Abstract
A 70-year-old man with non-ischaemic dilated cardiomyopathy presented with symptoms of fatigue, chills and unintentional weight loss over the past 2 months. Initial evaluation revealed anaemia, peripheral leucocytosis and elevated inflammatory markers. Results of an oesophagogastroduodenoscopy, colonoscopy, blood bacterial and fungal cultures and bone marrow biopsy were negative. An 18F-FDG positron-emission tomography-CT demonstrated an indeterminate, intensely FDG-avid 5 cm × 2 cm × 5.6 cm × 6.7 cm mass centred within the junction of the superior vena cava and right atrium, suggestive of probable malignancy versus an inflammatory thrombus. After multidisciplinary consideration, patient underwent a diagnostic minithoracotomy and a thick fibrotic mediastinal mass was visualised and evacuated. The encapsulated mass contained thick, white creamy liquid that appeared to be purulent/necrotic material. The biopsies of the capsule wall on frozen section demonstrated fungal elements consistent with Aspergillosis species. Fungal culture confirmed diagnosis of Aspergillus fumigatus.
Keywords: infectious diseases, cardiothoracic surgery
Background
Invasive aspergillosis is an infrequent life-threatening infection. Classically, it affects immunocompromised hosts in the settings of myeloablative chemotherapy for haematological malignancies or antirejection therapy for solid organ transplants. Healthcare-associated invasive aspergillosis presenting as surgical site infection is rare and may occur in both immunocompetent and immunocompromised hosts. It may present as superficial surgical site infection or deep organ-space infection.1 Establishing an aetiological diagnosis is often challenging given the non-specific clinical presentation. This case highlights the importance of a targeted diagnostic and therapeutic approach that lead to the diagnosis of delayed postvenous reconstruction mediastinal aspergilloma with possible endovascular invasion in an immunocompetent host.
Case presentation
A 70-year-old man presented to Mayo Clinic Rochester with complains of fatigue, malaise, chills and unintentional weight loss of 8 lbs over of the past 2 months. He had a complicated medical history that included non-ischaemic dilated cardiomyopathy (ejection fraction: 25%) and status postcardiac arrest with subsequent dual-chamber implantable cardioverter defibrillator (ICD) implantation. Other significant comorbidities including atrial fibrillation, factor V Leiden deficiency, history of lower extremity deep vein thrombosis (DVT), hypertension, hyperlipidaemia, thoracic aortic aneurysm, obstructive sleep apnoea and postpolio syndrome. He developed recurrent superior vena cava (SVC) syndrome secondary to indwelling ICD leads and had to undergo multiple surgeries including removal of ICD generator and leads, percutaneous transluminal angioplasty, SVC stenting and new dual-chamber ICD implantation. A year before his presentation to our institution, he developed SVC syndrome again and had to undergo another explantation of the ICD leads along with SVC reconstruction and right internal jugular vein to right atrial bypass with epicardial defibrillator placement.
Investigations
Initial evaluation for the current presentation demonstrated microcytic anaemia (Hemoglobin: 6.3 g/dL, mean corpuscular volume (MCV): 80.1 fL) and peripheral leucocytosis (12.7×109/L) along with elevated inflammatory markers (ESR 120 mm/hour, C-reactive protein (CRP) 153.3 mg/L). To investigate weight loss, he underwent oesophagogastroduodenoscopy and colonoscopy and both were unremarkable. Duodenal biopsy was unrevealing, with no evidence of Whipple's disease, coeliac sprue or giardiasis. Blood cultures were negative as well. Given his persistent haematological abnormalities, he underwent a bone marrow biopsy, which did not reveal any evidence of haematological malignancy or lymphoproliferative disorder. Bacteria, fungal and mycobacteria stains and cultures of bone marrow were negative as well.
