Abstract
We present a case of a 60-year-old woman with an invasive spinal infection with Staphylococcus pseudintermedius associated with a 15-year-old spinal fixation device and epidemiological contact with dogs. It was confirmed on blood culture and culture from pus from the epidural abscess and successfully treated using similar treatment as for a Staphylococcus aureus infection—6 weeks of intravenous flucloxacillin 2 g four times daily with a 6 week follow-on course of oral clindamycin 450 mg three times daily. This case represents the first reported deep abscess forming infection with this recently discovered organism. This case highlights that (1) S. pseudintermedius has a potential for invasive zoonotic infection, (2) treatment as for S. aureus appears adequate for resolution of the case, (3) the increased use of the matrix-assisted laser desorption/ionisation time-of-flight identification technique is leading to more specific identification of previously unrecognised organisms.
Keywords: bone and joint infections, medical management, spinal cord, neurosurgery
Background
Staphylococcus pseudintermedius is a coagulase and DNase-positive Staphylococcus that is a commensal and pathogen affecting animals, particularly dogs, but rarely humans.
Human infections have been infrequently reported but usually associated with immunocompromised patients, superficial soft tissue infections, or line-related or catheter-related infections.
Traditionally, staphylococci are speciated using biochemical tests, particularly the coagulase test. Coagulase-positive staphylococci are typically presumed to be S. aureus. With the advent of new proteomic identification techniques such as matrix-assisted laser desorption/ionisation time-of-flight (MALDI-TOF) mass spectrometry, more specific organism identification is possible with previously unrecognised organisms increasingly identified.
Case presentation
A 60-year-old woman presented to a district general hospital with a 3-day history of increasing weakness and paraesthesia in her hands, spreading to her feet, abdominal wall and left thigh. In the preceding 24 hours, she also developed shooting pains radiating from her neck to her mid-scapular region. She had also had a fall 1 week previously, but prior to that she was asymptomatic and at her functional baseline.
In terms of medical history, she had severe osteoarthritis requiring several joint replacements, a C5/6 traumatic spinal fracture requiring fixation 15 years previously, and primary biliary cirrhosis.
Normally, she required a wheelchair to mobilise, but was able to stand and transfer independently. She was unable to do so this time due to her pain and weakness. She kept two dogs at home.
On examination, there were no murmurs or signs of endocarditis. Neurologically, there was evidence of weakness in a C6–T1 distribution, with no sensory impairment. Reflexes were normal in the biceps and supinator, although reduced in the triceps. The overall neurological picture was consistent with a cervical cord compression with myelopathy. Soft tissue examination revealed pressure sores on her right elbow and presacral regions and abrasions from a recent fall, with no signs of superinfection.
Investigations
Whole spine MRI performed on admission demonstrated an anterior epidural spinal collection causing compression at C6/7 with extension superiorly to C3 and inferiorly to T3, associated with metal work from previous fracture (see figures 1-3).
Figure 1.
