Abstract
Ectopic varices (ECV) occur along the gastrointestinal (GI) tract outside the common variceal sites and represent 2%–5% of all GI variceal bleeds with mortality rates up to 40%. Management is challenging because of inaccessibility and increased risk of rebleeding. We report what is to our knowledge the first clinical use of a new microvascular plug (MVP) with transjugular intrahepatic portosystemic shunt (TIPSS) for a bleeding duodenal varix (DV). A 68-year-old man presented with melena. Endoscopy demonstrated a grade II varix in the second part of the duodenum with red wale sign. TIPSS was performed and portogram revealed a single DV. Poststent placement venogram revealed a persistent varix and hence a 5–7 mm MVP was deployed. Subsequent imaging showed cessation of blood through the DV. The patient had no further bleeding. TIPSS with embolisation is an effective treatment for ECV. This MVP offers advantages due to its size and compatibility and can be redeployed in case of suboptimal placement.
Keywords: varices, Gi bleeding, cirrhosis
Background
Ectopic varices (ECVs) represent 2%–5% of all gastrointestinal (GI) tract variceal bleeding cases; however, ECVs have a fourfold increased risk of bleeding when compared with oesophageal varices and have a mortality rate up to 40%. Management of bleeding ECVs can be challenging both because of inaccessibility and initial difficulty in diagnosis, which is further complicated by increased risk of rebleeding with standard endoscopic techniques. We report the use of a new microvascular plug (MVP) device with transjugular intrahepatic portosystemic shunt (TIPSS) for a bleeding duodenal varix.
Case presentation
A 68-year-old man with history of alcohol abuse presented to the emergency room with complaints of black tarry stool. He also complained of nausea and one episode of blood-tinged vomiting. He denied any abdominal pain, yellowish discolouration of the skin, any change in bowel habits or weight loss. The patient reported that he has been taking over-the-counter ibuprofen two to three times a day as needed for the last 4 weeks for his low back pain. The patient also admitted to being a current smoker with a 30 pack-years smoking history. He drank four to five beers every day and also had history of intravenous drug use in the past. There was no significant family history.
Investigations
Initial lab work was significant for a haemoglobin and haematocrit of 7.9 g/dL (13.5–18 g/dL) and 24.2% (40%–54%) with MCV of 81 fL (78–100 fL). His platelet counts were low at 65×109/L. The patient also had some elevation of liver enzymes, with an Aspartate Aminotransferase of 92 U/L (5–40 U/L), Alanine Aminotransferase of 62 U/L (7–52 U/L), alkaline phosphatase of 75 U/L (34–104 U/L), total bilirubin of 1.1 mg/dL (0.3–1 mg/dL) and direct component of 0.4 mg/dL (0.0–0.2 mg /dL), with Lactate Dehyrogenase of 250 U/L (125–220 U/L). Hepatitis C antibody was found to be positive with PCR-based testing revealing a viral load of 1 050 000 IU/mL. Hepatitis A serology was negative, whereas both hepatitis B surface antibody and hepatitis B core antibody were positive. Right upper quadrant ultrasound demonstrated diffuse heterogeneous echogenicity of liver parenchyma with a 2.5 cm hypoechoic mass in the left lobe. A CT scan of the abdomen revealed a nodular liver consistent with cirrhosis along with multiple enhancing lesions with a maximum size of 2.0×1.8 cm. The portal vein and its tributaries were patent. Upper endoscopy showed no oesophageal varices. There was an evidence of a solitary grade II (>5 mm) varix in the second part of the duodenum with the red wale sign, and there was no evidence of active bleeding (figure 1).
Figure 1.
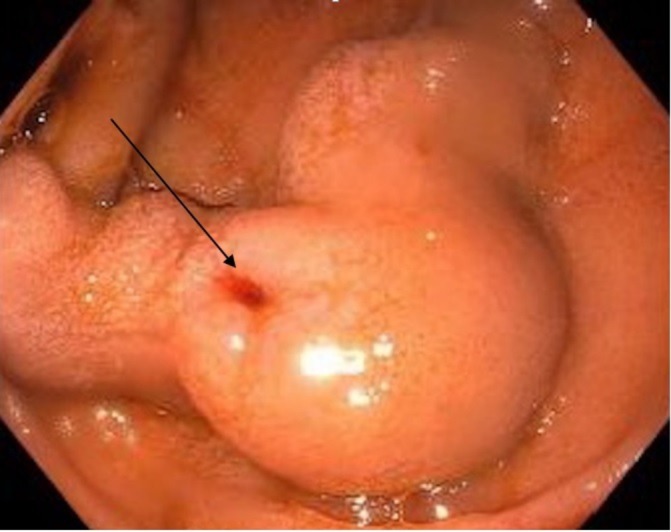
Oesophagogastroduodenoscopy demonstrating a large grade II (>5 mm) varix in the second part of the duodenum with the ‘Red Wale sign’ (arrow).
