Abstract
Purpose: To determine whether diabetes mellitus has an independent impact on major limb outcomes at 1 year after endovascular treatment of lower extremity peripheral artery disease (PAD). Methods: The study involved 1906 consecutive patients (mean age 66 years; 1469 men) enrolled in the observational Excellence in Peripheral Artery Disease (XLPAD) registry (ClinicalTrials.gov identifier NCT01904851) between January 2005 and October 2015 after undergoing index endovascular procedures in 2426 limbs for arterial occlusive disease. Patient outcomes included 12-month target limb amputation (above ankle) and target limb revascularization as well as all-cause death. Kaplan-Meier analysis and adjusted Cox proportional hazard models were used for time-to-event analysis of outcomes for the entire study sample as well as for the critical limb ischemia (CLI) and claudication subgroups. Results of the Cox regression models are reported as the hazard ratio (HR) and 95% confidence interval (CI). Results: Diabetics undergoing endovascular procedures had higher rates of comorbid conditions (p<0.001), CLI (p<0.001), heavily calcified lesions (p=0.002), multivessel disease (p=0.030), and fewer infrapopliteal runoff vessels (p<0.001). Regression analysis after adjusting for confounders revealed significantly higher target limb major amputation in diabetics compared with nondiabetics (HR 5.02, 95% CI 1.44 to 17.56, p=0.011). However, repeat revascularization rates were similar. When considering CLI and claudication subgroups, diabetes was associated with a nonsignificant increased risk of 12-month major amputation only for patients presenting with CLI (HR 3.48, 95% CI 0.97 to 12.51, p=0.056). Diabetes was also associated with an increased risk of 12-month all-cause mortality in the overall study sample (HR 4.64, 95% CI 2.01 to 10.70, p<0.001) and in the CLI subgroup (HR 14.15, 95% CI 3.16 to 63.32, p<0.001) but not in the claudication subgroup (HR 1.42, 95% CI 0.45 to 4.54, p=0.552). Conclusion: Diabetes increases the risk of major amputation and all-cause death at 12 months following endovascular revascularization in patients with symptomatic PAD. These risks are especially heightened in patients presenting with CLI.
Keywords: amputation; balloon angioplasty; diabetes mellitus; mortality; peripheral artery disease; reintervention, stenosis; stent; target lesion revascularization
Introduction
Diabetes mellitus is a growing problem worldwide, and in the United States it is linked to the epidemic of obesity.1,2 Diabetes is a major risk factor for peripheral artery disease (PAD)3–5 and leads to more diffuse calcified disease6 and higher rates of lower extremity amputation.7 It affects the entire vascular system, particularly the lower extremity vessels below the knee.7 At this time, it is unclear whether diabetic patients have a worse outcome than nondiabetics following lower limb endovascular interventions. Several studies have indicated that diabetes is a predictor of poor outcomes following such procedures with poorer patency rates8 and cardiovascular outcomes.9,10 However, other studies suggested that diabetics have similar outcomes to nondiabetics following stenting11 or atherectomy (directional12 or rotational13) despite more advanced comorbidities. In these studies, and after adjusting for multiple comorbidities, diabetics had a similar patency rate, freedom from target lesion revascularization and clinical improvement compared with nondiabetics.
This study analyzed registry data from PAD patients who underwent lower limb endovascular interventions to determine whether diabetes has an independent impact on target limb major amputation and repeat revascularization at 1-year follow-up.
Methods
Study Design and Patient Sample
The study involved 1906 consecutive patients (mean age 66.5 years; 1469 men) enrolled retrospectively (95%) and prospectively (5%) in the observational Excellence in Peripheral Artery Disease (XLPAD) registry (ClinicalTrials.gov identifier NCT01904851) after undergoing index endovascular procedures in 2426 limbs between January 2005 and October 2015. The XLPAD registry employs REDCap, a web-based data acquisition system, to gather data from 11 sites contributing consecutive patients treated with endovascular techniques for lower extremity arterial disease. The registry data collection and adjudication procedures have been previously described.14,15 Of note, angiograms of all enrolled patients are reviewed by the core laboratory at the Veteran Affairs Medical Center, Dallas, TX, USA.
