Abstract
Survival rates following myocardial infarction have increased in recent years but current treatments for post-infarction recovery are inadequate and cannot induce regeneration of damaged hearts. Regenerative medicine could provide disease-reversing treatments by harnessing modern concepts in cell and developmental biology. A recently-established paradigm in regenerative medicine is that regeneration of a tissue can be achieved by reactivation of the coordinated developmental processes that originally formed the tissue. In the heart, the epicardium has emerged as an important regulator of cardiac development and reactivation of epicardial developmental processes may provide a means to enable cardiac regeneration. Indeed, in adult mouse hearts, treatment with thymosin β4 and other drug-like molecules reactivates the epicardium and improves outcomes after myocardial infarction by inducing regenerative paracrine signalling, neovascularisation and de novo cardiomyocyte production. However, there are considerable limitations to current methods of epicardial reactivation that prevent direct translation into clinical practice. Here, we describe the rationale for targeting the epicardium and the successes and limitations of this approach. We consider how several recent advances in epicardial biology could be used to overcome these limitations. These advances include insight into epicardial signalling and heterogeneity, epicardial modulation of inflammation and epicardial remodelling of extracellular matrix.
Introduction
Coronary heart disease is a leading cause of worldwide morbidity and mortality1. Despite improved survival rates following myocardial infarction (MI), current treatments are unable to reverse loss of cardiac function after MI1–3. By harnessing modern concepts in cell and developmental biology, regenerative medicine could provide novel treatments to repair diseased hearts4.
The adult mammalian heart has long been regarded as non-proliferative and terminally differentiated but recent evidence has demonstrated that cardiomyocytes are capable of low level turnover, particularly after MI5–7. However, the limited proliferative capacity of the mammalian heart, beyond early neonatal stages8, is grossly insufficient to regenerate the ∼1 billion cardiomyocytes lost in a typical MI9. Moreover, regeneration of myocardial tissue is a complex process requiring appropriate integration of newly produced cardiomyocytes, production and integration of non-cardiomyocyte cell types, regenerative paracrine signalling, and moderation of inflammation that promotes excessive fibrosis. Therefore, even if cardiomyocytes could be easily replenished, such approaches, in isolation, are unlikely to induce sufficient regeneration.
Several different approaches to cardiac regeneration have been studied (Table 1), both in preclinical and clinical trials, described in10. These are principally based upon inducing cardiomyocyte cell cycle re-entry11, directly reprogramming cardiac-resident non-myocytes (fibroblasts)12 or cell therapy-based strategies. The latter approach has applied a range of somatic13 and haematopoietic14 cell types, resident cardiac progenitor cells15–18, embryonic (ESCs) or induced pluripotent stem cells (iPSCs). Despite numerous multi-centre clinical trials of transplanted autologous bone marrow stem cells, transdifferentiation into cardiac muscle appears not to occur and any clinical improvement is modest19, attributable to the local release of paracrine factors that enhance repair, attenuate fibrosis and improve cardiac functional recovery. Resident cardiac progenitor cells were anticipated by many to confer superior benefit.
Table 1. An overview of different approaches to cardiac regeneration, including epicardial reactivation.
Abbreviations: AAV9, adeno-associated virus 9; BMP4, bone morphogenetic protein 4; CDC, cardiosphere-derived cell; CM, cardiomyocyte; CPC, cardiac progenitor cell; ESC-CM, embryonic stem cell-derived cardiomyocyte; FSTL1, Follistatin-like 1; HGF, hepatocyte growth factor; iCM, induced cardiomyocyte-like cells; IGF-1, insulin-like growth factor 1; iPSC-CM, induced pluripotent stem cell-derived cardiomyocyte; LVEF, left ventricular ejection fraction; MI, myocardial infarction; miR, microRNA; modRNA, modified RNA; NRG-1, neuregulin-1; Tβ4, thymosin β4.
| Approach | Advantages | Disadvantages | Notable examples |
|---|---|---|---|
| Induce cardiomyocyte cell cycle re-entry | |||
| Induce proliferation of mature CMs to replace CMs lost in heart disease. | • Autologous (eliminates risk of rejection and requirement for immunosuppression). • No requirement for stimulating CM differentiation and maturation. • New CMs are likely to have good mechanical, vascular and electrical integration. |
• Potential off-target effects of treatment (particularly oncogenesis). • Difficult to achieve the magnitude of required CM proliferation for clinically meaningful benefit. • Inherent difficulties of gene therapy approaches – non-genetic approaches (eg. paracrine factors) more desirable. |
• Progressive improvement in infarct size and cardiac functional parameters occurred in cyclin D2 transgenic mice29. • Activation of the Hippo/YAP promitogenic signalling pathway improved heart function and survival after MI11. • AAV9 gene transfer of miR-590-3p and miR-199a-3p stimulated CM proliferation, reduced infarct size and improved cardiac functional parameters after MI30. • Inhibition of miR-15 induced cardiac proliferation and modestly improved cardiac function31. • In a small clinical trial, infusion of human recombinant NRG-1 was well-tolerated and improved cardiac function. NRG-1 has pro-proliferative effects via the ErbB2/ErbB4 receptor – oncogenic potential is an important concern32. • Deletion of Meis1, a cardiomyocyte cell cycle regulator, extends the postnatal window of proliferation and regeneration, although the ability to induce the effect in the adult heart was not reported33. • Reconstituting the potent cardiogenic activity of FSTL1 in an epicardial patch promoted myocardial regeneration following MI, in mouse and pig34. |
| Cell therapy | |||
| Produce ESC- or iPSC-derived CMs in vitro and deliver to the myocardium. | • Successful long-term engraftment of substantial numbers of ESC-CMs has been achieved in animal models. • Potentially highly reproducible. • Highly specific effects. |
• ESC-CMs: allogeneic and ethical concerns. • iPSC-CMs: logistical and regulatory concerns. • Possibility of teratoma formation. • Stem cell-derived CMs are immature - improper mechanical, electrical and vascular integration. |
• Human ESC-CMs could be produced on a clinical scale. Delivery of human ESC-CMs to infarcted non-human primate hearts produced extensive remuscularisation25. |
| Isolate resident or non-resident cardiac progenitor cells, expand in vitroand deliver to the myocardium. | • Autologous CPCs can be obtained from biopsies collected during surgery. • Clinical trials have demonstrated safety (but safety might be compromised at the higher engraftment rates that would be desirable for improved treatment). |
• Limited efficacy and inconsistent results in clinical trials. • Unclear mechanism of action – paracrine effects are likely to be important, in which case paracrine factor therapy may have advantages over cell-based therapy. • CMs may not appropriately integrate. |
