Abstract
Squamous cell carcinoma of uterine cervix is potentially a curable disease; however, many patients treated with definitive chemoradiotherapy develop distant metastases, with few of them having a single metastatic deposit. There are no guidelines for the treatment of patients with oligometastatic cervical cancer.
We present a case of a patient with International Federation of Gynecology and Obstetrics (FIGO) Stage IIB squamous cell carcinoma of uterine cervix. She was successfully treated with concurrent chemoradiotherapy with definitive intent. One year later, she developed a solitary pulmonary nodule for which she underwent resection followed by chemotherapy. She is free of any local or distant disease at 5 years of regular follow-up.
Keywords: gynecological cancer, cervical cancer
Background
Cervical cancer is the fourth most common malignancy among women in developing countries and majority of them are diagnosed in advanced stage, beyond surgical treatment.1 There has been a considerable improvement in long-term survival of patients with locally advanced cervical cancer.2 3 However, still there is a large proportion of patients who develop distant metastases, and few of them present with an oligometastatic disease. There are no guidelines for such patients. If treated aggressively, a small proportion of patients can still be cured. We present a case of a woman, who completed treatment with chemoradiotherapy and later on developed a solitary right lung base metastasis.
Case presentation
A 54-year-old woman, known asthmatic, mother of 3 children, presented with postmenopausal vaginal bleeding in July 2010. There was no fever, itching or any history of contraceptive or antibiotic use. She had insignificant family history. Vaginal examination showed a barrel-shaped cervix, vaginal fornices were obliterated and shallow and a nodule was palpable in the left parametrium.
Considering the clinical findings, it was planned to examine the patient under anaesthesia (EUA). On EUA, a friable growth coming out of cervix was seen, a hysteroscope was introduced simultaneously which revealed cervix and uterus filled with growth. Curettage was performed and friable pieces of growth were obtained and sent for histopathology.
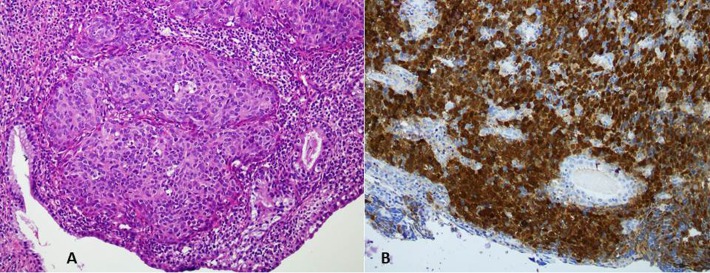
Sections from endocervical curettings revealed a tumour composed of sheets and groups of squamous epithelial cells, having large round to oval nuclei with prominent nucleoli showing pleomorphism and hyperchromasia. The cytoplasm was eosinophilic, forming intercellular bridges. Tumour was infiltrating into the muscle tissue. Scattered chronic inflammatory cells were seen. On immunohistochemical staining, the tumour cells show positivity for cytokeratin 5/6 and p16 (figure 1A,B).
Figure 1.
(A) H&E staining shows an infiltrating tumour arranged in nests with overlying endocervical columnar lining (B) Tumour cells are positive for p16 immunohistochemical stain.
Metastatic workup was negative for any distant disease. She was staged as International Federation of Gynecology and Obstetrics (FIGO) IIB, grade III squamous cell carcinoma cervix for which she was treated with pelvic radiotherapy and concurrent cisplatin chemotherapy (40 mg/m2). Pelvic radiation consisted of a dose of 45 Gy in 25 fractions via external beam radiation therapy followed by 3 sessions of high-dose rate intracavitary brachytherapy. At the end of treatment, the cervical mass had disappeared. On her first post-treatment follow-up in December 2010, there was no evidence of local disease on clinical examination, and CT scan of chest, abdomen and pelvis was also negative for any locoregional or distant disease.