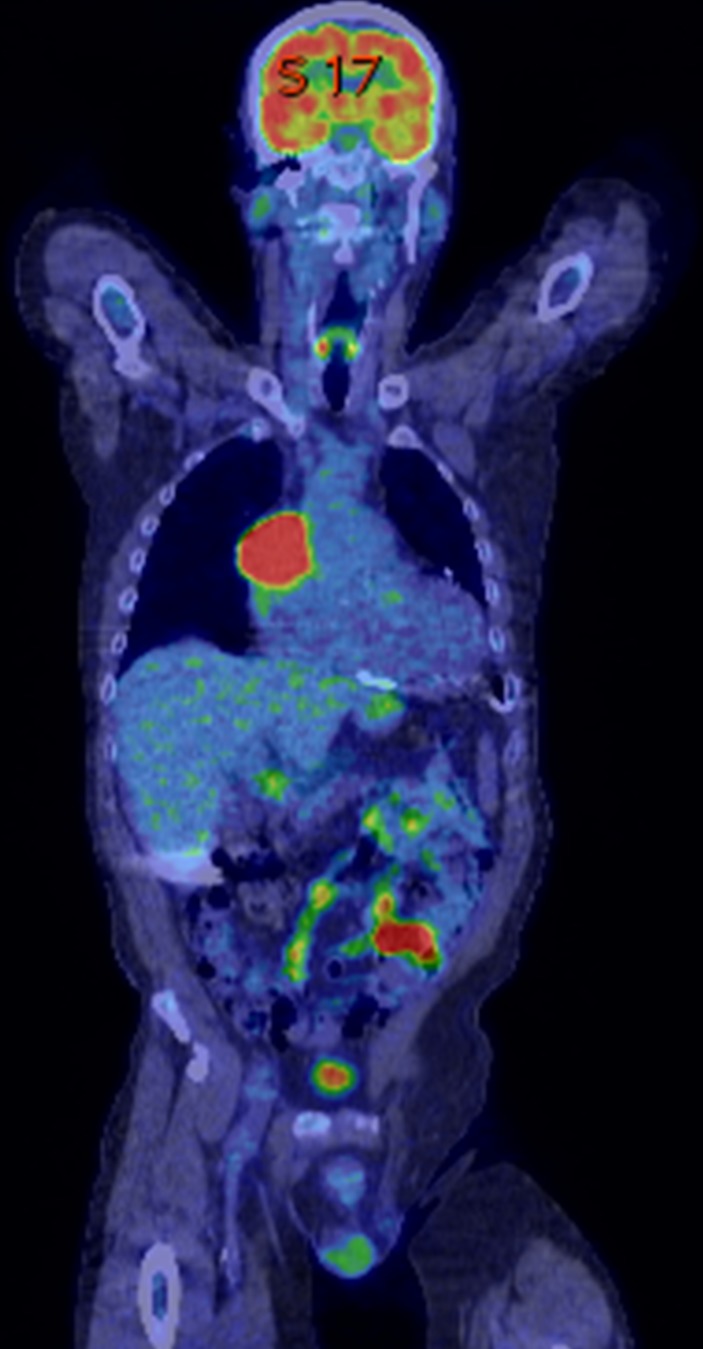
An 18F-FDG positron-emission tomography-CT (PET-CT) demonstrated an indeterminate, intensely FDG-avid 5 cm × 2 cm × 5.6 cm × 6.7 cm mass centred within the junction of the SVC and right atrium (RA). Appearance of mass was suggestive of probable malignancy versus inflammatory thrombus (see figure 1). Additionally, CT angiography of chest showing an ill-defined mass (arrow) at the SVC and right atrium junction (see figure 2). Due to concern for infection, several other lab tests were obtained including Aspergillus serum antigen, Cryptococcus serum antigen, urine Histoplasma antigen and blood serology for Sporothrix, Coccidioides, Histoplasma, Blastomyces, and Coxiella burnetti. All of these were reported. Fungal and mycobacterial blood culture were obtained and remained negative.
Figure 1.
An indeterminant, intensely FDG avid (standardized pptake value (SUV), maximum 14.2), 5.2 cm × 5.6 cm × 6.7 cm mass is centred within the junction of the superior vena cava and right cardiac atrium.
Figure 2.
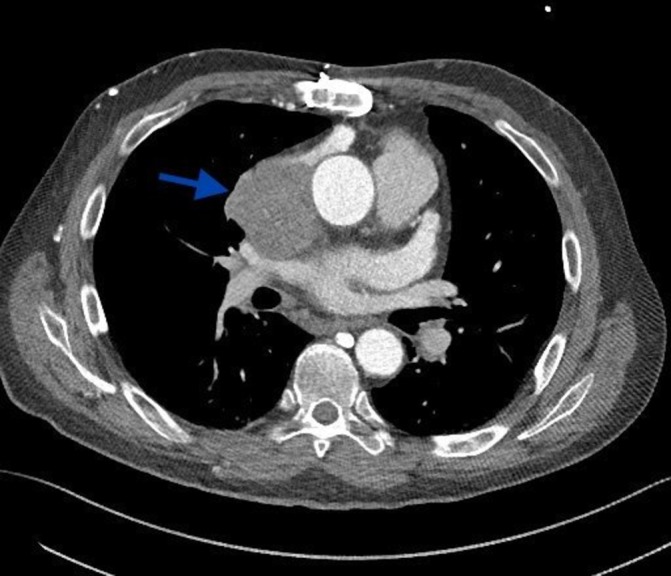
CT angiography of chest showing an ill-defined mass (arrow) at the superior vena cava and right atrium junction.