• In a randomised, open-label phase 1 trial (‘SCIPIO’), c-kit+ CPC-treated patients had a small improvement in LVEF20. However, The Lancet has issued an “expression of concern” regarding data integrity.21. In independent murine studies, c-kit+ CPCs made only minimal23, or no24, contribution to CMs. • In the ‘CADUCEUS’ trial, intracoronary infusion of autologous CDCs after MI was safe and reduced scar mass and regional contractile dysfunction; however the patient cohort was small22. |
| Enhance activity of endogenous CPCs | |||
| Stimulate resident CPCs in situ to increase regenerative activity. | • Potential to regenerate a range of cell types in addition to CMs. • Cell-free (less costly, fewer logistical difficulties and perhaps safer than cell-based therapies). |
• Potential off-target effects. • Robust regenerative technique not yet described. |
• “Priming” with Tβ4 activated the epicardium, resulting in de novo CMs and improved functional cardiac parameters28. • Intra-myocardial injection of modRNA encoding VEGF-A enhanced mobilisation of epicardial progenitor cells and improved heart function and survival in mice35. •In vivo injection of HGF and IGF-1 into murine hearts to mobilise and amplify resident c-kit+ CPCs improved function and survival after MI36. |
| Direct reprogramming of non-cardiomyocytes | |||
| Use cardiac reprogramming factors to induce conversion of non-cardiomyocytes (e.g. fibroblasts) into iCMs in vivo. | • Convert excessive fibroblasts (which induce scarring) into functional iCMs. • Cell-free (less costly, fewer logistical difficulties and perhaps safer than cell-based therapies). |
• Requires gene transfer by integrating viruses. • Low reprogramming efficiency. • Functional integration of iCMs is not established. • Difficult to confirm fibroblast-to-iCM reprogramming in vivo in patients. • Requires development of appropriate models for translation into humans. |
• Retroviral gene transfer and expression of Gata4, Mef2c, and Tbx15 (GMT)12 or additionally Hand2 (GHMT)37 induced reprogramming of fibroblasts into CMs and improved cardiac function after MI in mice. • Reprogramming with a combination of Hand2, Nkx2.5, Gata4, Mef2c, and Tbx5 (HNGMT) has a >50-fold higher efficiency than with GMT alone38. • Enhanced efficiency of reprogramming with Tβ4 treatment39, illustrating potential benefit of combining approaches. |
Whereas the findings from the SCIPIO trial appear promising20, concerns have been raised over some of the data21. Early evidence from the CADUCEUS trial22 suggests that cardiosphere-derived autologous stem cells confer modest functional improvement. In contrast, recent studies in rodents, where lineage tracing technologies can be applied, revealed minimal contribution of c-kit+ cells to cardiac regeneration23,24; indeed, the value of c-kit as a marker of cardiac stem cell potential was questioned when c-kit positive cells were revealed to be an endothelial cell population with no cardiomyogenic contribution24. A recent proof-of-concept study in a non-human primate model highlighted the safety and potential for extensive remuscularisation using human ESC-derived cardiomyocytes25. Given the ethical concerns that limit application of ESCs, the hopes of cardiac cell therapy may, therefore, depend upon improving functional maturation of iPSC-derived cardiomyocytes26.
An emerging paradigm in regenerative medicine is that repair of tissue can be achieved by reactivation of the coordinated developmental processes that originally formed the tissue27. Of note, the epicardium has been described as an important regulator of myocardial development and experimental treatments that reactivate embryonic epicardial processes improve outcomes after an experimental model of MI in adult mice28. However, these treatments have major limitations and are unlikely to be clinically useful in their current form. We discuss the rationale for targeting the epicardium and explore the successes and limitations of pre-clinical studies that demonstrate proof-of-principle of epicardial reactivation for cardiac regeneration. Moreover, we consider how recent evidence highlighting several novel concepts related to epicardial biology could help to overcome previous limitations. These avenues for future research may aid progress towards the ultimate aim of clinical induction of cardiac regeneration in the adult human.
Why target the epicardium?
The epicardium is essential for mammalian cardiac development
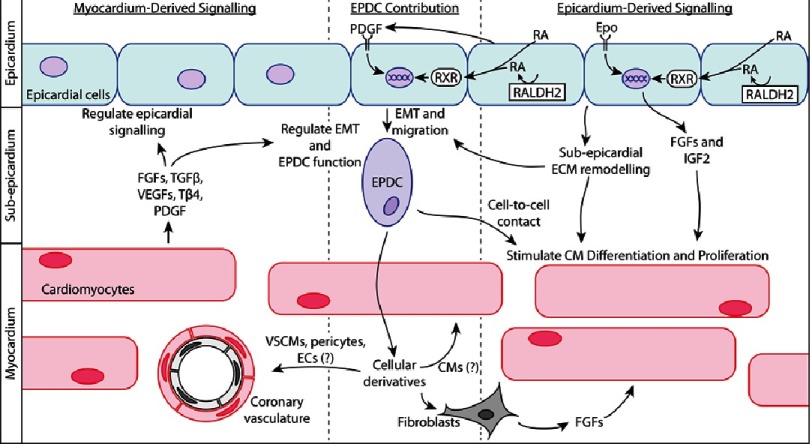
The epicardium develops principally from the mesodermal proepicardial organ soon after cardiac looping40,41. The epicardium then makes essential cellular and signalling contributions to cardiac development (Figure 1).
Figure 1. Epicardial cellular contribution and reciprocal epicardial-myocardial signalling are critical for cardiac development and may similarly determine epicardial potential for cardiac regeneration.
Abbreviations: CM, cardiomyocyte; EC, endothelial cell; EMT, epithelial to mesenchymal transition; EPDC, epicardium-derived cell; Epo, erythropoietin; FGF, fibroblast growth factor; IGF2, insulin-like growth factor 2; PDGF, platelet-derived growth factor; RA, retinoic acid; RALDH2, retinaldehyde dehydrogenase 2; RXR, retinoid X receptor; Tβ4, thymosin β4; TGFβ, transforming growth factor β; VEGF, vascular endothelial growth factor; VSMCs, vascular smooth muscle cells.
The cellular contribution is mediated by a subpopulation of epicardial cells that undergoes epithelial-to-mesenchymal transition (EMT) to produce epicardium-derived cells (EPDCs). EPDCs then migrate into the myocardium and differentiate into several cardiac lineages41. In development, it is widely accepted that EPDCs make essential contributions to cardiac interstitial fibroblasts, along with adventitial fibroblasts, vascular smooth muscle cells and pericytes of the coronary vessels41. However, the contribution of EPDCs to cardiomyocyte and coronary endothelial lineages is more controversial. Modern fate map studies primarily use Cre-LoxP-based genetic lineage tracing, whereby Cre recombinase is expressed with a lineage-specific gene to induce genetic recombination and consequent lineage-specific expression of a reporter gene (eg. enhanced green fluorescent protein or β-galactosidase).
These genetic changes are inherited by daughter cells (‘indelible labelling’) and can be assessed by histological techniques. Using Tbox18 (Tbx18) and Wilms’ tumour-1 (Wt1) as epicardial marker genes for such genetic lineage tracing, a subset of cardiomyocytes was found to be derived from Tbx18+ or Wt1+ cells42,43. However, an important consideration in genetic lineage tracing is the specificity and sensitivity of the marker gene. In this regard, Tbx18 is expressed in epicardial cells (high sensitivity) but is also expressed in the interventricular septum and left ventricular cardiomyocytes (low specificity)44. Similarly, a tamoxifen-inducible Wt1CreERT2 drives incomplete epicardial recombination (low sensitivity) and Wt1 is reported to be expressed in coronary endothelial cells (low specificity)45,46. Since Tbx18 and Wt1 expression is not strictly limited to the epicardium, cells labelled by such genetic lineage tracing could derive from sources other than epicardial cells, and therefore the epicardial contribution to the cardiomyocyte lineage has been disputed44,45.