Treatment
Intervention radiology (IR)-guided TIPSS was performed using a 0.035 inch glide wire and a 5 French (Fr) multipurpose angled catheter. Free and wedged hepatic pressures were measured with a mean portosystemic pressure of 19 mm Hg. The intrahepatic parenchymal tract was dilated and an 8 cm × 10 mm Viatorr stent was placed, and a post-TIPSS venogram was obtained, which demonstrated excellent shunting of portal blood flow via the stent. The post-TIPSS venous pressure measurement revealed a reduction in mean portosystemic pressure to 4 mm Hg. Selective sonography of the varix still showed the presence of a dominant varix extending into the lumen of the duodenum. A 5 Fr multipurpose catheter was used to apply a 5–7 mm MVP to occlude the varix. Postapplication imaging showed cessation of blood through the varix (figure 2).
Figure 2.
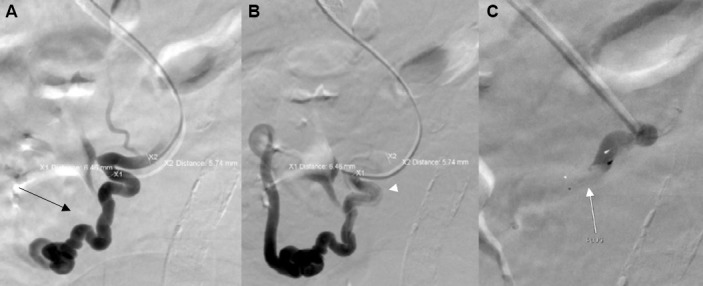
(A) Portogram showing single dominant duodenal varix (black arrow). (B) Premicrovascular plug deployment selective venogram (white arrowhead). (C) Post plug deployment over the wire venogram demonstrating instant occlusion (white arrow).
Outcome and follow-up
The patient had no further episodes of GI bleeding and was discharged home after 3 days with close outpatient follow-up.
Discussion
ECVs are dilated splanchnic (mesoportal) veins or are dilated portosystemic collaterals that can occur along the entire GI tract outside the common pathological variceal sites.1 These ECVs have been reported to occur at several GI sites such as the small intestine, colon, rectum and peritoneum. Duodenum varices comprise 17% of ECVs, with duodenum bulb being the most common site followed by the second portion of the duodenum.2 These have been found to be associated with extrahepatic as well as intrahepatic portal hypertension. In patients with portal hypertension the veins of Retzius, which are the collaterals between the portal vein and the Inferior vena cava at the retroperitoneum, are an important site of formation of these varices.3 The incidence of bleeding duodenal varices is low, but they may result in severe life-threatening haemorrhage.4 Treatment options include endoscopic modalities, IR-guided interventions and surgery, but there is no standardised approach due to limited availability of case series and reports. Procedures such as endoscopic sclerotherapy have significant rebleeding rates and are also associated with ulceration, which may predispose to severe life-threatening haemorrhage.5 Endoscopic variceal ligation for bleeding duodenal varices is challenging because of the difficulty in maintaining the field of vision. Also due to high rebleeding rates, the haemostasis is usually temporary and frequently necessitates an additional procedure to control bleeding.6
TIPSS is considered the first-line treatment for refractory variceal bleeding, with many studies reporting favourable outcomes in patients with bleeding ECVs.7 Although patients with recurrent oesophagogastric variceal bleeding can be treated successfully with TIPSS by reducing the portosystemic pressure gradient to less than 12 mm Hg or more than 50% from baseline, these outcomes have not been observed in ECVs. These ECVs may rebleed despite a reduction of portosystemic pressure gradient <12 mm Hg or by 25%–50% of baseline value with TIPSS.8 This may be explained by the fact that the major determinant of the risk of bleeding is the variceal tension that is in turn influenced by the diameter of the varix, which is significantly larger in these cases (Laplace equation).
