Abstract
A 59-year-old male with a history of lifelong asthma, allergic rhinitis and hypercholesterolaemia presented to the emergency department for management of severe substernal chest pain with radiating pain to his left arm, nausea and diaphoresis. Physical examination was unrevealing and a cardiac workup including cardiac enzymes, ECG, chest radiographs were negative for an underlying ischaemic event. A subsequent gastrointestinal workup including oesophageal manometry and oesophagogastroduodenoscopy revealed elevated lower oesophageal pressures and histopathology suggestive of mast cell proliferation, respectively. These findings were suggestive of oesophageal mastocytosis. Treatment with omeprazole-sodium bicarbonate, cetirizine, montelukast and oral budesonide promptly ameliorated his symptoms which have not recurred.
Keywords: gastrointestinal system, oesophagus
Background
Chest pain remains one of the most common presenting complaints to our emergency departments warranting a barrage of tests including cardiac enzymes, radiographs, ECGs and close observation. Moreover, if the workup is equivocal more invasive tests such as cardiac catheterisation may be warranted in the appropriate clinical setting. However, non-cardiac chest pain (NCCP) may present similarly to a myocardial infarction but is often overlooked. Peptic ulcer disease, oesophagitis and gastro-oesophageal reflux disease (GERD) are common culprits of NCCP. A rare and easily missed diagnosis of oesophageal mastocytosis may be missed even with appropriate oesophagogastroduodenoscopy (EGD) and biopsy if proper staining and clinical acumen are lacking. A missed diagnosis may lead to more invasive, costly and unnecessary testing. Therefore, early recognition of oesophageal mastocytosis may preclude unnecessary and at times invasive testing.
Case presentation
A 59-year-old never-smoker male with a past medical history of lifelong asthma, allergic rhinitis, hypercholesterolaemia and a pre-excitation Wolff-Parkinson-White (WPW) pattern without arrhythmias developed squeezing substernal chest pain shortly after awakening, followed by diaphoresis and bradycardia. The chest pain was not relieved by liquid antacids and increased from 4/10 to 9/10 which prompted his wife to call for emergent paramedic support. His initial ECG in the field showed bradycardia and delta waves. There was no relief with the initial sublingual nitroglycerin. However, a second dose administered in the ambulance provided complete relief of the pain. Of note, he mentioned several other episodes of chest pain, some occurred postprandial and were relieved by antacids. His primary care physician had recently advised discontinuing omeprazole therapy that he had been taking for presumed GERD. He noted an episode of chest pain 1 week prior while riding his bicycle with minimal exertion after breakfast. At that time, a 5 min rest relieved the chest pain. His only other complaint is that his seasonal allergies have become more prominent as of late. He reports no history of odynophagia, dysphagia, haemoptysis, fevers or chills.
Physical examination revealed a fit-appearing male in no acute distress. Temperature was 99.7°F, blood pressure was 140/70, a pulse of 63 beats/min, respiratory rate of 20 breaths/min and an oxygen saturation of 99% on room air. Cardiac examination revealed a normal rate and regular rhythm, no murmurs, rubs or gallops were noted. No chest wall tenderness to palpation. Pulmonary exam revealed good breath sounds throughout the lungs with minimal rales noted in the lower lobes bilaterally.
Investigations
Initial lab results demonstrated three negative sets of cardiac enzymes, troponin T <0.03 ng/mL (reference range: <0.03 ng/mL) and a creatinine kinase (CK)-MB of 4 ng/mL (reference range: <5.1 ng/mL). A basic metabolic profile was unremarkable except for a low phosphorus of 2.6 mg/dL. A complete blood count was normal with a haemoglobin of 14.1 g/dL (reference range: 13.0–16.5 g/dL), white blood cell count of 4.60 g/dL (reference range: 4.0–10.0 thou/cu mm) and a platelet count of 159 thou/cu mm (reference range: 150–450 thou/cu mm). An ECG revealed normal sinus rhythm and rate with delta waves. No ST depression or elevations were appreciated. An upright chest radiograph revealed no cardiopulmonary abnormalities. Given his history of chest pain at rest and exertion, further workup of a cardiac cause was warranted.
A subsequent myocardial perfusion scan revealed symmetric contractility in all myocardial segments with an ejection fraction of 58%, no rest or poststress perfusion defects and a sum difference score of 0. Given the negative likelihood of a cardiac aetiology of the chest pain, further workup focused on the gastrointestinal tract.