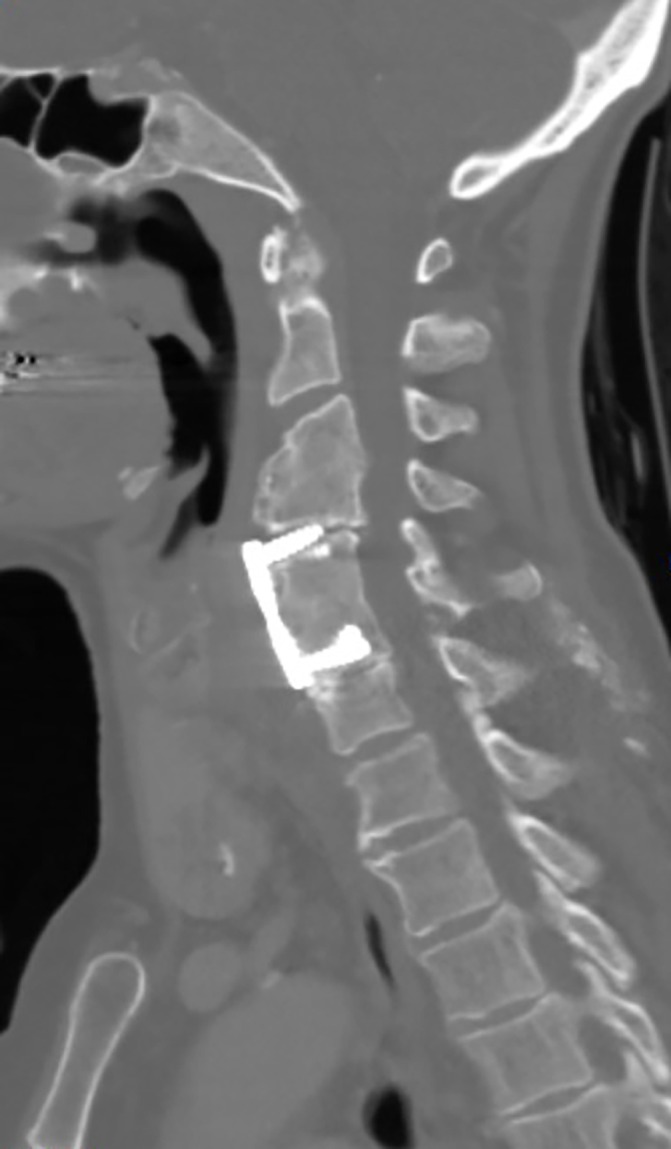
CT scan with contrast of the cervical spine demonstrating the existing metal fixation device.
Figure 2.
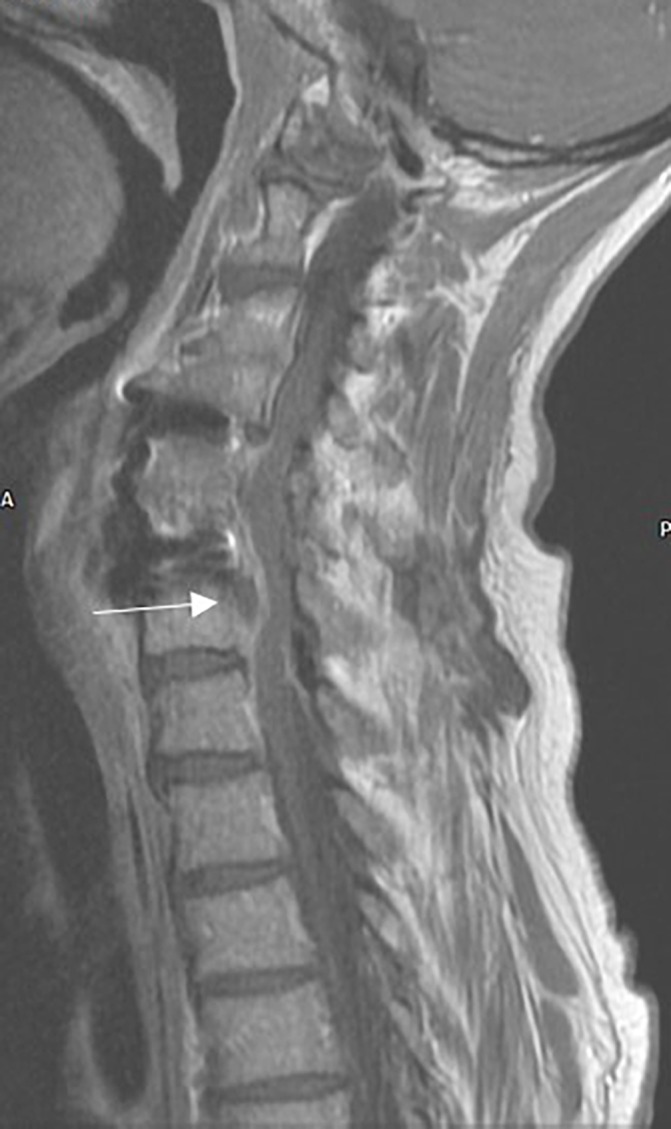
T1-weighted MRI scan (sagittal orientation) with contrast demonstrating the epidural collection (highlighted with arrow) associated with the metal fixation device.