For the present analysis, patients were divided into 2 groups according to presence or absence of diabetes based on clinical diagnosis and/or use of medications prescribed for the treatment of diabetes. Patients were followed for 12 months after an index lower limb endovascular revascularization procedure. Outcomes in this study were target limb amputation (above ankle), target limb reintervention (endovascular or surgical), and all-cause mortality.
Diabetics comprised 56.9% of the study cohort. A third of all patients (37.3%) presented with critical limb ischemia (CLI). The majority of lesions were in the femoropopliteal segment (81.2%). Chronic total occlusions accounted for 51.9% of the target lesions. Baseline demographic, clinical, lesion, and treatment characteristics for the entire cohort and by subgroups are shown in Table 1.
Table 1.
Baseline Patient and Lesion Characteristics and Postprocedure Medical Therapy Between Patients With and Without Diabetes Mellitus (DM).a
| All | DM | No DM | p | |
|---|---|---|---|---|
| Patient characteristics | n=1906 | n=1084 | n=822 | |
| Age, y | 66.5±9.9 | 66.2±9.6 | 66.8±10.3 | 0.219 |
| Men | 1469 (77.4) | 860 (79.3) | 609 (74.9) | 0.024 |
| Race | ||||
| White | 1344 (70.8) | 718 (66.2) | 626 (77.0) | <0.001 |
| African American | 328 (17.3) | 193 (17.8) | 135 (16.6) | |
| Hispanic | 178 (9.4) | 145 (13.4) | 33 (4.1) | |
| Other | 47 (2.5) | 28 (2.6) | 19 (2.3) | |
| Rutherford category | ||||
| 1 | 184 (9.7) | 91 (8.4) | 93 (11.4) | <0.001 |
| 2 | 93 (4.9) | 40 (3.7) | 53 (6.5) | |
| 3 | 913 (48.1) | 453 (41.8) | 460 (56.6) | |
| 4 | 273 (14.4) | 164 (15.1) | 109 (13.4) | |
| 5 | 434 (22.9) | 336 (31.0) | 98 (12.1) | <0.001 |
| Critical limb ischemia | 707 (37.3) | 500 (46.1) | 207 (25.5) | <0.001 |
| Coronary artery disease | 1238 (65.3) | 789 (72.8) | 449 (55.2) | <0.001 |
| Prior MI | 441 (23.3) | 285 (26.3) | 156 (19.2) | <0.001 |
| Chronic kidney disease | 345 (18.2) | 274 (25.3) | 71 (8.7) | <0.001 |
| Heart failure | 329 (17.3) | 238 (22.0) | 91 (11.2) | <0.001 |
| Hypertension | 1731 (91.3) | 1048 (96.7) | 683 (84.0) | <0.001 |
| Hyperlipidemia | 1623 (85.6) | 993 (91.6) | 630 (77.5) | <0.001 |
| LDL, mg/dL | 82.0±34.8 | 77.3±34.4 | 88.5±34.3 | <0.001 |
| Ankle-brachial index | 0.68±0.23 | 0.68±0.24 | 0.69±0.22 | 0.184 |
| Lesion characteristics (per limb) | n=2426 | n=1358 | n=1068 | |
| Target vessels | ||||
| Femoropopliteal | 1969 (81.2) | 1028 (75.7) | 941 (88.1) | <0.001 |
| Infrapopliteal | 424 (17.5) | 313 (23.1) | 111 (10.4) | <0.001 |
| Multivessel diseaseb | 660 (27.2) | 393 (28.9) | 267 (25.0) | 0.030 |
| Iliac intervention | 234 (9.7) | 92 (6.8) | 142 (13.3) | <0.001 |
| Infrapopliteal runoff | 2.1±0.8 | 2.0±0.8 | 2.2±0.8 | <0.001 |
| Vessel diameter, mm | 4.9±1.1 | 4.8±1.2 | 5.1±1.0 | <0.001 |
| Lesion length, mm | 136.8±99.4 | 134.8±98.9 | 139.2±100.0 | 0.366 |
| Chronic total occlusions | 1259 (51.9) | 699 (51.5) | 560 (52.4) | 0.638 |
| Heavy calcificationc | 1190 (49.5) | 704 (51.8) | 486 (45.5) | 0.002 |
| Endovascular treatment | ||||
| Angioplasty | 2113 (87.1) | 1192 (87.8) | 921 (86.2) | 0.261 |
| Atherectomy | 1046 (43.1) | 574 (42.3) | 472 (44.2) | 0.341 |
| BMS | 693 (28.6) | 378 (27.8) | 315 (29.5) | 0.369 |
| DES | 146 (6.0) | 61 (4.5) | 85 (8.0) | <0.001 |
| Postprocedure medical therapy | ||||
| Dual antiplatelet therapy | 1972 (81.3) | 1124 (82.7) | 848 (79.4) | 0.035 |
| Lipid-lowering drugs | 1932 (79.6) | 1131 (83.3) | 801 (75.0) | <0.001 |
| Angiotensin blockers | 1330 (55.0) | 875 (64.4) | 455 (42.6) | <0.001 |
Abbreviations: BMS, bare metal stent; DES, drug-eluting stent; LDL, low-density lipoprotein; MI, myocardial infarction.