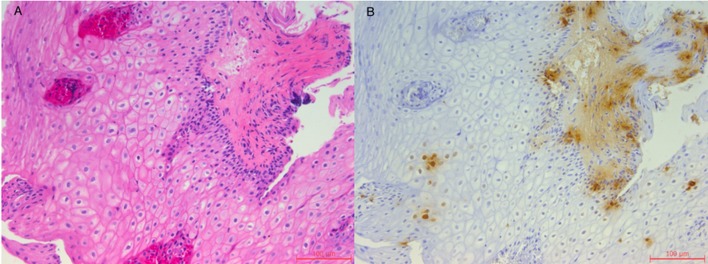
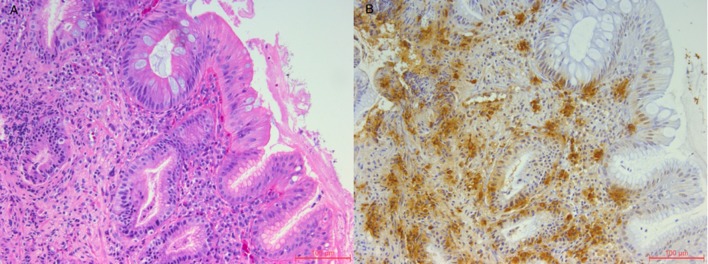
High-resolution manometry showed distal oesophageal spasms in 3/10 swallows with simultaneous contractions seen with amplitudes of 140 mm Hg and normal lower oesophageal sphincter relaxation. A subsequent EGD revealed oesophageal mucosal changes suspicious of eosinophilic oesophagitis. Biopsies of the oesophagus showed a relatively normal mid-oesophagus (figure 1A) with changes suggestive of intestinal metaplasia (no dysplasia) in the lower oesophagus (figure 2A). No Helicobacter pylori was identified on immunohistochemical staining. Given the relatively normal findings and no identifiable cause of this chest pain, it was requested that the biopsy samples undergo tryptase staining for mast cells. This showed mast cells focally in the distal oesophagus as high as 80 mast cells per high powered field (HPF) (figure 2B) and 2–6 mast cells per HPF in the mid-oesophagus (figure 1B).
Figure 1.
(A) H&E stain of the mid-oesophagus (20×) showing normal oesophageal mucosa. (B) Tryptase stain of the mid-oesophagus (20×) showing mast cells in a scattered distribution ranging from 2 to 6 cell/high powered field.
Figure 2.
(A) H&E stain of the lower oesophagus (20×) showing intestinal metaplasia. (B) Tryptase stain of the lower oesophagus (20×) showing focal mast cell accumulation ranging from 60 to 80 cell/high powered field.
Differential diagnosis
Our initial working diagnosis focused on a cardiac cause of his chest pain given his history of what appeared to be unstable and intermittently stable angina. However, given the negative cardiac workup, our differential diagnosis included a gastrointestinal cause of his symptoms. Considering this patient had episodes of nocturnal chest pain, we included GERD as a cause which can cause pain on recumbency. Oesophagitis either caused by reflux or eosinophilic oesophagitis were included, the latter being likely given his significant allergy history and asthma. Chest radiographs were not suggestive of any pulmonary cause of his chest pain and given his negative history of smoking, weight loss or haemoptysis, a malignancy as a cause of chest pain was excluded.
GERD
oesophagitis
eosinophil oesophagitis
oesophageal spasm
gastritis
peptic ulcer disease
achalasia
pulmonary embolism
pleuritis.
Treatment
Our patient was initially started on omeprazole-sodium bicarbonate 40 mg twice daily, cetirizine 10 mg daily, montelukast 10 mg nightly and oral budesonide 500 mcg daily initially which was tapered to 250 mcg daily over 1 month to be continued indefinitely.
Outcome and follow-up
He is currently doing well and his symptoms of at rest and exertional chest pain have all resolved with the above therapy.
Discussion
Oesophageal mastocytosis remains a relatively enigmatic disease characterised by the increased presence of mast cells in the oesophageal mucosa.1 Mast cells play a key role in the host–environment interface as they respond to a wide repertoire of stimuli including allergens, bacteria, parasites and stress.2 Therefore, the presence of these cells is important in normal function, and it has been demonstrated that the normal gut mucosa contains 3.79–7.6 cell/HPF.1 3 4 However, mast cell proliferation and activation by allergens, peptides or stress have been implicated in altering the gut physiology. In fact, a study by Barbara et al demonstrated that patients with irritable bowel syndrome have an increased number of mast cells in their intestinal mucosa.2 In another report by Lee et al, the presence of 66 mast cell/HPF was implicated as the cause of NCCP in a young, relatively healthy woman.4 The activation of these mast cells lead to an increase in secretion of mediators and subsequent activation of proximal nerve fibres leading to perceived abdominal or oesophageal pain. This increased neuronal activation has also been shown to have neuromuscular implications. In fact, studies have demonstrated an increased oesophageal contractility and oesophageal pressure in patients with mast cell proliferation.5 6 Here, we present a case of a healthy male with NCCP due to oesophageal mastocytosis as evident by the presence of 80 mast cells/HPF on biopsy.