Figure 3.
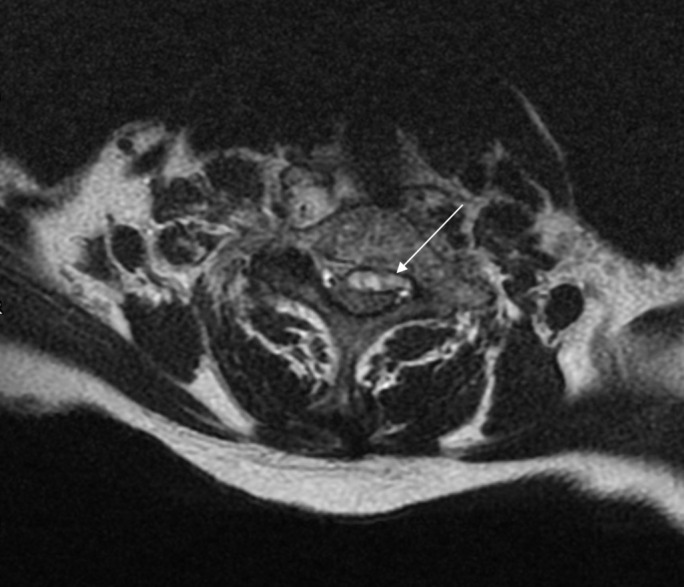
T2-weighted MRI scan (axial orientation) at C6/7 level demonstrating compression of the spinal cord by the collection (highlighted by arrow). Note the lack of high-signal cerebrospinal fluid around the cord.
Blood tests from the initial presentation demonstrated an inflammatory response with a white cell count of 13.66×109/L (4.0–11.0), a neutrophil count of 8.23×109/L (2.0–7.0) and a C-reactive protein level of 194.2 mg/L (0–5). The alkaline phosphatase (ALP) level was 1040 IU/L (30–130) with otherwise normal liver function tests. An ALP level 3 months earlier was 1480 IU/L, and therefore this likely reflects comorbid primary biliary cirrhosis.
Blood cultures taken on presentation grew a Gram-positive coccus in one set of aerobic and anaerobic bottles. When cultured on a blood agar plate, it grew small (~2 mm) round white colonies, typical of staphylococci. This was subsequently identified by MALDI-TOF (Bruker) as S. pseudintermedius with sensitivities determined by disc diffusion method (using EUCAST MIC breakpoints) as follows: resistant to penicillin; sensitive to flucloxacillin, erythromycin, clindamycin, co-trimoxazole, vancomycin and rifampicin. Biochemical tests, such as the coagulase test, were not performed as these have largely been superseded by the MALDI-TOF.
The identified S. pseudintermedius was initially thought to be a contaminant.
Treatment
The patient was transferred within 24 hours to a regional neurosurgical centre. She received urgent decompressive surgery with revision of the discectomy and removal of the aforementioned C6/7 fixation metal work, which was intimately involved with the epidural collection.
The patient was initially treated empirically with intravenous co-amoxiclav 1.2 g three times a day and intravenous vancomycin 1 g twice daily. Repeat blood cultures taken 1 day after she was started on antibiotics were negative. However, cultures from the pus collection and metal work grew the same S. pseudintermedius (three out of three operative samples), confirmed by MALDI-TOF (Bruker) at the tertiary centre. The sensitivities were identical as above, confirmed via automated susceptibility testing (BD Phoenix). Postoperative MRI demonstrated resolution of the epidural collection with osteomyelitis of the C3–7 vertebrae, along with severe loss of intervertebral disc material with severe spinal stenosis at C3/4–C6/7. A subsequent transthoracic echocardiogram demonstrated no evidence of infective endocarditis.
The patient's antibiotics were subsequently rationalised to intravenous flucloxacillin 2 g four times daily on day 6 of admission, to continue as an inpatient for 6 weeks post-surgery. Afterwards, she was switched to 6 weeks oral clindamycin 450 mg by mouth three times daily.