Continuous data are presented as the means ± standard deviation; categorical data are given as the counts (percentage).
Multivessel disease is defined as both femoropopliteal disease and below the knee disease.
Heavy calcification is defined as the presence of at least 5 mm of calcification on both sides of the vessel.
Definitions and Statistical Analysis
Kaplan-Meier analysis and Cox proportional hazard models were used for time-to-event analysis of the 3 outcomes on a periprocedure basis adjusted for baseline patient and lesion characteristics. The Cox regression model for all-cause death was adjusted for baseline patient characteristics only. Patient characteristics included age, gender, ethnicity, race, comorbid conditions (coronary artery disease, heart failure, hypertension, hyperlipidemia, and prior myocardial infarction), Rutherford category, and ankle-brachial index (ABI). Lesion characteristics included indicators of multivessel disease, concurrent iliac intervention with an infrainguinal revascularization, and heavy calcification. Multivessel disease was defined as both femoropopliteal and below-the-knee disease (≥50% angiographic stenosis). Heavy calcification was defined as presence of at least 5 mm of calcification on both sides of the vessel. Cox models were also adjusted for the number of runoff vessels and prescribed dual antiplatelet, lipid-lowering, and angiotensin-converting enzyme/angiotensin receptor antagonist (ACE/ARB) therapies. Interaction terms were tested in adjusted Cox models to examine the effect of diabetes with other baseline comorbid conditions. However, the interaction terms were included in the final models only if they were statistically significant (p<0.05). Chronic kidney disease was not included in the model due to multicollinearity with other baseline comorbidities. Cox models were run only when a homogeneity assumption across 2 groups, diabetics and nondiabetics, was met. In other words, in Kaplan-Meier analysis, survival curves between 2 groups do not cross over.
Continuous variables were described using the mean ± standard deviation and evaluated for difference between groups with t tests. Categorical variables were presented as the frequency (percentage) and were assessed using a chi-square test. Curves in the Kaplan-Meier graphs were compared using the log-rank test. Missing data on age, ABI, and runoff were imputed using a multiple imputation technique described by Little and Rubin16 under the missing at random assumption. Subgroup analyses were performed for patients presenting with CLI (Rutherford category ≥4) vs claudication (Rutherford category ≤3). Cox models were used for subgroup analysis. The threshold of statistical significance was p<0.05. All statistical analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC, USA).
Results
Diabetics had significantly higher rates of concomitant coronary artery disease, heart failure, hypertension, hyperlipidemia, and chronic kidney disease compared with nondiabetics (Table 1), and a higher percentage of the diabetic patients presented with CLI. While patients with diabetes had smaller reference vessel diameters, more heavily calcified and multivessel disease, and fewer runoff vessels than nondiabetics, there were no significant group differences in the incidence of chronic total occlusion or mean lesion lengths. Diabetes was significantly associated with a higher rate of infrapopliteal vessel interventions. The diabetes group was more likely to receive dual antiplatelet therapy, lipid-lowering, and ACE/ARB therapies postprocedure and less likely to receive an iliac intervention during the index procedure compared with the nondiabetic group. Endovascular strategies employed for treating diabetic and nondiabetic patients were similar, except for higher use of drug-eluting stents in nondiabetics. Baseline characteristics of CLI and claudicants subgroups are shown in Supplemental Table S1 (supplementary material available at http://journals.sagepub.com/doi/suppl/10.1177/1526602817705135).