NCCP can be categorised as either GERD-related NCCP or non-GERD-related NCCP with the later encompassing oesophageal dysmotility, visceral hypersensitivity, altered autonomic activity and psychological disorders.1 4 7 8 However, even in patients with non-GERD-related NCCP, a definitive diagnosis is often unattainable. As evident in our case, the lack of a thorough workup and consideration of special staining may lead to a missed diagnosis. Moreover, the EGD revealed a suspicion of eosinophilic oesophagitis (figure 1); yet, on histopathology no eosinophils were identifiable. It was not until utilisation of a tryptase stain for mastocytes that the appropriate diagnosis attained.
Because activation of mast cells lead to the release of preformed granules such as histamine, tryptase and eventual production of leukotrienes, which subsequently illicit immediate reactions, treatment should be aimed at stabilising mast cells and ameliorating the downstream complications of mast cell activation.2 We began treatment with montelukast and cetirizine which block the action of leukotriene and histamine, respectively. In addition, budesonide was initiated to decrease mucosal inflammation. With the aforementioned therapy, our patient’s chest pain resolved with no new recurrences.
Patient’s perspective.
When I experienced severe chest pain that morning, I thought that I was having ‘the big one’, that is, a heart attack. After receiving the second sublingual nitroglycerin spray in the ambulance, I felt the most incredible ‘clunk’ in my chest with sudden and complete relief of my chest pain. I remember looking at the paramedic and saying ‘You guys are good!’ Another interesting moment was when the primary team was doing the manometry study, and I saw all their eyes go wide when I felt a minor belch. They asked if that hurt, which it did not. I am very grateful to the attending and her primary team for her diagnosis and excellent management, as I have remained symptom free and enjoy a very active life.
Learning points.
Oesophageal mastocytosis remains a rare cause of non-cardiac and non-gastro-oesophageal reflux disease-related chest pain, one that may easily be overlooked. However, in a patient with the appropriate allergy history and a negative oesophageal mucosa biopsy, one should have a low threshold to consider staining for mast cells.
Activation of mast cells and subsequent degranulation activates proximal oesophageal nerves which may impact neuromuscular function leading to an increased distal oesophageal pressure and contractility.
Treatment for oesophageal mastocytosis is tailored towards prevention of mast cell activation. This includes antihistamine and antileukotriene agents.
Footnotes
Contributors: DW and JL drafted the initial body of the manuscript. JL obtained the pathology images and gave relevant descriptions. SG helped in drafingt and editing the final version of our manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Lee H, Chung H, Park JC, et al. . Heterogeneity of mucosal mast cell infiltration in subgroups of patients with esophageal chest pain. Neurogastroenterol Motil 2014;26:786–93. 10.1111/nmo.12325 [DOI] [PubMed] [Google Scholar]
- 2.Barbara G, Stanghellini V, De Giorgio R, et al. . Functional gastrointestinal disorders and mast cells: implications for therapy. Neurogastroenterol Motil 2006;18:6–17. 10.1111/j.1365-2982.2005.00685.x [DOI] [PubMed] [Google Scholar]
- 3.Yu Y, Ding X, Wang Q, et al. . Alterations of mast cells in the esophageal mucosa of the patients with non-erosive reflux disease. Gastroenterology Res 2011;4:70–5. 10.4021/gr284w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee K, Kwon HJ, Kim IY, et al. . Esophageal mast cell infiltration in a 32-year-old woman with noncardiac chest pain. Gut Liver 2016;10:152–5. 10.5009/gnl14294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park SW, Lee H, Lee HJ, et al. . Esophageal mucosal mast cell infiltration and changes in segmental smooth muscle contraction in noncardiac chest pain. Dis Esophagus 2015;28:512–9. 10.1111/dote.12231 [DOI] [PubMed] [Google Scholar]
- 6.Gao G, Ouyang A, Kaufman MP, et al. . ERK1/2 signaling pathway in mast cell activation-induced sensitization of esophageal nodose C-fiber neurons. Dis Esophagus 2011;24:194–203. 10.1111/j.1442-2050.2010.01127.x [DOI] [PubMed] [Google Scholar]
- 7.Coss-Adame E, Rao SS. A Review of esophageal chest pain. Gastroenterol Hepatol 2015;11:759–66. [PMC free article] [PubMed] [Google Scholar]
- 8.Van Handel D, Fass R. The pathophysiology of non-cardiac chest pain. J Gastroenterol Hepatol 2005;20 Suppl:S6–S13. 10.1111/j.1440-1746.2005.04165.x [DOI] [PubMed] [Google Scholar]