Analogous inadequacy of marker genes may underlie the controversy surrounding the (pro)epicardial contribution to the coronary endothelium47. A substantial contribution to the coronary endothelium is made by recently-identified proepicardial sub-populations that express neither Wt1 nor Tbx1847, thus, previous studies using these marker genes did not include the relevant sub-population (low sensitivity) and therefore the previous conclusion of a lack of (pro)epicardial contribution to the coronary endothelium may be incorrect42–44,47–49. While alternative sources, notably the sinus venosus48 and endocardium50, have also been described, those endothelial cells contributed from the proepicardium appear to derive from a distinct subpopulation, characterised by expression of Scleraxis (Scx) and Semaphorin3D (Sema3D)47.
In addition to cellular contributions, the epicardium also participates in reciprocal, bi-directional signalling between the epicardium and myocardium51 (Figure 1). Epicardium-derived signalling functions include promoting cardiomyocyte proliferation and differentiation and stimulating coronary vascularisation27; as such, epicardium-derived factors are essential for the formation of mature myocardium52. Retinoic acid and erythropoietin signalling within the epicardium stimulates epicardial production of fibroblast growth factor (FGF) family members and insulin-like growth factor 2 (IGF2), which govern normal development in the underlying myocardium53–60. Epicardial signalling also stimulates epicardial EMT, facilitating epicardial cellular contribution to the myocardium61,62. Epicardial EMT facilitates direct contact between EPDCs and myocardial cells, which enhances cardiomyocyte proliferation, maturation and alignment63,64. Finally, modification of extracellular matrix (ECM) composition by the epicardium and EPDCs has important effects on both epicardial and myocardial development51,65–67.
Conversely, the myocardium also signals to the epicardium to control epicardial activities, such as EMT and migration and differentiation of EPDCs27,68. This occurs by myocardial secretion of signalling factors, which include FGF family members69, transforming growth factor β (TGFβ)70,71, vascular endothelial growth factors (VEGFs)72, thymosin β4 (Tβ4)73 and platelet-derived growth factor (PDGF)61,74. Thus, the epicardium and myocardium form a reciprocal signalling unit that is essential for cardiac development.
The epicardium is essential for non-mammalian cardiac regeneration
The epicardium is not only important in mammalian cardiac development but is also critical for cardiac regeneration in species such as zebrafish, which are able to fully recover after substantial cardiac injury. During zebrafish cardiac regeneration, the epicardium re-expresses embryonic genes Retinaldehyde dehydrogenase 2 (Raldh2), a regulator of retinoic acid synthesis, and Tbx18 resulting in epicardial EMT and EPDC migration to vascularise the myocardium75. By contrast, in adult mammals, epicardial proliferation and signalling occurs after injury but, without therapeutic intervention, this is unable to invoke substantial regeneration76,77.
Rationale for targeting the epicardium
Epicardial programmes underpin cardiac development and re-activation of these programmes mediates regeneration in zebrafish. However, mammalian epicardial reactivation requires enhancement to be therapeutically useful. Importantly, the epicardium can uniquely contribute to coordinated repair by providing a range of cell types and signalling factors.
Prior successes and limitations in reactivating the quiescent adult mammalian epicardium
Thymosin β4 treatment improves outcomes after myocardial infarction in mice
Thymosin β4 (Tβ4) is a 43-amino acid actin monomer-binding peptide, which is important for both systemic and coronary vascular development73,78. In the adult mammalian heart, Tβ4 treatment stimulates reactivation of the epicardium after MI and results in regenerative neovascularisation79, recapitulating its embryonic role. Furthermore, ‘priming’ by treatment with Tβ4 before MI enables EPDCs to form de novo cardiomyocytes28, and induces a robust neovascularisation79,80. In the short time frame of 28 days after MI, Tβ4 treatment reduced infarct volume and improved ejection fraction28. New cardiomyocytes must integrate properly into the myocardium in order to maintain structural integrity and avoid arrhythmogenic electrophysiological heterogeneity. After Tβ4 stimulation, epicardium-derived cardiomyocytes formed adherens and gap junctions, suggesting structural integration28; moreover, functional integration of the de novo cardiomyocytes was demonstrated by synchronous [Ca2+]i transients between de novo and pre-exisiting cardiomyocytes28. However, given the complexities of coupling between cardiomyocytes, as well as other functional links such as proposed cardiomyocyte-fibroblast coupling81, more comprehensive assessment of de novo cardiomyocyte integration might be required in order to conclude complete integration.
Limitations of Thymosin β4 and other treatments
EPDCs from the adult mouse after Tβ4 treatment are not molecularly identical to embryonic EPDCs, despite functional similarities82. Reactivated adult EPDCs display a heterogeneous molecular profile, defined by both cardiac progenitor and mesenchymal stem cell markers, including Sca-1, CD29, CD90, PDGFRβ and CD44, thus are significantly different from their embryonic counterparts obtained at mouse embryonic day 12.5 (E12.5), despite the common expression of the early embryonic epicardial gene Wt182. Consequences of the molecular differences are unclear but this finding suggests that Tβ4 priming does not achieve true recapitulation of embryonic processes, which perhaps limits maximal therapeutic benefit.
In contrast to Tβ4 treatment before MI, Tβ4 treatment after MI does not produce de novo cardiomyocytes, although it does confer modest benefits, probably via paracrine effects83. This is a considerable limitation for translation into clinical practice because it presents a requirement for prophylactic treatment in order to regenerate de novo cardiomyocytes after MI. Although such prophylactic treatment is possible, particularly if at-risk patients were targeted, widespread long-term Tβ4 treatment may give cause for concern over issues such as safety, cost, patient compliance, and feasibility of frequent administration by necessarily non-oral routes.
Perhaps the most important limitation of Tβ4 treatment is low efficiency of de novo cardiomyocyte production from EPDCs (∼0.59% of Wt1+ EPDCs)28. In addition to Tβ4, two other drug-like molecules, prokineticin-2 and VEGF-A modified RNA (modRNA), are also able to reactivate the epicardium but they too have important limitations35,84,85. On balance, these experimental treatments demonstrate the principle of epicardial reactivation for cardiac regeneration but several difficulties prevent direct translation into clinical practice.
Novel research avenues that could improve epicardial reactivation strategies
Understanding the regulatory pathways that restrict epicardial cell behaviour
Extrapolating from our understanding of epicardial processes in development and from recent insights into their redeployment for regenerative benefit by zebrafish following injury, we can clearly appreciate the shortcomings of the cardiac healing response in mammals. Therapeutically instilling developmental or cardioregenerative mechanisms into the refractory mammalian heart may support a greater degree of muscle regeneration. As far as engaging epicardial involvement in regeneration is concerned, at least three key processes need to be targeted: (i) reactivation and restoration of pluripotency; (ii) EMT and inward migration; (iii) cell fate determination. While partial success in stimulating each of these steps has been achieved, notably with Tβ4, the extent, as previously stated, is inadequate, particularly of EMT and in directing cardiomyocyte cell fate. While screening for potent small molecules86 may lead to a breakthrough, candidate approaches, based on understanding embryonic and zebrafish mechanisms, may also prove fruitful. Taking EMT as an example, the principal drivers of epicardial EMT in the embryo are FGFs, notably basic FGF and TGFβ68, as discussed above. Furthermore, the regenerative capacity of zebrafish is FGF-dependent; expression of a dominant-negative FGF receptor blocks epicardial EMT, neovascularisation and regeneration75. Identifying suitable targets to achieve enhanced activation of these pathways in epicardial cells may be beneficial. Understanding the pathways that regulate cardiac cell differentiation during development may also reveal novel targets to enhance the complement of pro-regenerative cell types, at the expense of the predominant fibroblast fate.