For limb-related outcomes, Kaplan-Meier estimates showed a higher risk for 12-month major amputation compared with patients without diabetes for the entire study sample (3.16% vs 0.46%, p<0.001) and in the CLI (6.30% vs 1.57%, p=0.003) and claudicant (2.47% vs 0.86%, p=0.049) subgroups (Figure 1). However, 12-month repeat revascularization was not significantly different between diabetics and nondiabetics in the entire study sample (17.11% vs 17.14%, p=0.772) or in either the CLI (18.29% vs 21.57%, p=0.439) or claudicant (16.21% vs 15.74%, p=0.801) subgroup (Figure 2).
Figure 1.
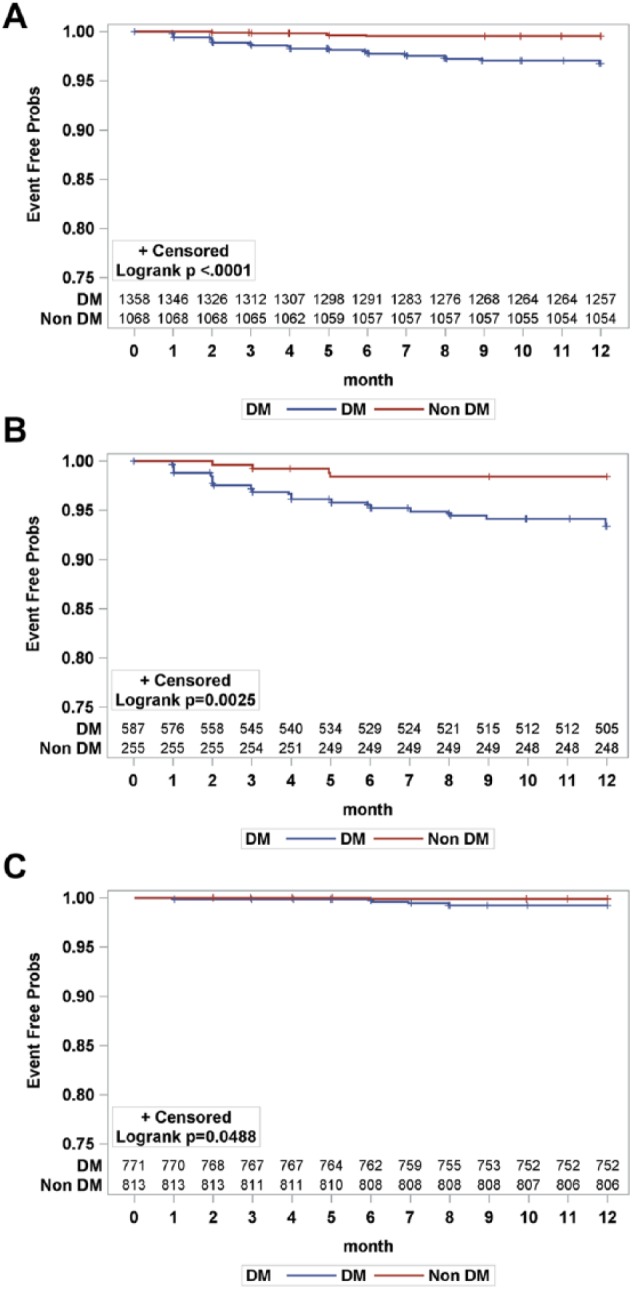
Kaplan-Meier curves for target limb major amputation at 12 months for diabetics (DM) and nondiabetics (non DM): (A) entire sample, (B) critical limb ischemia subgroup, and (C) claudicant subgroup. Seventeen observations were excluded due to missing date of target limb amputation. The standard error did not exceed 10% at 12 months for both groups. Probs, probability.
Figure 2.

Kaplan-Meier curves for repeat endovascular and surgical revascularization per procedure at 12 months for diabetics (DM) and nondiabetics (non DM): (A) entire sample, (B) critical limb ischemia subgroup, and (C) claudicant subgroup. Nineteen observations were excluded due to missing date of repeat endovascular and surgical revascularization. The standard error did not exceed 10% at 12 months for both groups. Probs, probability.
