] [PubMed] [Google Scholar]
- 9.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 10.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evan DB. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 11.Caplin ME, Pavel M, Ruszniewski P. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:1556–1557. doi: 10.1056/NEJMc1409757. [DOI] [PubMed] [Google Scholar]
- 12.Wynick D, Anderson JV, Williams SJ, Bloom SR. Resistance of metastatic pancreatic endocrine tumours after long-term treatment with the somatostatin analogue octreotide (SMS 201–995) Clin Endocrinol (Oxf) 1989;30:385–388. doi: 10.1111/j.1365-2265.1989.tb00436.x. [DOI] [PubMed] [Google Scholar]
- 13.Reubi JC, Kvols LK, Waser B, Nagorney DM, Heitz PU, Charboneau JW, Reading CC, Moertel C. Detection of somatostatin receptors in surgical and percutaneous needle biopsy samples of carcinoids and islet cell carcinomas. Cancer Res. 1990;50:5969–5977. [PubMed] [Google Scholar]
- 14.Kvols LK, Buck M, Moertel CG, Schutt AJ, Rubin J, O’Connell MJ, Hahn RG. Treatment of metastatic islet cell carcinoma with a somatostatin analogue (SMS 201-995) Ann Intern Med. 1987;107:162–168. doi: 10.7326/0003-4819-107-2-162. [DOI] [PubMed] [Google Scholar]
- 15.Kvols LK, Moertel CG, O’Connell MJ, Schutt AJ, Rubin J, Hahn RG. Treatment of the malignant carcinoid syndrome. Evaluation of a long-acting somatostatin analogue. N Engl J Med. 1986;315:663–666. doi: 10.1056/NEJM198609113151102. [DOI] [PubMed] [Google Scholar]
- 16.Gardner-Roehnelt NM. Update on the management of neuroendocrine tumors: focus on somatostatin antitumor effects. Clin J Oncol Nurs. 2012;16:56–64. doi: 10.1188/12.CJON.56-64. [DOI] [PubMed] [Google Scholar]
- 17.Laznicek M, Laznickova A, Maecke HR. Receptor affinity and preclinical biodistribution of radiolabeled somatostatin analogs. Anticancer Res. 2012;32:761–766. [PubMed] [Google Scholar]
- 18.Reubi JC, Schar JC, Waser B, Wenger S, Heppeler A, Schmitt JS, Macke HR. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med. 2000;27:273–282. doi: 10.1007/s002590050034. [DOI] [PubMed] [Google Scholar]
- 19.Janson ET, Gobl A, Kalkner KM, Oberg K. A comparison between the efficacy of somatostatin receptor scintigraphy and that of in situ hybridization for somatostatin receptor subtype 2 messenger RNA to predict therapeutic outcome in carcinoid patients. Cancer Res. 1996;56:2561–2565. [PubMed] [Google Scholar]
- 20.Bakker WH, Krenning EP, Reubi JC, Breeman WA, Setyono-Han B, de Jong M, Kooij PP, Bruns C, van Hagen PM, Marbach P, et al. In vivo application of [111In-DTPA-D-Phe1]-octreotide for detection of somatostatin receptor-positive tumors in rats. Life Sci. 1991;49:1593–1601. doi: 10.1016/0024-3205(91)90053-e. [DOI] [PubMed] [Google Scholar]
- 21.Krenning EP, Kwekkeboom DJ, Bakker WH, Breeman WA, Kooij PP, Oei HY, van Hagen M, Postema PT, de Jong M, Reubi JC, et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med. 1993;20:716–731. doi: 10.1007/BF00181765. [DOI] [PubMed] [Google Scholar]
- 22.Maecke HR, Reubi JC. Somatostatin receptors as targets for nuclear medicine imaging and radionuclide treatment. J Nucl Med. 2011;52:841–844. doi: 10.2967/jnumed.110.084236. [DOI] [PubMed] [Google Scholar]
- 23.Ambrosini V, Fani M, Fanti S, Forrer F, Maecke HR. Radiopeptide imaging and therapy in Europe. J Nucl Med. 2011;52(Suppl 2):42S–55S. doi: 10.2967/jnumed.110.085753. [DOI] [PubMed] [Google Scholar]
- 24.Treglia G, Castaldi P, Rindi G, Giordano A, Rufini V. Diagnostic performance of Gallium-68 somatostatin receptor PET and PET/CT in patients with thoracic and gastroenteropancreatic neuroendocrine tumours: a meta-analysis. Endocrine. 2012;42:80–87. doi: 10.1007/s12020-012-9631-1. [DOI] [PubMed] [Google Scholar]