Due to negative blood work, cardiovascular surgery performed an exploratory right minithoracotomy. On intraoperative exploration, a thick fibrotic, encapsulated mediastinal mass was visualised that contained thick, white creamy liquid that appeared to be purulent/necrotic material (see figure 3). The entire mass was evacuated, and a sample was sent for multiple frozen sections as well as cultures, including fungal culture. The biopsies of the capsule wall on frozen section demonstrated narrow branching fungal elements consistent with Aspergillus-like mould. Cultures confirmed the suspected diagnosis of Aspergillus fumigatus.
Figure 3.
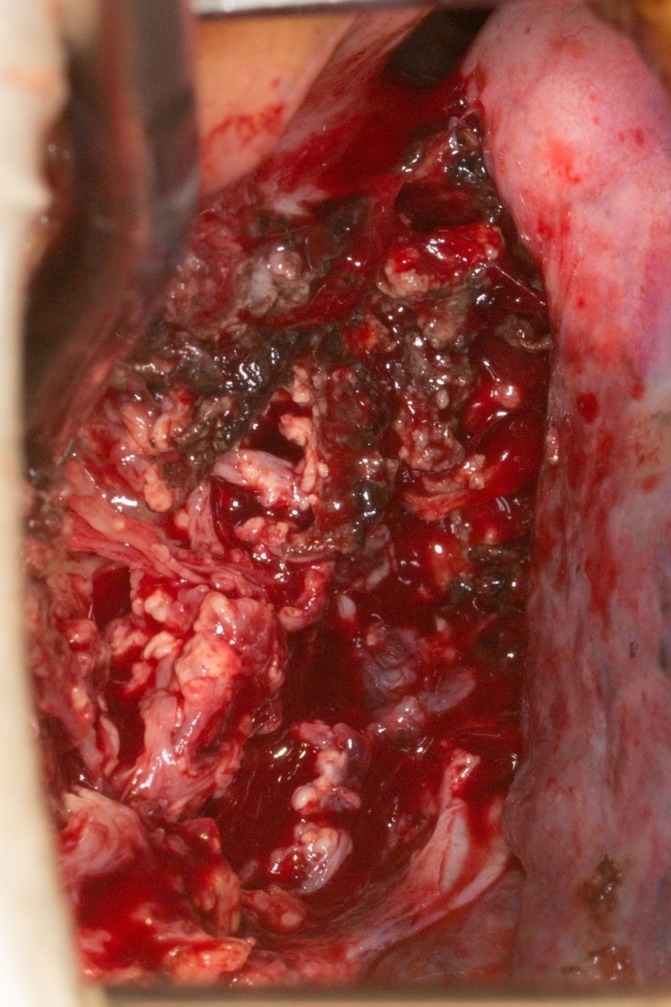
Intraoperative findings of a fibrotic mass with tissue necrosis and purulent material in the encapsulated cavity.
Treatment
The patient was empirically started on voriconazole and caspofungin. However, voriconazole was switched to isavuconazole due to development of QT interval prolongation. Repeat CT of the chest done 3 days after surgery revealed findings consistent with debridement of the inflammatory mass along the SVC/RA, with air and fluid collection in the area. The intracardiac involvement of the mass showed subtle areas of enhancement, which would suggest propagating SVC thrombus with superimposed Aspergillus infection (Aspergilloma), with the enhancing components suggesting areas of inflammation/infection and the non-enhancing areas representing thrombus. Due to high risk of another surgical intervention, medical management with dual antifungal therapy for 6 weeks with follow-up as an outpatient to make further decisions was chosen for subsequent management.
Outcome and follow-up
The patient tolerated the dual antifungal therapy with caspofungin and isavuconazole. The former was discontinued after 7 weeks, and the latter was continued as long-term oral suppressive therapy. A CT scan of the chest at the 7-week follow-up showed improvement with some residual inflammatory changes. Patient was healthy and doing well at the 6-month follow-up.