Outcome and follow-up
Following successful source control of the infection and an appropriate antibiotic plan, the patient was transferred to a spinal rehabilitation centre. Three months later, she had completed her antibiotic course, and was self-caring independently. Her neurological function was improving but still impaired with residual weakness only in her left second and third fingers and no sensory loss. By 6 months, she had fully recovered to baseline neurological function and was awaiting a planned replacement fixation of her cervical spine.
Discussion
S. pseudintermedius is a coagulase and DNase-positive Gram-positive bacteria, first characterised in 2005 after differentiation from the similar bacteria S. intermedius.1 It is a common commensal organism of animals, particularly dogs2 and is a cause of soft tissue infections in such animals.3 It has virulence factors similar to S. aureus, although these have yet to be fully characterised.3 In addition, it seems to have a higher rate of methicillin resistance than S. aureus, with rates of resistance reported as high as 66.5% in some veterinary clinical cohorts.4
It rarely causes disease in humans. A retrospective study of isolates associated with dog bites demonstrated mis-identification of S. pseudintermedius as S. aureus in 12.87% of cases (16/101) by identification of S. pseudintermedius specific genes by PCR.5 Isolated case reports have identified S. pseudintermedius as a cause of a wound infection in an immunocompromised patient (isolated from pus sample),6 a postoperative infection of a frontal sinus (isolated from pus sample),7 in patients with infections of recently implanted cardiac defibrillator devices (isolated from pus samples)8 9 and a vascular catheter line infection in a bone marrow transplant patient (isolated from blood culture taken from line).10 More recently, a case series has identified S. pseudintermedius as a cause of infection in several superficial soft-tissue infections, a prosthetic knee and a dialysis vascular access fistula.11 Most of these cases had epidemiological exposure of the patient to dogs. Of the clinical cases referenced above, 6/38 (15.8%) had an isolate resistant to methicillin. In the cases where outcome is reported, all (5/5) made a complete recovery from the infection with appropriate antibiotic treatment based on sensitivities.
This case illustrates the first reported case of a deep abscess forming infection by S. pseudintermedius. All previous reported cases have described infection in patients with immunodeficiency or the presence of recently inserted prosthetic material. In this case, the fracture fixation device in the patient's cervical spine, which formed the focus around which the epidural collection developed, had been implanted 15 years previously. The case was also unusual in that it was associated with bacteraemia. No reported case to date has demonstrated a bloodstream infection, except in those with infected vascular access devices.
We hypothesise that the organism entered the bloodstream via pressure sores due to contact with the patient's dog. The organism then spread haematogenously to the prosthetic device, causing formation of a collection, before causing bacteraemia and spinal compression.
There are some limitations to this case. Speciation of staphylococci (including S. pseudintermedius) by MALDI-TOF is usually excellent, with a specificity level for identification of S. pseudintermedius of 97%.12 This makes a false positive result unlikely, but nevertheless this remains a possibility. Ideally, identification using PCR to genetically sequence the isolate would have been performed, but this was not available in this case.
The implications of this case are threefold. First, this illustrates the rise in incidence of a canine commensal with pathogenic zoonotic potential that is difficult to distinguish from the more common S. aureus. From this case, it seems that the organism has potential for invasive disease similar to that caused by S. aureus.
Second, beyond aetiological interest, the identification of this organism may change clinical practice due to the higher rate of methicillin resistance in S. pseudintermedius. This may warrant consideration of vancomycin as an empirical antibiotic until sensitivities are known.
Finally, the case highlights that the use of MALDI-TOF allows more precise and specific identification of bacterial organisms than that available previously. This does, however, mean that physicians need to be vigilant when faced with an unfamiliar identified species from the MALDI-TOF. In particular, verification of the significance of such an unfamiliar organism should be required before discounting it as a contaminant.
Learning points.
Staphylococcus pseudintermedius is a known pathogen of animals, but is increasingly recognised as a potential cause of zoonotic infection, particularly associated with canine contact.
Treatment as for S. aureus infection seems adequate in this case (ie, 6 weeks intravenous antibiotics, followed by 6 weeks of oral antibiotics based on sensitivities). However, the background rate of methicillin resistance might prompt consideration of alternative antibiotics, such as vancomycin, as an empirical antibiotic choice until the full sensitivities are known.