Understanding the intrinsic response of the epicardium to myocardial injury
Despite its recognised failure to regenerate, the adult mammalian heart displays an intrinsic response to injury which, to date, has only been superficially characterised. Whilst scarring, ventricular dilatation and hypertrophic responses are widely recognised87, other elements have been largely overlooked. One such endogenous response is the reactivation of the epicardium, in the form of re-expression of embryonic epicardial genes28 and expansion via proliferation28 and infiltration of haematopoietic cells40. These responses would logically appear to be beneficial, an attempt at self-repair, for example by secretion of pro-regenerative paracrine factors77 and induction of new vessel growth79. An appreciation of what these pathways contribute, and how they may be enhanced, may facilitate a greater degree of regeneration. However, not all intrinsic responses constructively influence repair, fibrosis being a prime example; while moderate fibrosis is essential, at least initially, to prevent cardiac rupture, excessive fibrosis leads to permanent scarring at the expense of myocardial replenishment87. Although some embryonic epicardial processes are redeployed27, others are either ineffectively induced or even actively suppressed. A notable, recently-identified example of this is expression of the secreted protein, Follistatin-like 1 (FSTL1)34. FSTL1 is a potent cardiogenic factor that is actively expressed by embryonic and adult epicardial cells. Curiously, epicardial FSTL1 declines following myocardial infarction but application of human FSTL1 via an epicardial patch was able to stimulate cardiomyocyte proliferation, to improve cardiac function and survival, in mouse and swine MI models34. These findings suggest that the loss of epicardial FSTL1 is a maladaptive response to injury, and that its restoration can reverse myocardial death and remodelling following MI. Thus, understanding endogenous responses empowers regenerative strategies but requires additional insight into whether to therapeutically enhance or override intrinsic mechanisms.
The epicardium is a complex heterogeneous structure
The epicardium was, for a long time, regarded as a simple mesothelial layer with little heterogeneity, but recent work indicates hereto unappreciated complexities41,47. Epicardial sub-populations have been classified according to factors such as activation by signalling molecules (e.g. Notch or Tβ4) or expression of molecular markers (e.g. Wt1, Sca-1 or c-kit)27,28,82,88. However, one limitation of the literature is that different studies often assess different activating factors, molecular markers or developmental time points. The extent of overlap between different populations reported in separate studies is, therefore, not immediately apparent. For example, there is considerable molecular heterogeneity among the EPDCs that are reactivated by Tβ482 and it is therefore likely that some of the Tβ4-stimulated epicardial cells could also be classified into sub-populations described elsewhere on the basis of other distinguishing factors. A detailed understanding of the different cell types that populate the epicardium might allow for more precise targeting of sub-populations relevant to regeneration. The first study to systematically characterise epicardial heterogeneity, using a single cell transcriptomic approach, confirmed that at least three distinct sub-populations of tcf21+ epicardial cells exist in zebrafish89. The specific functional roles of these sub-populations in cardiac development, homeostasis and regeneration remains to be explored and, crucially, the extent to which this heterogeneity is conserved, or potentially more complex, in mammals remains to be determined.
Assessment of murine epicardial structure has recently revealed additional heterogeneity in the form of clusters of CD45+ Wt1− haematopoietic cells, encased in ECM, which resemble stem cell niches40. After MI, the CD45+ cells proliferated, encapsulating ECM was degraded by matrix metalloproteinases, and proliferative CD45+ cells were released into the underlying myocardium. Although the precise extent of this movement into the myocardium was not assessed, these results hint at a possible role for the CD45+ cells that deserves further investigation40, specifically to investigate whether the CD45+ cells contribute adversely or beneficially to cardiac regeneration. This study both challenges the prevailing dogma that the epicardium derives solely from the proepicardial organ and identifies a novel sub-population of epicardial cells that might be a useful therapeutic target.
Controlling the inflammatory response to cardiac injury
In zebrafish and newts, regeneration occurs with only short-lived fibrosis. By contrast, MI in adult mammalian hearts results in inflammation, fibrosis and permanent scarring90. Regeneration of the adult mammalian heart may, therefore, require moderating the response away from excessive inflammation and fibrosis, which preclude regeneration, and towards cellular regeneration and integration.
Although use of Tβ4, prokineticin-2 and VEGF-A modRNA to reactivate epicardial developmental programmes is beneficial, it does not necessarily follow that epicardial activity, in the absence of developmental reactivation, is beneficial. Indeed, it was reported that epicardial activity may underlie, at least in part, the inflammatory fibrosis of the endogenous response to MI91. After identifying the CCAAT/enhancer binding protein (C/EBP) transcription factor family as important in epicardial activation, it was found that viral gene transfer of dominant-negative mutant C/EBP improved ejection fraction and fibrosis after MI and reduced local neutrophil count; however, the causal link between lowering the inflammatory infiltrate and improving outcome was not directly demonstrated91.
Thymosin β4 may also be useful for controlling inflammation. One study found that treatment of mice after MI with a biologically-occurring form of Tβ4 (Tβ4-sulfoxide) increased the local macrophage count at day 2 post-MI but reduced the macrophage count at day 7 compared to control92. In combination with the finding of reduced infarct volume after Tβ4 treatment, these results were interpreted to mean that Tβ4-sulfoxide hastens early phagocytic debris removal after MI and enhances subsequent clearance of immune cells. Although the epicardium may not have a direct role in these anti-inflammatory effects, these findings, along with its proven role in cardiomyocyte protection93, suggest that Tβ4 might be clinically useful for prevention of cardiac scarring, even if its role in stimulation of production of de novo cardiomyocytes by EPDCs is limited80,92,94.
The concept of beneficial effects of early macrophages in cardiac regeneration is supported by recent evidence showing that macrophage depletion prevents the regeneration that normally occurs in neonatal mice after MI95. The pro-regenerative macrophages had pro-angiogenic effects, although the underlying mechanisms remain unclear. Molecular profiling of the early macrophages revealed no clear bias towards M1-type or M2-type macrophages, instead pointing to a transient, pro-regenerative phenotype and secretion of distinct soluble factors that may facilitate myocardial regeneration. Although these findings identify a potentially important new macrophage subset, the non-canonical characteristics of the pro-regenerative neonatal macrophages might limit translation into clinical therapy96.
Thus, inflammation, fibrosis and scarring remain as major obstacles to cardiac regeneration, and the underlying mechanisms are complex. Improved understanding of the inflammatory response to cardiac injury, including the role played by the epicardium, might enable targeted modification of adverse fibrosis while preserving beneficial debris removal by macrophages97,98.
Epicardium-controlled remodelling of the extracellular matrix
The ECM is a dynamic network of fibrous and non-fibrous proteins that can control cell function through several signalling mechanisms65,99. In development, the ECM has important effects on the epicardium and elsewhere in the heart99. For example, binding of epicardial α4-integrin to ECM ligands in the sub-epicardium inhibits EMT and migration, and influences epicardial cell differentiation66. Moreover, remodelling of the ECM recapitulates embryonic programmes to facilitate regeneration of limbs, tails and fins in fish and amphibians100–103.