Discussion
Aspergillus species are ubiquitous in nature, and human infection mostly occurs through inhalation. Invasive pulmonary aspergillosis can affect patients with different stages of immunosuppression2 and has been associated with high mortality rates. Cardiac involvement with Aspergillus species is rare, and timely diagnosis requires a high index of suspicion. Cardiac aspergillosis may manifest as endocarditis or diffuse pancarditis.3 Aspergillus endocarditis is usually associated with a cardiovascular prosthesis, such as prosthetic valves, but may involve non-valvular endocardial surfaces in patients with structural heart defects.3
Postsurgical invasive aspergillosis is rare but devastating complication of cardiac surgery and has been reported in immunocompetent patients. Previously reported cases of postcardiac surgery aspergillosis include open heart procedures such as coronary artery bypass and heart transplant,1 4 5 deep sternal wound infection, aortitis6 and patch infection after repaired tetralogy of Fallot.2 7 8 These infections are typically seen within the first 30 days following the surgery due to low immunity but can be indolent3 in some cases occurring several months after surgery. In most patients, the fungal spores seem to have originated from the air during surgery, but contamination from paranasal sinuses, bronchopulmonary lesions, haematogenous dissemination and contaminated grafts is also possible.7 Airborne transmission of Aspergillus infection has also been reported due to contamination of the operating room environment7 9 from geographically close hospital renovation activities, and it is vital to emphasise the need for implementing environmental control according to Centers for Disease Control and Prevention (CDC) guidelines.10
Our patient is the first reported case of delayed mediastinal infection with Aspergillus after a venous reconstruction surgery. After the pathology results were reported in our patient, we went back and found that he had documented airway colonisation with Aspergillus prior to surgery in 2012. It is conceivable that patient suffered some airway trauma during his intubation/extubation that lead to a breach in continuity of airway lining with invasion of fungal organisms and the slowly growing mass. Purported risk factors for postsurgical Aspergillus mediastinitis9 11–14 include old age, obesity, chronic obstructive pulmonary disease, diabetes mellitus, history of previous cardiac surgery, use of steroids or antibiotics during the hospital admission, preoperative renal failure, blood transfusions, re-exploration and emergent surgery. Our patient had many of these risk factors including old age, obesity, history of previous cardiac surgery, use of antibiotics and urgent nature of surgical intervention.
There is one prior case report of aspergilloma of the heart in an immunocompetent patient.15 This was a 22-year-old patient with chronic hepatitis B virus infection presenting with weight loss and pain in the lower extremities. Doppler of the lower extremities revealed thrombus in the right common iliac and right external iliac arteries. A transthoracic echocardiogram revealed a large tumour involving the inferior and inferolateral wall of the left ventricle. A transcatheter cardiac biopsy was done and showed Aspergillus. The authors suggested a functional immunocompromised status due to the high viral load of hepatitis B virus. The patient was treated with voriconazole for cardiac aspergilloma and entecavir for hepatitis B virus infection. A repeat transthoracic echocardiogram after 3 months of treatment showed a reduction in the size of the mass.
Cardiac aspergillosis is associated with high mortality, and early diagnosis requires a high index of clinical suspicion.3 10 Diagnostic clues may include slowly progressive and destructive deep surgical site infection with negative bacterial cultures. Medical therapy alone is associated with a mortality rate of 32% to 57%.3 Poor response to antifungal therapy alone may be, in part, due to limited ability of antifungal agents to penetrate endocardial vegetations and fungal mass. Successful intracavitary treatment using CT-guided percutaneously placed catheters to instil amphotericin B deoxycholate has been reported in a small number of patients.10
The treatment duration for patients with postsurgical aspergillosis remains undefined.2 It is recommended that after extensive surgical debridement, antifungals should be administered for at least 3 months from the last evidence of active disease.3 Voriconazole remains the antifungal agent of choice. There are case reports that describe favourable outcome at 1-year interval with shorter 6 weeks course of antifungal therapy after aggressive surgical debridement.1 Moreover, patients with sternal wound infection with Aspergillus may be successfully treated with medical therapy alone, using induction with an amphotericin B product and continuation with oral itraconazole therapy.4 10
Combination therapy of an echinocandin with an azole is better studied in patients with neutropaenia.16 In our case with the concern for incomplete resection, a more aggressive approach with combination therapy with caspofungin and voriconazole was used. However, due to a prolonged corrected QT (QTc) interval with voriconazole use, it was switched to isavucunazole for long-term oral suppressive therapy. Lifelong antifungal therapy was chosen to reduce the likelihood for infection relapse.2
Learning points.
Delayed surgical site infections due to Aspergillus species are extremely rare and can be difficult to diagnose. Diagnostic clues may include slowly progressive and destructive deep surgical site infection with negative bacterial cultures.
Postcardiac surgery aspergillosis is a life-threatening complication, classically described after open heart surgery or heart transplant.1 5 However, vascular reconstruction patient may be at risk as in our case.
Appropriate utilisation of positron-emission tomography/CT scanning can be helpful in early diagnosis of deep mediastinal infection.
Complete surgical resection and appropriate antifungal therapy may improve outcomes.