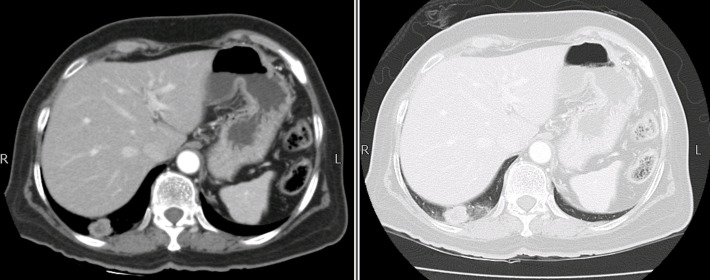
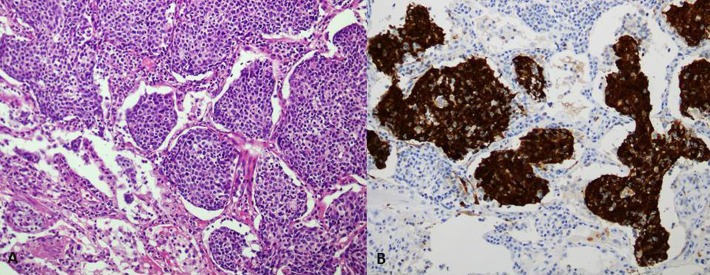
She was on regular follow-up and in June 2011 on routine follow-up CT scan of chest, abdomen and pelvis, there was a 3×3 cm nodule in right lung base (figure 2). The case was discussed in multidisciplinary team meeting and recommended to undergo right lower lobectomy for solitary lung lesion. Histopathology was consistent with poorly differentiated metastatic carcinoma (figure 3A,B). After surgery, she was given six cycles of carboplatin (area under curve (AUC) 6) and paclitaxel (175 mg/m2) chemotherapy, which she completed in November 2011.
Figure 2.
Axial CT scan showing right lung base nodule.
Figure 3.
(A) Metastatic squamous cell carcinoma to lung filling the alveoli. (B) Tumour cells showing nuclear positivity for p16 immunostain.
Outcome and follow-up
The patient visited the clinics regularly where local examination was done every 3 months along with an annual CT chest, abdomen and pelvis. After 5 years of surveillance, she is free of any disease on examination and on imaging.
Discussion
Pulmonary metastasectomy is a recognised therapeutic modality for the treatment of metastatic disease. Surgical resections have been widely performed on patients with pulmonary metastasis from soft tissue sarcoma and colorectal cancer. However, the role of metastasectomy in metastatic squamous cell carcinoma of uterine cervix is not well explored. There are currently no standard surgical options for pulmonary metastasis.
Okiror et al showed that pulmonary metastasectomy confers an improved survival in patients with metastatic sarcoma. A total of 80 pulmonary metastasectomies were performed on 66 patients. The median number of metastases resected was three, and there were no postoperative in-hospital deaths. The median overall survival (OS) was 25.5 months; however, recurrence of metastases significantly affected survival.4
Historically, the clinical practices with respect to colorectal cancers have been reviewed thoroughly focusing on the detection of lung and liver metastasis and their subsequent resections.5 In a retrospective study, Hattori et al evaluated outcomes after pulmonary resection of colorectal metastases in patients with or without a curative hepatic metastasectomy. The 5-year OS of the lung metastasectomy group was significantly better than that of the liver and lung metastasectomy group.6 Similarly, a Japanese study showed that surgical resection offers a chance to prolong survival in colorectal cancer patients with resectable pulmonary metastases.7
Apart from sarcomas and adenocarcinomas, the role of metastasectomy in metastatic squamous cell carcinoma is not well established. A systematic review of studies examining outcomes for patients with head and neck squamous cell carcinoma who underwent pulmonary metastasectomy for metachronous pulmonary metastases was conducted in the UK, but it only provided limited evidence in the favour of pulmonary metastasectomy.8
Little is known about the long-term survival after pulmonary metastasectomy for gynaecological malignancies. Tumour histopathology, duration of disease-free interval after the treatment of primary site, number of pulmonary metastases and absence of extra pulmonary disease have been identified as independent prognostic factors in the management of metastatic gynaecological malignancies.9 10 There are few single institutional reports highlighting this subject. Adachi et al reported a 5-year OS of 81.7% in 37 patients with isolated lung metastases (<3 nodules). The histopathology of gynaecological tumours was not mentioned in this study.11
A retrospective analysis of 103 patients conducted at Mayo Clinic showed that pulmonary resection for metastatic gynaecological cancer in selected patients is safe and effective. However, the majority of the patients had disease in uterine corpus, while cervix was involved in only seven. Furthermore, merely five patients had disease with squamous cell histology.12 A study by Anraku et al stratified the 5-year OS for each histological type. Squamous cell carcinomas of cervix had better 5-year OS when compared with cervical adenocarcinomas. The same study showed that patients with a disease-free interval of 12 months or more are good candidates for metastasectomy, provided there is adequate control of the primary tumour without extra pulmonary metastasis.9
Our patient was found to have a solitary metastatic nodule in lung on routine follow-up and systemic investigations. Considering her good performance status, she was offered metastasectomy. Anticipating the presence of microscopic disease elsewhere based on biopsy-proven metastasis in lung, she was given postoperative chemotherapy consisting of carboplatin at a dose of AUC 6 and paclitaxel at doses of 175 mg/m2 every three weeks. She tolerated this treatment uneventfully and is disease free after 5 years of treatment. However, there is no data in the contemporary literature emphasising the significance of adjuvant chemotherapy after metastasectomy.