Recently it was suggested that dynamic spatiotemporal ECM remodelling by the epicardium plays an important role in cardiac regeneration in zebrafish and newts104,105. In situ hybridisation and transgenic reporter analyses revealed that deposition of fibronectin-1 and fibronectin-1b by epicardial cells in zebrafish was dynamically upregulated during heart regeneration104. Furthermore, concomitant expression of integrin β3 and αV also occurred in cardiomyocytes, perhaps facilitating the fibronectin signalling to enhance mobilisation and integration of cardiomyocytes. Importantly, fibronectin-1-defective zebrafish displayed impaired myocardial regeneration and increased fibrosis104.
These findings suggest that the ECM acts as an important intermediate messenger for regenerative epicardium-to-myocardium signalling. Similarly, DNA microarray profiling and subsequent gene ontology analysis has identified an enrichment of genes associated with ECM in the regenerating hearts of newts and zebrafish105. Of particular interest were the ECM components hyaluronic acid, tenascin C and fibronectin, which were upregulated in the epicardium of the regenerating newt heart. Tenascin C induced cell cycle re-entry of primary cultured newt cardiomyocytes and may facilitate migration with ‘counter-adhesive domains’. It was speculated that the ‘regeneration-specific matrix’ may also facilitate migration by altering tissue stiffness through hyaluronic acid-dependent ECM hydration105.
However, while these studies show that ECM remodelling is important in newt and zebrafish cardiac regeneration, the implications for adult mammalian non-regenerative hearts are unclear. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation, both around the injury site and throughout the whole organ, as well as by cardiomyocyte migration106–109. This differs mechanistically from the adult mammalian response to cardiac injury, even if partial regeneration is induced. It is therefore difficult to extrapolate between species. On the other hand, comparative gene ontology suggested that species differences in ECM remodelling may be partly responsible for the different regenerative capacities105. Modification of injury-induced epicardial ECM remodelling might therefore present a method of converting the characteristics of the non-regenerative response towards those seen in regenerative hearts. Tissue engineering of pro-regenerative biomimetic matrices might be one approach to exploiting these novel findings105.
The role of pericardial fluid
The pericardial sac enhances epicardial activation by constraining heart-derived signalling factors in close proximity to the epicardium88. Myocardially secreted FGF-1110 and FGF-2 levels111 in the pericardial fluid of MI patients were found to correlate with the severity of ischaemia and a possible role in mediating collateral growth, although a direct involvement of the epicardium in this process has not been explored.
A recent study in patients profiled the miRNA content of pericardial fluid and identified a number of previously implicated heart failure markers112; this was interpreted to be an active and selective paracrine mechanism, involving non-coding RNAs as well as growth factors, to mediate cross-talk between cardiac cell types, which likely includes epicardial cells. Administration of pericardial fluid into the pericardial cavity of non-infarcted mouse was sufficient to induce epicardial proliferation and re-expression of embryonic genes88.
Insulin-like growth factor 1 (IGF1), hepatocyte growth factor (HGF) and High mobility group box 1 protein (HMGB1), factors known to induce resident cardiac progenitors, were significantly elevated in pericardial fluid from MI patients88. Thus, pericardial fluid contains trophic factors which might be harnessed to invoke epicardial activation and repair. In a recent study, clusterin, was found to be secreted in exosomes of MI patients; addition of clusterin to the pericardial sac of mice post-MI enhanced epicardial EMT and arteriogenesis and led to improved cardiac function113. These studies demonstrate that intra-pericardial injection may be an effective delivery method for any future treatments to augment epicardial contribution to myocardial regeneration114.
Translation into humans
The preclinical experiments described above have necessarily used non-human animal models, with the non-regenerative adult mouse heart implicitly assumed to be similar to adult human hearts. Nevertheless, experiments on human epicardial cells are required for translation of therapies into clinical practice. Several studies have used cultured primary epicardial cells, taken from right atrial appendages of human patients during right coronary artery bypass115,116; in vitro characterisation of the cells demonstrated basic similarities between mouse and human. Some species similarities were also noted in an in situ analysis of primary embryonic and foetal tissue samples117, in terms of marker expression and, broadly speaking, in its formation and apparent role during development. However, consistent differences were also reported between species both in fetal and adult epicardium; the embryonic human epicardium is not a simple squamous epithelium, as previously reported118. Instead, the external layer of flat mesothelial cells overlies a thin basal lamina with an underlying layer of connective tissue, the subepicardial space, containing elastic fibres as well as large vessels. In the adult human myocardium, the subepicardial space consists mainly of adipose tissue, which surrounds coronary vessels. By contrast, murine epicardium does not contain adipose tissue and comprises only a monolayer of mesothelial cells on a thin layer of connective tissue formed from elastic fibres118. These species differences suggest that experiments on human tissue may be important for translation from animal models to humans.
Furthermore, at least in the fetal human epicardium, atrial-ventricular differences in cellular behaviour were reported between epicardial cells. The ventricular, but not atrial, epicardium exhibited greater cell alignment and spindle-like morphology and ex vivo ventricular cells spontaneously differentiate and lose epicardial identity, whereas atrial-derived cells remained more ‘epithelial-like’117. The utility of cultured human atrial EPDCs as a model may therefore be limited not only by low availability but also by dissimilarity to the epicardial cells that may be stimulated in vivo. If atrial epicardial cells differ from ventricular epicardial cells, cells from atrial appendages may not necessarily represent the population of cells that partakes in the majority of reactivation after MI, albeit the extent of contribution in vivo from the individual chambers has not been addressed.
In an attempt to overcome these difficulties, epicardial-like cells have been generated from human pluripotent stem cells (hPSCs)119. One approach used stage-specific activation of bone morphogenetic protein (BMP) and WNT signalling pathways to generate cells with epicardial morphology expressing the epicardial markers WT1, TBX18 and RALDH2. These epicardial-like cells could be induced to undergo EMT to produce populations displaying characteristics of fibroblast and vascular smooth muscle cell lineages116. An independent approach used a two-stage process to generate similar epicardial-like cells. The hPSCs were first converted to lateral plate mesoderm and then differentiated into epicardial-like cells by stimulation of BMP, WNT and retinoic acid signalling pathways120. These approaches can provide unlimited sources of cultured human epicardial-like cells which could be used to optimise potential treatments or could be combined with chemical genetic screening techniques to identify novel regenerative compounds and provide insight into poorly-understood signalling pathways86,121.
Conclusion
High rates of cardiovascular disease mean that safe, effective and feasible cardiac regeneration could transform clinical practice. Reactivation of epicardial developmental processes is an attractive approach to coordinated regeneration of the heart and, if refined based on emerging concepts in epicardial biology, could provide novel strategies for regenerative epicardial reactivation.
Funding sources
NS is supported by the British Heart Foundation Ian Fleming Senior Basic Science Research Fellowship.