Footnotes
Contributors: All authors have contributed significantly to manuscript drafting, revision and discussion. Case report planning: SF and MRS. Acquisition of data: SF, MRS, NA and OA. Analysis and interpretation of data: SF, MRS, NA and OA. Drafting of the manuscript: SF and OA. Critical revision of the manuscript for important intellectual content: MRS, OA and SF. Case report supervision: MRS.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.El-Sayed Ahmed MM, Almanfi A, Aftab M, et al. . Aspergillus mediastinitis after orthotopic heart transplantation: a case report. Tex Heart Inst J 2015;42:468–70. 10.14503/THIJ-14-4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen J, Guinea J, Torres-Narbona M, et al. . Post-surgical invasive aspergillosis: an uncommon and under-appreciated entity. J Infect 2010;60:162–7. 10.1016/j.jinf.2009.11.005 [DOI] [PubMed] [Google Scholar]
- 3.Rajbanshi BG, Hughes JE, DeSimone DC, et al. . Surgical excision of invasive aspergillosis of the right ventricle presenting as intractable ventricular arrhythmia and right ventricular mass. Mayo Clin Proc 2012;87:926–8. 10.1016/j.mayocp.2012.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forestier E, Remy V, Lesens O, et al. . A case of Aspergillus mediastinitis after heart transplantation successfully treated with liposomal amphotericin B, caspofungin and voriconazole. Eur J Clin Microbiol Infect Dis 2005;24:347–9. 10.1007/s10096-005-1327-5 [DOI] [PubMed] [Google Scholar]
- 5.Grossi P, Farina C, Fiocchi R, et al. . Prevalence and outcome of invasive fungal infections in 1,963 thoracic organ transplant recipients: a multicenter retrospective study. Italian Study Group of Fungal Infections in thoracic organ transplant recipients. Transplantation 2000;70:112–6. [PubMed] [Google Scholar]
- 6.Leffert RL, Hackett RL. Aspergillus aortitis following replacement of aortic valve. J Thorac Cardiovasc Surg 1967;53:866–74. [PubMed] [Google Scholar]
- 7.Hedayati MT, Khodavaisy S, Alialy M, et al. . Invasive aspergillosis in intensive care unit patients in Iran. Acta Medica 2013;56:52–6. 10.14712/18059694.2014.24 [DOI] [PubMed] [Google Scholar]
- 8.Kawasaki S, Naitoh Y, Takagaki Y, et al. . [Two cases of Aspergillus endocarditis after cardiac surgery in childhood]. Kyobu Geka 1996;49:673–6. [PubMed] [Google Scholar]
- 9.Lutz BD, Jin J, Rinaldi MG, et al. . Outbreak of invasive Aspergillus infection in surgical patients, associated with a contaminated air-handling system. Clin Infect Dis 2003;37:786–93. 10.1086/377537 [DOI] [PubMed] [Google Scholar]
- 10.Florio M, Marroni M, Morosi S, et al. . Nosocomial Aspergillus flavus wound infections following cardiac surgery. Infez Med 2004;12:270–3. [PubMed] [Google Scholar]
- 11.Ghotaslou R, Parvizi R, Safaei N, et al. . A case of Aspergillus fumigatus mediastinitis after heart surgery in Madani Heart Center, Tabriz, Iran. Prog Cardiovasc Nurs 2008;23:133–5. 10.1111/j.1751-7117.2008.00003.x [DOI] [PubMed] [Google Scholar]
- 12.Vandecasteele SJ, Boelaert JR, Verrelst P, et al. . Diagnosis and treatment of Aspergillus flavus sternal wound infections after cardiac surgery. Clin Infect Dis 2002;35:887–90. 10.1086/342699 [DOI] [PubMed] [Google Scholar]
- 13.Nevado Portero J, Ruiz Borrell M, Gomez Izquierdo L. Contained aortic rupture secondary to post-surgery mediastinitis due to Aspergillus fumigatus. Eur Heart J 2007;28:153 10.1093/eurheartj/ehl178 [DOI] [Google Scholar]
- 14.Diez C, Koch D, Kuss O, et al. . Risk factors for mediastinitis after cardiac surgery - a retrospective analysis of 1700 patients. J Cardiothorac Surg 2007;2:23 10.1186/1749-8090-2-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soman SO, Vijayaraghavan G, Padmaja NP, et al. . Aspergilloma of the heart. Indian Heart J 2014;66:238–40. 10.1016/j.ihj.2013.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caballero MJ, Mongardon N, Haouache H, et al. . Aspergillus mediastinitis after cardiac surgery. Int J Infect Dis 2016;44:16–19. 10.1016/j.ijid.2016.01.014 [DOI] [PubMed] [Google Scholar]