TIPSS with embolisation has emerged as an effective method for the treatment of these varices, and while TIPSS leads to reduction of portal pressures the embolisation procedure causes the occlusion of feeding vessels of the varix to achieve effective bleeding control.8 For this particular patient we used a new, 5–7 mm MVP device for embolisation, which likely provided effective occlusion due to its larger size, which was more suitable for these larger varices. The MVP has several features that gives it an advantage over other common embolisation techniques like coils. While it allows for an immediate and stable target vessel occlusion and is a single device that is compatible with the routinely used catheters, its improved navigability makes delivery easier. An angiogram to assess the position of the device and occlusion of the target artery before detachment can be performed, and in case of suboptimal placement the device can be resheathed and redeployed. Additionally, the use of coils for embolisation can be complicated by coil migration and erosion, which can be more concerning in these cases as ECVs have thin walls and thin overlying mucosa.1 In the past another plug device, the Amplatzer Vascular Plug (AGA Medical, Golden Valley, Minnesota, USA), has been successfully used for vascular embolisation along with TIPSS, but the introduction of this device required a different, larger catheter (6–8 Fr).9 This shortcoming has been further addressed by the MVP device as it can be introduced and deployed using a regular 5 Fr catheter, hence omitting the need for an additional, larger catheter. To our knowledge, this is the first reported use of this new MVP device for the successful treatment of bleeding ECVs. These devices provide us with an attractive new option but require further large-scale studies to explore their future potential. They may have a potential significant role in the management of variceal bleeding along with other established modalities.
Learning points.
Ectopic varices (ECVs), although a rare cause of gastrointestinal bleeding, can cause significant morbidity and mortality.
Management of ECVs is challenging due to their inaccessibility and increased risk of rebleeding with standard endoscopic techniques.
To our knowledge this is the first reported use of this new microvascular plug (MVP) device for the successful treatment of bleeding ECVs.
Transjugular intrahepatic portosystemic shunt with MVP devices provide us with an attractive new option for the management of variceal bleeding.
Footnotes
Contributors: RB: Collection of data, manuscript writing, drafting the article. GB: Revision of manuscript, material support. EB: Radiology inputs, material support. RK: Conception and design, revising the article for important intellectual content, critical revisions, clinical oversight and approval.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Saad WE, Lippert A, Saad NE, et al. . Ectopic varices: anatomical classification, hemodynamic classification, and hemodynamic-based management. Tech Vasc Interv Radiol 2013;16:108–25. 10.1053/j.tvir.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 2.Sato T, Akaike J, Toyota J, et al. . Clinicopathological features and treatment of ectopic varices with portal hypertension. Int J Hepatol 2011;2011:1–9. 10.4061/2011/960720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopera JE, Arthurs B, Scheuerman C, et al. . Bleeding duodenal: varices treatment by TIPS and transcatheter embolization. Cardiovasc Intervent Radiol 2008;31:431–4. 10.1007/s00270-004-0161-y [DOI] [PubMed] [Google Scholar]
- 4.Sato T. Treatment of ectopic varices with portal hypertension. World J Hepatol 2015;7:1601–5. 10.4254/wjh.v7.i12.1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vidal V, Joly L, Perreault P, et al. . Usefulness of transjugular intrahepatic portosystemic shunt in the management of bleeding ectopic varices in cirrhotic patients. Cardiovasc Intervent Radiol 2006;29:216–9. 10.1007/s00270-004-0346-4 [DOI] [PubMed] [Google Scholar]
- 6.Shiraishi M, Hiroyasu S, Higa T, et al. . Successful management of ruptured duodenal varices by means of endoscopic variceal ligation: report of a case. Gastrointest Endosc 1999;49:255–7. 10.1016/S0016-5107(99)70498-0 [DOI] [PubMed] [Google Scholar]
- 7.Kochar N, Tripathi D, McAvoy NC, et al. . Bleeding ectopic varices in cirrhosis: the role of transjugular intrahepatic portosystemic stent shunts. Aliment Pharmacol Ther 2008;28:294–303. 10.1111/j.1365-2036.2008.03719.x [DOI] [PubMed] [Google Scholar]
- 8.Vangeli M, Patch D, Terreni N, et al. . Bleeding ectopic varices--treatment with transjugular intrahepatic porto-systemic shunt (TIPS) and embolisation. J Hepatol 2004;41:560–6. 10.1016/j.jhep.2004.06.024 [DOI] [PubMed] [Google Scholar]
- 9.Pattynama PM, Wils A, van der Linden E, et al. . Embolization with the Amplatzer Vascular Plug in TIPS patients. Cardiovasc Intervent Radiol 2007;30:1218–21. 10.1007/s00270-007-9089-3 [DOI] [PMC free article] [PubMed] [Google Scholar]