References
- 1.Mozaffarian D, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2015 doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Gerber Y, et al. Contemporary trends in heart failure with reduced and preserved ejection fraction after myocardial infarction: a community study. Am J Epidemiol. 2013;178(8):1272–80. doi: 10.1093/aje/kwt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jameel MN, Zhang J. Heart failure management: the present and the future. Antioxid.Redox.Signal. 2009;11(8):1989–2010. doi: 10.1089/ars.2009.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin Z, Pu WT. Strategies for cardiac regeneration and repair. Sci Transl Med. 2014;6(239):239rv1. doi: 10.1126/scitranslmed.3006681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergmann O, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324(5923):98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angert D, et al. Repair of the injured adult heart involves new myocytes potentially derived from resident cardiac stem cells. Circ Res. 2011;108(10):1226–37. doi: 10.1161/CIRCRESAHA.110.239046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Senyo SE, et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493(7432):433–6. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porrello ER, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331(6020):1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotech. 2005;23(7):845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 10.van Berlo JH, Molkentin JD. An emerging consensus on cardiac regeneration. Nat Med. 2014;20(12):1386–93. doi: 10.1038/nm.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin Z, et al. Cardiac-specific YAP activation improves cardiac function and survival in an experimental murine MI model. Circ Res. 2014;115(3):354–63. doi: 10.1161/CIRCRESAHA.115.303632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian L, et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012 doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menasche P. Skeletal myoblasts as a therapeutic agent. Prog.Cardiovasc Dis. 2007;50(1):7–17. doi: 10.1016/j.pcad.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Balsam LB, Robbins RC. Haematopoietic stem cells and repair of the ischaemic heart. Clin.Sci (Lond) 2005;109(6):483–492. doi: 10.1042/CS20050087. [DOI] [PubMed] [Google Scholar]
- 15.Beltrami AP, et al. Adult Cardiac Stem Cells Are Multipotent and Support Myocardial Regeneration. Cell. 2003;114(6):763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 16.Oh H, et al. Cardiac muscle plasticity in adult and embryo by heart-derived progenitor cells. Ann N Y.Acad Sci. 2004;1015:182–189. doi: 10.1196/annals.1302.015. [DOI] [PubMed] [Google Scholar]
- 17.Smith RR, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115(7):896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 18.Laugwitz KL, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433(7026):647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher SA, et al. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2015;9:Cd006536. doi: 10.1002/14651858.CD006536.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolli R, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378(9806):1847–57. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.The Lancet E. Expression of concern: the SCIPIO trial. Lancet. 2014;383(9925):1279. doi: 10.1016/S0140-6736(14)60608-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malliaras K, et al. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction) J Am Coll Cardiol. 2014;63(2):110–22. doi: 10.1016/j.jacc.2013.08.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Berlo JH, et al. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509(7500):337–41. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sultana N, et al. Resident c-kit(+) cells in the heart are not cardiac stem cells. Nat Commun. 2015;6:8701. doi: 10.1038/ncomms9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chong JJ, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510(7504):273–7. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talkhabi M, Aghdami N, Baharvand H. Human cardiomyocyte generation from pluripotent stem cells: A state-of-art. Life Sci. 2015 doi: 10.1016/j.lfs.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 27.Smart N, Riley PR. The epicardium as a candidate for heart regeneration. Future.Cardiol. 2012;8(1):53–69. doi: 10.2217/fca.11.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smart N, et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474:640–644. doi: 10.1038/nature10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassink RJ, et al. Cardiomyocyte cell cycle activation improves cardiac function after myocardial infarction. Cardiovasc Res. 2008;78(1):18–25. doi: 10.1093/cvr/cvm101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eulalio A, et al. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492(7429):376–81. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 31.Porrello ER, et al. MiR-15 family regulates postnatal mitotic arrest of cardiomyocytes. Circ Res. 2011;109(6):670–9. doi: 10.1161/CIRCRESAHA.111.248880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jabbour A, et al. Parenteral administration of recombinant human neuregulin-1 to patients with stable chronic heart failure produces favourable acute and chronic haemodynamic responses. Eur J Heart Fail. 2011;13(1):83–92. doi: 10.1093/eurjhf/hfq152. [DOI] [PubMed] [Google Scholar]
- 33.Mahmoud AI, et al. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature. 2013;497(7448):249–53. doi: 10.1038/nature12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei K, et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature. 2015;525(7570):479–85. doi: 10.1038/nature15372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zangi L, et al. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat Biotechnol. 2013;31(10):898–907. doi: 10.1038/nbt.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urbanek K, et al. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res. 2005;97(7):663–73. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 37.Song K, et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485(7400):599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Addis RC, et al. Optimization of direct fibroblast reprogramming to cardiomyocytes using calcium activity as a functional measure of success. J Mol Cell Cardiol. 2013;60:97–106. doi: 10.1016/j.yjmcc.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srivastava D, et al. Cardiac repair with thymosin beta4 and cardiac reprogramming factors. Ann.N.Y.Acad.Sci. 2012;1270:66–72. doi: 10.1111/j.1749-6632.2012.06696.x. [DOI] [PubMed] [Google Scholar]
- 40.Balmer GM, et al. Dynamic haematopoietic cell contribution to the developing and adult epicardium. Nat Commun. 2014;5:4054. doi: 10.1038/ncomms5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riley PR. An epicardial floor plan for building and rebuilding the mammalian heart. Curr.Top.Dev.Biol. 2012;100:233–251. doi: 10.1016/B978-0-12-387786-4.00007-5. [DOI] [PubMed] [Google Scholar]
- 42.Cai CL, et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454(7200):104–108. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou B, et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454(7200):109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christoffels VM, et al. Tbx18 and the fate of epicardial progenitors. Nature. 2009;458(7240):E8–E9. doi: 10.1038/nature07916. [DOI] [PubMed] [Google Scholar]
- 45.Rudat C, Kispert A. Wt1 and Epicardial Fate Mapping. Circ.Res. 2012 doi: 10.1161/CIRCRESAHA.112.273946. [DOI] [PubMed] [Google Scholar]