The largest report to date, a multi-institutional study including cervical cancer patients exclusively, reported a 5-year disease-free survival (DFS) rate of 32.9% after pulmonary metastasectomy. Patients with one or two pulmonary metastases had a 5-year DFS rate of 42.2% compared with 0% for patients with three or four metastases. Patients with squamous cell cancers had a 5-year DFS rate of 47.4%, compared with 0% for patients with adenosquamous cell cancers or adenocarcinoma.10
These existing reports on the role of metastasectomy in cases of squamous cell carcinoma of cervix with oligometastatic disease emphasise the need for an aggressive treatment approach, considering it as potentially a curative treatment. However, there is a swift need for multicenter collaboration that will establish the clinical guidelines for the better management of such patients.
Learning points.
International Federation of Gynecology and Obstetrics (FIGO) stage IV B squamous cell carcinoma of uterine cervix with solitary metastasis can be approached aggressively with a curative intent.
Like established therapeutic roles in sarcomas and colorectal cancer, local treatment of distant metastasis has a probable role in the management of carcinoma of cervix.
Multidisciplinary team care is the key to success.
Footnotes
Contributors: NA is the primary oncologist of the patient. ANA assisted in finalizing the treatment plan of this patient. MAM and BMQ contributed significantly in writing the report.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bhurgri Y, Pervez S, Kayani N, et al. Time trends in the incidence of cancer cervix in Karachi South, 1995-2002. Asian Pac J Cancer Prev 2008;9:533–6. [PubMed] [Google Scholar]
- 2.Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol 2008;26:5802–12. 10.1200/JCO.2008.16.4368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med 1999;340:1144–53. 10.1056/NEJM199904153401502 [DOI] [PubMed] [Google Scholar]
- 4.Okiror L, Peleki A, Moffat D, et al. Survival following pulmonary metastasectomy for sarcoma. Thorac Cardiovasc Surg 2016;64:146–9. 10.1055/s-0035-1546430 [DOI] [PubMed] [Google Scholar]
- 5.Treasure T, Milošević M, Fiorentino F, et al. History and present status of pulmonary metastasectomy in colorectal cancer. World J Gastroenterol 2014;20:14517–26. 10.3748/wjg.v20.i40.14517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hattori N, Kanemitsu Y, Komori K, et al. Outcomes after hepatic and pulmonary metastasectomies compared with pulmonary metastasectomy alone in patients with colorectal cancer metastasis to liver and lungs. World J Surg 2013;37:1315–21. 10.1007/s00268-013-1954-4 [DOI] [PubMed] [Google Scholar]
- 7.Suzuki H, Kiyoshima M, Kitahara M, et al. Long-term outcomes after surgical resection of pulmonary metastases from colorectal cancer. Ann Thorac Surg 2015;99:435–40. 10.1016/j.athoracsur.2014.09.027 [DOI] [PubMed] [Google Scholar]
- 8.Young ER, Diakos E, Khalid-Raja M, et al. Resection of subsequent pulmonary metastases from treated head and neck squamous cell carcinoma: systematic review and meta-analysis. Clin Otolaryngol 2015;40:208–18. 10.1111/coa.12348 [DOI] [PubMed] [Google Scholar]
- 9.Anraku M, Yokoi K, Nakagawa K, et al. Pulmonary metastases from uterine malignancies: results of surgical resection in 133 patients. J Thorac Cardiovasc Surg 2004;127:1107–12. 10.1016/j.jtcvs.2003.10.011 [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto K, Yoshikawa H, Shiromizu K, et al. Pulmonary metastasectomy for uterine cervical cancer: a multivariate analysis. Ann Thorac Surg 2004;77:1179–82. 10.1016/j.athoracsur.2003.06.023 [DOI] [PubMed] [Google Scholar]
- 11.Adachi M, Mizuno M, Mitsui H, et al. The prognostic impact of pulmonary metastasectomy in recurrent gynecologic cancers: a retrospective single-institution study. Nagoya J Med Sci 2015;77:363–72. [PMC free article] [PubMed] [Google Scholar]
- 12.Clavero JM, Deschamps C, Cassivi SD, et al. Gynecologic cancers: factors affecting survival after pulmonary metastasectomy. Ann Thorac Surg 2006;81:2004–7. 10.1016/j.athoracsur.2006.01.068 [DOI] [PubMed] [Google Scholar]