The MALDI-TOF identification system is identifying organisms that would otherwise have been labelled as other species. It is important for physicians to be alert to this and to verify the significance of unfamiliar organisms.
Footnotes
Contributors: CD collected the case data and was the primary author of the manuscript. NP and MS assisted with the drafting of the paper, reviewed the final draft and provided the figures for inclusion in the paper. BA provided input into the design of the paper, assisted with the drafting of the paper, reviewed the drafts and provided senior oversight of the writing process.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Devriese LA, Vancanneyt M, Baele M, et al. . Staphylococcus pseudintermedius sp. nov., a coagulase-positive species from animals. Int J Syst Evol Microbiol 2005;55:1569–73. 10.1099/ijs.0.63413-0 [DOI] [PubMed] [Google Scholar]
- 2.Rubin JE, Chirino-Trejo M. Prevalence, sites of colonization, and antimicrobial resistance among Staphylococcus pseudintermedius isolated from healthy dogs in Saskatoon, Canada. J Vet Diagn Invest 2011;23:351–4 http://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=21398462&site=ehost-live 10.1177/104063871102300227 [DOI] [PubMed] [Google Scholar]
- 3.van Duijkeren E, Catry B, Greko C, et al. . Review on methicillin-resistant Staphylococcus pseudintermedius. J Antimicrob Chemother 2011;66:2705–14. 10.1093/jac/dkr367 [DOI] [PubMed] [Google Scholar]
- 4.Kawakami T, Shibata S, Murayama N, et al. . Antimicrobial susceptibility and methicillin resistance in Staphylococcus pseudintermedius and Staphylococcus schleiferi subsp. coagulans isolated from dogs with pyoderma in Japan. J Vet Med Sci 2010;72:1615–9. 10.1292/jvms.10-0172 [DOI] [PubMed] [Google Scholar]
- 5.Börjesson S, Gómez-Sanz E, Ekström K, et al. . Staphylococcus pseudintermedius can be misdiagnosed as Staphylococcus aureus in humans with dog bite wounds. Eur J Clin Microbiol Infect Dis 2015;34:839–44. 10.1007/s10096-014-2300-y [DOI] [PubMed] [Google Scholar]
- 6.Savini V, Barbarini D, Polakowska K, et al. . Methicillin-resistant Staphylococcus pseudintermedius infection in a bone marrow transplant recipient. J Clin Microbiol 2013;51:1636–8. 10.1128/JCM.03310-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stegmann R, Burnens A, Maranta CA, et al. . Human infection associated with methicillin-resistant Staphylococcus pseudintermedius ST71. J Antimicrob Chemother 2010;65:2047–8. 10.1093/jac/dkq241 [DOI] [PubMed] [Google Scholar]
- 8.Riegel P, Jesel-Morel L, Laventie B, et al. . Coagulase-positive Staphylococcus pseudintermedius from animals causing human endocarditis. Int J Med Microbiol 2011;301:237–9. 10.1016/j.ijmm.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 9.Van Hoovels L, Vankeerberghen A, Boel A, et al. . First case of Staphylococcus pseudintermedius infection in a human. J Clin Microbiol 2006;44:4609–12. 10.1128/JCM.01308-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuang CY, Yang YL, Hsueh PR, et al. . Catheter-related bacteremia caused by Staphylococcus pseudintermedius refractory to antibiotic-lock therapy in a hemophilic child with dog exposure. J Clin Microbiol 2010;48:1497–8. 10.1128/JCM.02033-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Somayaji R, Priyantha MA, Rubin JE, et al. . Human infections due to Staphylococcus pseudintermedius, an emerging zoonosis of canine origin: report of 24 cases. Diagn Microbiol Infect Dis 2016;85:471–6. 10.1016/j.diagmicrobio.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 12.Decristophoris P, Fasola A, Benagli C, et al. . Identification of Staphylococcus intermedius Group by MALDI-TOF MS. Syst Appl Microbiol 2011;34:45–51. 10.1016/j.syapm.2010.11.004 [DOI] [PubMed] [Google Scholar]