The adjusted Cox models found that the risk of major amputation at 12 months increased by 502% in patients with diabetes compared with patients without [hazard ratio (HR) 5.02, 95% CI 1.44 to 17.56, p=0.011], while there was no significant difference in 12-month need for repeat revascularization (HR 0.83, 95% CI 0.65 to 1.05, p=0.119). In the CLI subgroup, diabetics had a higher major amputation rate, which was not significant (HR 3.48, 95% CI 0.97 to 12.51, p=0.056), and a similar repeat revascularization rate at 12 months. For the claudication subgroup, no significant differences were observed between diabetics and nondiabetics for either of the endpoints.
Kaplan-Meier analysis of all-cause mortality estimated that the diabetes group had significantly higher 12-month mortality in the overall sample and in the CLI subgroup (Figure 3). In an adjusted Cox model, diabetics had almost 4 times higher mortality risk than nondiabetic patients in the entire study sample (HR 4.64, 95% CI 2.01 to 10.70, p<0.001). In the CLI subgroup, diabetics had a 14-fold higher risk of death (HR 14.15, 95% CI 3.16 to 63.32, p<0.001); in the claudicant subgroup, there was no difference in the risk of death between diabetics and nondiabetics (HR 1.42, 95% CI 0.45 to 4.54, p=0.552).
Figure 3.

Kaplan-Meier curves for death at 12 months for diabetics (DM) and nondiabetics (non DM): (A) entire sample, (B) critical limb ischemia subgroup, and (C) claudicant subgroup. Nineteen observations were excluded due to missing data on time of death event. The standard error did not exceed 10% at 12 months for both groups. Probs, probability.
Discussion
This study indicates that diabetes is associated with a higher rate of major amputation at 12 months but not repeat revascularization following endovascular treatment of lower limb PAD. Additionally, for patients presenting with CLI, diabetes significantly increases the risk of major amputations of the target limb over the same time period.
Diabetes has been linked to more diffuse and calcified lower limb lesions and a higher occurrence of major adverse cardiovascular events.3,4,7 Jude et al7 reported a significant increase in mortality in diabetics compared with nondiabetics (51.7% vs 25.6%, p=0.002). Also, diabetic patients in their study died at a younger age. In a cohort of 1.9 million individuals followed for a median of 5.5 years, patients with diabetes presented most commonly with PAD (16.2%) and heart failure (14.1%).5 In addition, diabetes was associated with ischemic stroke, angina, and nonfatal myocardial infarction but inversely associated with abdominal aortic aneurysm and subarachnoid hemorrhage.
The positive association between diabetes and the heightened risk of target limb major amputation in the CLI subgroup confirms that diabetics with advanced limb ischemia are more likely to progress to amputation than nondiabetics following endovascular treatment of PAD.7 However, endovascular therapy of occlusive PAD in diabetics has shown good limb salvage rates and symptom improvement.12,13,17,18
It is unclear how diabetes affects the outcomes of endovascular intervention for lower extremity PAD. In 1 study,19 predictors of patency following superficial femoral artery stenting were evaluated in a cohort of 117 patients (165 limbs) at a mean follow-up of 15.3±3.2 months. The primary patency rates at 6, 12, 18, and 24 months were 78%, 66%, 42%, and 22%, respectively. Independent predictors associated with reintervention were TransAtlantic Inter-Society Consensus II C or D lesions, stent length >8 cm, number of patent tibial arteries, and diabetes. In a study of 437 patients (525 limbs) treated with balloon angioplasty with or without adjunctive stenting in 38%, 50% of patients were diabetic.20 Among claudicants, diabetics had significantly lower assisted primary patency (p<0.01) and a higher incidence of restenosis (p=0.04). Among limb ischemia patients, limb salvage was significantly worse for diabetics than nondiabetics.