- 46.Duim SN, et al. Cardiac endothelial cells express Wilms’ tumor-1: Wt1 expression in the developing, adult and infarcted heart. J Mol Cell Cardiol. 2015;81:127–35. doi: 10.1016/j.yjmcc.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Katz TC, et al. Distinct compartments of the proepicardial organ give rise to coronary vascular endothelial cells. Dev.Cell. 2012;22(3):639–650. doi: 10.1016/j.devcel.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Red-Horse K, et al. Coronary arteries form by developmental reprogramming of venous cells. Nature. 2010;464(7288):549–553. doi: 10.1038/nature08873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smart N, Dube KN, Riley PR. Epicardial progenitor cells in cardiac regeneration and neovascularisation. Vascul.Pharmacol. 2012 doi: 10.1016/j.vph.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Wu B, et al. Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell. 2012;151(5):1083–96. doi: 10.1016/j.cell.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masters M, Riley PR. The epicardium signals the way towards heart regeneration. Stem Cell Res. 2014;13(3 Pt B):683–92. doi: 10.1016/j.scr.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smart N, Dube KN, Riley PR. Coronary vessel development and insight towards neovascular therapy. Int.J.Exp.Pathol. 2009;90(3):262–283. doi: 10.1111/j.1365-2613.2009.00646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kastner P, et al. Genetic analysis of RXR alpha developmental function: convergence of RXR and RAR signaling pathways in heart and eye morphogenesis. Cell. 1994;78(6):987–1003. doi: 10.1016/0092-8674(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 54.Sucov HM, et al. RXR alpha mutant mice establish a genetic basis for vitamin A signaling in heart morphogenesis. Genes Dev. 1994;8(9):1007–18. doi: 10.1101/gad.8.9.1007. [DOI] [PubMed] [Google Scholar]
- 55.Chen TH, et al. Epicardial Induction of Fetal Cardiomyocyte Proliferation via a Retinoic Acid-Inducible Trophic Factor. Developmental Biology. 2002;250(1):198–207. doi: 10.1006/dbio.2002.0796. [DOI] [PubMed] [Google Scholar]
- 56.Subbarayan V, et al. RXRalpha overexpression in cardiomyocytes causes dilated cardiomyopathy but fails to rescue myocardial hypoplasia in RXRalpha-null fetuses. J Clin Invest. 2000;105(3):387–94. doi: 10.1172/JCI8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stuckmann I, Evans S, Lassar AB. Erythropoietin and retinoic acid, secreted from the epicardium, are required for cardiac myocyte proliferation. Dev.Biol. 2003;255(2):334–349. doi: 10.1016/s0012-1606(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 58.Merki E, et al. Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proc.Natl.Acad Sci USA. 2005;102(51):18455–18460. doi: 10.1073/pnas.0504343102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brade T, et al. Retinoic acid stimulates myocardial expansion by induction of hepatic erythropoietin which activates epicardial Igf2. Development. 2011;138(1):139–148. doi: 10.1242/dev.054239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guadix JA, et al. Wt1 controls retinoic acid signalling in embryonic epicardium through transcriptional activation of Raldh2. Development. 2011;138(6):1093–1097. doi: 10.1242/dev.044594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith CL, et al. Epicardial-Derived Cell Epithelial-to-Mesenchymal Transition and Fate Specification Require PDGF Receptor Signaling / Novelty and Significance. Circulation Research. 2011;108(12):e15–e26. doi: 10.1161/CIRCRESAHA.110.235531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.von GA, et al. WT1 regulates epicardial epithelial to mesenchymal transition through beta-catenin and retinoic acid signaling pathways. Dev.Biol. 2011;356(2):421–431. doi: 10.1016/j.ydbio.2011.05.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eid H, et al. Role of epicardial mesothelial cells in the modification of phenotype and function of adult rat ventricular myocytes in primary coculture. Circ.Res. 1992;71(1):40–50. doi: 10.1161/01.res.71.1.40. [DOI] [PubMed] [Google Scholar]
- 64.Weeke-Klimp A, et al. Epicardium-derived cells enhance proliferation, cellular maturation and alignment of cardiomyocytes. J.Mol.Cell Cardiol. 2010;49(4):606–616. doi: 10.1016/j.yjmcc.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 65.Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117(4):1183–1198. doi: 10.1242/dev.117.4.1183. [DOI] [PubMed] [Google Scholar]
- 66.Dettman RW, et al. Inhibition of [alpha]4-integrin stimulates epicardial-mesenchymal transformation and alters migration and cell fate of epicardially derived mesenchyme. Developmental Biology. 2003;257(2):315–328. doi: 10.1016/s0012-1606(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 67.Ieda M, et al. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev.Cell. 2009;16(2):233–244. doi: 10.1016/j.devcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morabito CJ, et al. Positive and Negative Regulation of Epicardial-Mesenchymal Transformation during Avian Heart Development. Developmental Biology. 2001;234(1):204–215. doi: 10.1006/dbio.2001.0254. [DOI] [PubMed] [Google Scholar]
- 69.Lavine KJ, et al. Fibroblast growth factor signals regulate a wave of Hedgehog activation that is essential for coronary vascular development. Genes and Development. 2006;20(12):1651–1666. doi: 10.1101/gad.1411406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Compton LA, et al. Coronary vessel development is dependent on the type III transforming growth factor beta receptor. Circ Res. 2007;101(8):784–791. doi: 10.1161/CIRCRESAHA.107.152082. [DOI] [PubMed] [Google Scholar]
- 71.Compton LA, et al. Transforming growth factor-beta induces loss of epithelial character and smooth muscle cell differentiation in epicardial cells. Dev.Dyn. 2006;235(1):82–93. doi: 10.1002/dvdy.20629. [DOI] [PubMed] [Google Scholar]
- 72.Tomanek RJ, et al. Multiple growth factors regulate coronary embryonic vasculogenesis. Dev.Dyn. 2001;221(3):265–273. doi: 10.1002/dvdy.1137. [DOI] [PubMed] [Google Scholar]
- 73.Smart N, et al. Thymosin b4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445(7124):177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 74.Kang J, et al. PDGF-A as an epicardial mitogen during heart development. Dev.Dyn. 2008;237(3):692–701. doi: 10.1002/dvdy.21469. [DOI] [PubMed] [Google Scholar]
- 75.Lepilina A, et al. A Dynamic Epicardial Injury Response Supports Progenitor Cell Activity during Zebrafish Heart Regeneration. Cell. 2006;127(3):607–619. doi: 10.1016/j.cell.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 76.van Wijk B, et al. Cardiac regeneration from activated epicardium. PLoS ONE. 2012;7(9):e44692. doi: 10.1371/journal.pone.0044692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou B, et al. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J.Clin.Invest. 2011;121(5):1894–1904. doi: 10.1172/JCI45529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rossdeutsch A, et al. Essential role for thymosin beta4 in regulating vascular smooth muscle cell development and vessel wall stability. Circ.Res. 2012;111(4):e89–102. doi: 10.1161/CIRCRESAHA.111.259846. [DOI] [PubMed] [Google Scholar]
- 79.Smart N, et al. Thymosin beta4 facilitates epicardial neovascularization of the injured adult heart. Ann.N.Y.Acad.Sci. 2010;1194:97–104. doi: 10.1111/j.1749-6632.2010.05478.x. [DOI] [PubMed] [Google Scholar]
- 80.Bollini S, Riley PR, Smart N. Thymosin beta4: multiple functions in protection, repair and regeneration of the mammalian heart. Expert Opin Biol Ther. 2015;15(Suppl 1):163–74. doi: 10.1517/14712598.2015.1022526. [DOI] [PubMed] [Google Scholar]
- 81.Kohl P, Gourdie RG. Fibroblast-myocyte electrotonic coupling: does it occur in native cardiac tissue? J Mol Cell Cardiol. 2014;70:37–46. doi: 10.1016/j.yjmcc.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bollini S, et al. Re-activated adult epicardial progenitor cells are a heterogeneous population molecularly distinct from their embryonic counterparts. Stem Cells Dev. 2014;23(15):1719–30. doi: 10.1089/scd.2014.0019. [DOI] [PubMed] [Google Scholar]