- 25.Sharma N, Naraev BG, Engelman EG, Zimmerman MB, Bushnell DL, Jr, O’Dorisio TM, O’Dorisio MS, Menda Y, Muller-Brand J, Howe JR, et al. Peptide Receptor Radionuclide Therapy Outcomes in a North American Cohort With Metastatic Well-Differentiated Neuroendocrine Tumors. Pancreas. 2016 doi: 10.1097/MPA.0000000000000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yalchin M, Oliveira A, Theocharidou E, Pencharz D, Navalkissoor S, Quigley AM, Walker M, Caplin M, Toumpanakis C. The Impact of Radiological Response to Peptide Receptor Radionuclide Therapy on Overall Survival in Patients With Metastatic Midgut Neuroendocrine Tumors. Clin Nucl Med. 2016 doi: 10.1097/RLU.0000000000001457. [DOI] [PubMed] [Google Scholar]
- 27.Shi C, Gonzalez RS, Zhao Z, Koyama T, Cornish TC, Hande KR, Walker R, Sandler M, Berlin J, Liu EH. Liver metastases of small intestine neuroendocrine tumors: Ki-67 heterogeneity and World Health Organization grade discordance with primary tumors. Am J Clin Pathol. 2015;143:398–404. doi: 10.1309/AJCPQ55SKOCYFZHN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaemmerer D, Peter L, Lupp A, Schulz S, Sanger J, Baum RP, Prasad V, Hommann M. Comparing of IRS and Her2 as immunohistochemical scoring schemes in gastroenteropancreatic neuroendocrine tumors. Int J Clin Exp Pathol. 2013;5:187–194. [PMC free article] [PubMed] [Google Scholar]
- 29.Daniel K, Maria A, Amelie L, Isabell L, Stefan S, Luisa P, Merten H, Vikas P, Gerd B, Paul BR. Somatostatin receptor immunohistochemistry in neuroendocrine tumors: comparison between manual and automated evaluation. Int J Clin Exp Pathol. 2014;7:4971–4980. [PMC free article] [PubMed] [Google Scholar]
- 30.Volante M, Brizzi MP, Faggiano A, La Rosa S, Rapa I, Ferrero A, Mansueto G, Righi L, Garancini S, Capella C, et al. Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: a proposal of scoring system correlated with somatostatin receptor scintigraphy. Mod Pathol. 2007;20:1172–1182. doi: 10.1038/modpathol.3800954. [DOI] [PubMed] [Google Scholar]
- 31.Team R. R: A language and environment for statistical computing. (3.3) Vienna, Austria: [Google Scholar]
- 32.Kloppel G. Classification and pathology of gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2011;18(Suppl 1):S1–16. doi: 10.1530/ERC-11-0013. [DOI] [PubMed] [Google Scholar]
- 33.Heidari P, Wehrenberg-Klee E, Habibollahi P, Yokell D, Kulke M, Mahmood U. Free somatostatin receptor fraction predicts the antiproliferative effect of octreotide in a neuroendocrine tumor model: implications for dose optimization. Cancer Res. 2013;73:6865–6873. doi: 10.1158/0008-5472.CAN-13-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicolas G, Giovacchini G, Muller-Brand J, Forrer F. Targeted radiotherapy with radiolabeled somatostatin analogs. Endocrinol Metab Clin North Am. 2011;40:187–204. ix–x. doi: 10.1016/j.ecl.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Oksuz MO, Winter L, Pfannenberg C, Reischl G, Mussig K, Bares R, Dittmann H. Peptide receptor radionuclide therapy of neuroendocrine tumors with (90)Y-DOTATOC: is treatment response predictable by pre-therapeutic uptake of (68)Ga-DOTATOC? Diagn Interv Imaging. 2014;95:289–300. doi: 10.1016/j.diii.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Kwekkeboom DJ, de Herder WW, van Eijck CH, Kam BL, van Essen M, Teunissen JJ, Krenning EP. Peptide receptor radionuclide therapy in patients with gastroenteropancreatic neuroendocrine tumors. Semin Nucl Med. 2010;40:78–88. doi: 10.1053/j.semnuclmed.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Frilling A, Clift AK. Therapeutic strategies for neuroendocrine liver metastases. Cancer. 2015;121:1172–1186. doi: 10.1002/cncr.28760. [DOI] [PubMed] [Google Scholar]
- 38.Bodei L, Mueller-Brand J, Baum RP, Pavel ME, Horsch D, O’Dorisio MS, O’Dorisio TM, Howe JR, Cremonesi M, Kwekkeboom DJ, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40:800–816. doi: 10.1007/s00259-012-2330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korner M, Waser B, Reubi JC. Does somatostatin or gastric inhibitory peptide receptor expression correlate with tumor grade and stage in gut neuroendocrine tumors? Neuroendocrinology. 2015;101:45–57. doi: 10.1159/000371804. [DOI] [PubMed] [Google Scholar]