Certain endovascular therapies, however, can improve limb outcome in diabetics. In the Pilot Trial of Cryoplasty or Conventional Balloon Post-Dilation of Nitinol Stents for Revascularization of Peripheral Arterial Segments (COBRA) trial,21 cryoplasty utilized as an adjunctive treatment to postdilate nitinol stents for revascularization of peripheral arterial segments in diabetic patients showed a significant reduction in binary restenosis compared to adjunctive treatment with balloon angioplasty alone (29.3% vs 55.8%, p=0.01; odds ratio 0.36, 95% CI 0.15 to 0.89). Also, recent data from a large registry containing 46.8% diabetics followed for 12 months showed no differences in patency rates in diabetes vs nondiabetics treated with directional atherectomy.12 Patency was assessed by duplex ultrasound using core laboratory adjudication. Despite more baseline comorbidities in the diabetic cohort, 12-month primary patency (77.0%) in diabetics was noninferior (p<0.001) to nondiabetics (77.9%). Also, freedom from clinically driven target lesion revascularization was similar between the groups (83.8% vs 87.5%).
In another study, 80 diabetic patients treated with rotational and aspiration atherectomy for femoropopliteal occlusive disease had similar outcomes to 92 nondiabetics.13 Both diabetics and nondiabetics had respectively similar lesion lengths (28.5 vs 26.2 mm) and frequencies of total occlusions (29% vs 32%) and restenotic lesions (15% vs 14%). At 1 year, the major adverse event rates (death, index limb amputation, myocardial infarction, target lesion revascularization, and target vessel revascularization) in diabetics was 25% compared with 31.5% in nondiabetics. The target lesion revascularization rate in diabetics through 12 months was 20% vs 28% in nondiabetics. However, most of the current evidence is limited to studies with small samples exploring the impact of specific PAD revascularization modalities on clinical outcomes in patients with diabetes, without adjusting for comorbidities and postprocedure antiplatelet and medical therapies that are known to impact patient outcomes. Moreover, combined endpoints reported in many of the above studies are a composite of cardiovascular and limb outcomes.
Our data indicate that target limb revascularization following lower extremity endovascular interventions in diabetics is similar to nondiabetics after adjusting for comorbid conditions and pharmacotherapy factors, however, with higher all-cause mortality. Our findings contradict some of previously published studies that indicate that diabetes is associated with worse repeat revascularization outcomes.19,20
Limitations
Despite the multicenter sources of consecutive patient data collection in our registry, it is observational in nature and hence subject to selection bias. The study also has other limitations. The diabetes group in the study included both type I and II, and the impact of diabetes type on the outcomes of endovascular interventions was not specifically investigated along all potential comorbid conditions. While our study considered both 12-month target limb revascularization and major amputation for the claudicant group, major amputation may not differ in this group at 12 months’ postintervention.
Conclusion
This study demonstrated that diabetic patients with advanced limb ischemia are at a higher risk of major amputation but have similar repeat revascularization rates at 12 months following endovascular treatment of PAD. Also, patients with diabetes have significantly higher all-cause mortality at 12 months.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the University of Texas Southwestern Medical Center for its support in establishing and managing the REDCap database software utilized in the XLPAD registry (Academic Information Systems NIH grant UL1-RR024982).
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Ehrin Armstrong reports receiving honoraria from Medtronic, Abbott Vascular, Boston Scientific Corporation, Cardiovascular Systems, Merck, and Spectranetics. Emmanouil Brilakis reports receiving consulting/speaker honoraria from Abbott Vascular, Asahi, Boston Scientific, Elsevier, Somahlution, St Jude Medical, and Terumo; research support from InfraRedx and Boston Scientific; and his spouse is an employee of Medtronic. Subhash Banerjee reports receiving research grants from Boston Scientific and the Medicines Company; consultant/speaker honoraria from Gilead, St Jude, Cordis, Boehinger Ingerheim, Sanofi, and Medtronic; spouse’s ownership of Mdcare Global; and intellectual property with HygeiaTel.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol. 2012;8:228–236. [DOI] [PubMed] [Google Scholar]