- 83.Zhou B, et al. Thymosin beta 4 treatment after myocardial infarction does not reprogram epicardial cells into cardiomyocytes. J.Mol.Cell Cardiol. 2011 doi: 10.1016/j.yjmcc.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Urayama K, et al. The prokineticin receptor-1 (GPR73) promotes cardiomyocyte survival and angiogenesis. Faseb j. 2007;21(11):2980–93. doi: 10.1096/fj.07-8116com. [DOI] [PubMed] [Google Scholar]
- 85.Urayama K, et al. Prokineticin receptor-1 induces neovascularization and epicardial-derived progenitor cell differentiation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(5):841–849. doi: 10.1161/ATVBAHA.108.162404. [DOI] [PubMed] [Google Scholar]
- 86.Vieira JM, Riley PR. Chemical genetics and its potential in cardiac stem cell therapy. Br J Pharmacol. 2013;169(2):318–27. doi: 10.1111/j.1476-5381.2012.01928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sutton MGSJ, Sharpe N. Left Ventricular Remodeling After Myocardial Infarction: Pathophysiology and Therapy. Circulation. 2000;101(25):2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 88.Limana F, et al. Myocardial infarction induces embryonic reprogramming of epicardial c-kit(+) cells: role of the pericardial fluid. J.Mol.Cell Cardiol. 2010;48(4):609–618. doi: 10.1016/j.yjmcc.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 89.Cao J, et al. Single epicardial cell transcriptome sequencing identifies Caveolin-1 as an essential factor in zebrafish heart regeneration. Development. 2015 doi: 10.1242/dev.130534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc.Res. 2002;53(1):31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 91.Huang GN, et al. C/EBP transcription factors mediate epicardial activation during heart development and injury. Science. 2012;338(6114):1599–603. doi: 10.1126/science.1229765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Evans MA, et al. Thymosin beta4-sulfoxide attenuates inflammatory cell infiltration and promotes cardiac wound healing. Nat Commun. 2013;4:2081. doi: 10.1038/ncomms3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bock-Marquette I, et al. Thymosin b4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432(7016):466–472. doi: 10.1038/nature03000. [DOI] [PubMed] [Google Scholar]
- 94.Smart N, et al. Myocardial regeneration: expanding the repertoire of thymosin beta4 in the ischemic heart. Ann.N.Y.Acad.Sci. 2012;1269(1):92–101. doi: 10.1111/j.1749-6632.2012.06708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aurora AB, et al. Macrophages are required for neonatal heart regeneration. J Clin Invest. 2014;124(3):1382–92. doi: 10.1172/JCI72181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Riley PR. Fanning the flames to regenerate the heart. J Clin Invest. 2014;124(3):961–4. doi: 10.1172/JCI74418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nahrendorf M, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204(12):3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation. 2010;121(22):2437–45. doi: 10.1161/CIRCULATIONAHA.109.916346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rienks M, et al. Myocardial extracellular matrix: an ever-changing and diverse entity. Circ Res. 2014;114(5):872–88. doi: 10.1161/CIRCRESAHA.114.302533. [DOI] [PubMed] [Google Scholar]
- 100.Gulati AK, Zalewski AA, Reddi AH. An immunofluorescent study of the distribution of fibronectin and laminin during limb regeneration in the adult newt. Dev Biol. 1983;96(2):355–65. doi: 10.1016/0012-1606(83)90173-2. [DOI] [PubMed] [Google Scholar]
- 101.Calve S, Simon HG. Biochemical and mechanical environment cooperatively regulate skeletal muscle regeneration. Faseb J. 2012;26(6):2538–45. doi: 10.1096/fj.11-200162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mercer SE, et al. Multi-tissue microarray analysis identifies a molecular signature of regeneration. PLoS ONE. 2012;7(12):e52375. doi: 10.1371/journal.pone.0052375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Govindan J, Iovine MK. Hapln1a is required for connexin43-dependent growth and patterning in the regenerating fin skeleton. PLoS ONE. 2014;9(2):e88574. doi: 10.1371/journal.pone.0088574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang J. Fibronectin is deposited by injury-activated epicardial cells and is necessary for zebrafish heart regeneration. Dev Biol. 2013;382(2):427–35. doi: 10.1016/j.ydbio.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mercer SE, Odelberg SJ, Simon HG. A dynamic spatiotemporal extracellular matrix facilitates epicardial-mediated vertebrate heart regeneration. Dev Biol. 2013;382(2):457–69. doi: 10.1016/j.ydbio.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jopling C, et al. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464(7288):606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kikuchi K, et al. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464(7288):601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kikuchi K, et al. tcf21+ epicardial cells adopt non-myocardial fates during zebrafish heart development and regeneration. Development. 2011;138(14):2895–2902. doi: 10.1242/dev.067041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Itou J, et al. Migration of cardiomyocytes is essential for heart regeneration in zebrafish. Development. 2012;139(22):4133–42. doi: 10.1242/dev.079756. [DOI] [PubMed] [Google Scholar]
- 110.Iwakura A, et al. Myocardial ischemia enhances the expression of acidic fibroblast growth factor in human pericardial fluid. Heart Vessels. 2000;15(3):112–116. doi: 10.1007/pl00007264. [DOI] [PubMed] [Google Scholar]
- 111.Fujita M, et al. Elevated basic fibroblast growth factor in pericardial fluid of patients with unstable angina. Circulation. 1996;94(4):610–613. doi: 10.1161/01.cir.94.4.610. [DOI] [PubMed] [Google Scholar]
- 112.Kuosmanen SM, et al. MicroRNA profiling of pericardial fluid samples from patients with heart failure. PLoS ONE. 2015;10(3):e0119646. doi: 10.1371/journal.pone.0119646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Foglio E, et al. Exosomal clusterin, identified in the pericardial fluid, improves myocardial performance following MI through epicardial activation, enhanced arteriogenesis and reduced apoptosis. Int J Cardiol. 2015;197:333–47. doi: 10.1016/j.ijcard.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 114.Yang Y, et al. MRI study of cryoinjury infarction in pig hearts: i. Effects of intrapericardial delivery of bFGF/VEGF embedded in alginate beads. NMR Biomed. 2012;25(1):177–88. doi: 10.1002/nbm.1736. [DOI] [PubMed] [Google Scholar]
- 115.van Tuyn J, et al. Epicardial cells of human adults can undergo an epithelial-to-mesenchymal transition and obtain characteristics of smooth muscle cells in vitro. Stem Cells. 2006:2006-0366. doi: 10.1634/stemcells.2006-0366. [DOI] [PubMed] [Google Scholar]
- 116.Bax NA, et al. Epithelial-to-mesenchymal transformation alters electrical conductivity of human epicardial cells. J.Cell Mol.Med. 2011 doi: 10.1111/j.1582-4934.2011.01266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Risebro CA, et al. Characterisation of the human embryonic and foetal epicardium during heart development. Development. 2015;142(21):3630–6. doi: 10.1242/dev.127621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Limana F, et al. Identification of myocardial and vascular precursor cells in human and mouse epicardium. Circ Res. 2007;101(12):1255–1265. doi: 10.1161/CIRCRESAHA.107.150755. [DOI] [PubMed] [Google Scholar]
- 119.Witty AD, et al. Generation of the epicardial lineage from human pluripotent stem cells. Nat Biotechnol. 2014;32(10):1026–35. doi: 10.1038/nbt.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Iyer D, et al. Robust derivation of epicardium and its differentiated smooth muscle cell progeny from human pluripotent stem cells. Development. 2015;142(8):1528–41. doi: 10.1242/dev.119271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bock-Marquette I, et al. Thymosin beta4 mediated PKC activation is essential to initiate the embryonic coronary developmental program and epicardial progenitor cell activation in adult mice in vivo. J.Mol.Cell Cardiol. 2009;46(5):728–738. doi: 10.1016/j.yjmcc.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]