- 2. Menke A, Casagrande S, Geiss L, et al. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314:1021–1029. [DOI] [PubMed] [Google Scholar]
- 3. Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006;47:921–929. [DOI] [PubMed] [Google Scholar]
- 4. Lüscher TF, Creager MA, Beckman JA, et al. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II. Circulation. 2003;108:1655–1661. [DOI] [PubMed] [Google Scholar]
- 5. Shah AD, Langenberg C, Rapsomaniki E, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rocha-Singh KJ, Zeller T, Jaff MR. Peripheral arterial calcification: prevalence, mechanism, detection, and clinical implications. Catheter Cardiovasc Interv. 2014;83:E212–E220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jude EB, Oyibo SO, Chalmers N, et al. Peripheral arterial disease in diabetic and nondiabetic patients: a comparison of severity and outcome. Diabetes Care. 2001;24:1433–1437. [DOI] [PubMed] [Google Scholar]
- 8. DeRubertis BG, Pierce M, Ryer EJ, et al. Reduced primary patency rate in diabetic patients after percutaneous intervention results from more frequent presentation with limb-threatening ischemia. J Vasc Surg. 2008;47:101–108. [DOI] [PubMed] [Google Scholar]
- 9. Bunte MC, House JA, Spertus JA, et al. Association between health status and long-term mortality after percutaneous revascularization of peripheral artery disease. Catheter Cardiovasc Interv. 2016;87:1149–1155. [DOI] [PubMed] [Google Scholar]
- 10. Jones WS, Patel MR, Tsai TT, et al. Anatomic runoff score predicts cardiovascular outcomes in patients with lower extremity peripheral artery disease undergoing revascularization. Am Heart J. 2015;170:400–408. [DOI] [PubMed] [Google Scholar]
- 11. Oberto S, Cetta F, Trabattoni P, et al. A comparison of SFA treatment with Zilver PTX in diabetics vs non diabetics. Clinical and functional results after 24 months. [published online March 20, 2015]. J Cardiovasc Surg (Torino). [DOI] [PubMed] [Google Scholar]
- 12. Garcia LA, Jaff MR, Rocha-Singh KJ, et al. A comparison of clinical outcomes for diabetic and nondiabetic patients following directional atherectomy in the DEFINITIVE LE claudicant cohort. J Endovasc Ther. 2015;22:701–711. [DOI] [PubMed] [Google Scholar]
- 13. Sixt S, Scheinert D, Rastan A, et al. One-year outcome after percutaneous rotational and aspiration atherectomy in infrainguinal arteries in patient with and without type 2 diabetes mellitus. Ann Vasc Surg. 2011;25:520–529. [DOI] [PubMed] [Google Scholar]
- 14. Banerjee S, Sarode K, Patel A, et al. Comparative assessment of guidewire and microcatheter vs a crossing device-based strategy to traverse infrainguinal peripheral artery chronic total occlusions. J Endovasc Ther. 2015;22:525–534. [DOI] [PubMed] [Google Scholar]
- 15. Banerjee S, Sarode K, Mohammad A, et al. Femoropopliteal artery stent thrombosis: report from the Excellence in Peripheral Artery Disease Registry. Circ Cardiovasc Interv. 2016;9:e002730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Little RJ, Rubin DB. Statistical Analysis With Missing Data. 2nd ed. Hoboken, NJ: Wiley; 2002. [Google Scholar]
- 17. Ferraresi R, Centola M, Ferlini M, et al. Long-term outcomes after angioplasty of isolated, below-the-knee arteries in diabetic patients with critical limb ischaemia. Eur J Vasc Endovasc Surg. 2009;37:336–342. [DOI] [PubMed] [Google Scholar]
- 18. Alexandrescu V, Hubermont G, Philips Y, et al. Combined primary subintimal and endoluminal angioplasty for ischaemic inferior-limb ulcers in diabetic patients: 5-year practice in a multidisciplinary ‘diabetic-foot’ service. Eur J Vasc Endovasc Surg. 2009;37:448–456. [DOI] [PubMed] [Google Scholar]
- 19. Yu JS, Park KM, Jeon YS, et al. Midterm outcome of femoral artery stenting and factors affecting patency. Vasc Specialist Int. 2015;31:115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bakken AM, Palchik E, Hart JP, et al. Impact of diabetes mellitus on outcomes of superficial femoral artery endoluminal interventions. J Vasc Surg. 2007;46:946–958. [DOI] [PubMed] [Google Scholar]
- 21. Banerjee S, Das TS, Abu-Fadel MS, et al. Pilot trial of cryoplasty or conventional balloon post-dilation of nitinol stents for revascularization of peripheral arterial segments: the COBRA trial. J Am Coll Cardiol. 2012;60:1352–1359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


