Abstract
Background
So-called juvenile stroke, i.e., stroke in a person aged 18 to 55, affects approximately 30 000 persons per year in Germany and is thus an important cause of mortality and permanent morbidity. The spectrum of causes of stroke is broader in this age group than in older patients and is also differently distributed.
Methods
This review is based on pertinent publications retrieved by a selective search in PubMed and on current guideline recommendations.
Results
Juvenile strokes are often caused by cardiogenic emboli (ca. 25%) and by vascular dissection (ca. 20%). Approximately 10% are due to rare causes such as vasculitis or thrombophilia, 25–50% remain cryptogenic, and 20–30% meet the criteria for an embolic stroke of undetermined source (ESUS). A rational diagnostic algorithm should be applied that is based on the relative frequencies of the potential causes. The acute treatment of ischemic stroke is the same for patients of all ages: the patient must be transferred as soon as possible to a hospital where a vascular recanalization procedure can be performed. From age 40 onward, there is a steep rise in vascular risk factors and therefore also in the resulting macro- and microangiopathy, which lead, in turn, to stroke. Only 40% of patients with juvenile stroke are ever able to return to their original occupation, and approximately one-third remain permanently unable to work.
Conclusion
The high rates of cryptogenic stroke and ESUS among patients with juvenile stroke indicate that uncertainties remain in the diagnosis and treatment of this entity. The identification of rare causes of juvenile stroke requires a major diagnostic effort. Which diagnostic tests are useful or necessary in which patients is a matter that is currently decided on an individual basis. This is true, above all, of the indication for long-term cardiac monitoring.
There is no uniform age definition of “juvenile” stroke in young adults: in the literature, age ranges of 18 to 40, 45, 50, or 55 years have been given for juvenile stroke (1). The incidence of arterial ischemic stroke increases exponentially with age and is comparatively low in young and middle-aged adults compared to the elderly (2.4 per 100 000 for 20- to 24-year-olds, 20 per 100 000 for 35- to 44-year-olds, and 1200 per 100 000 for 75- to 84-year-olds) (2, 3). About 15% of strokes in Germany occur in persons younger than 55 years, corresponding to about 30 000 strokes in young adults per year (3– 5). The health and psychosocial effects are particularly severe in this young age group
Types of young adult strokes include arterial ischemic stroke (approximately 70%; range, 42 to 98%), intracerebral hemorrhage (approximately 10%; range, 0 to 29%), subarachnoid hemorrhage (approximately 20%; range, 0 to 45%), and cerebral venous sinus thrombosis (0.5 to 1%) (6, 7). This review focuses on arterial ischemic stroke in young adults.
Etiological classification of young adult stroke is a major challenge for the treating physicians, as the spectrum of underlying causes is more heterogeneous, with a different frequency distribution, than in older stroke patients.
Acute care after ischemic stroke is independent of age and consists of the fastest possible transport to a hospital, where a vascular recanalization procedure (systemic thrombolysis and/or endovascular thrombectomy) and treatment in a stroke unit are possible (8, 9).
In this review, the possible causes of stroke in young adults are presented. The most frequent, and therefore most clinically relevant, etiologies and their guideline-appropriate therapies are described in detail in separate sections.
Methods
A selective literature search was carried out in PubMed for papers published from 1990, based on the clinical experience of the authors. Reviews, meta-analyses, randomized controlled studies, cohort studies, case–control studies, and case reports were included. The current guidelines of the German Society of Neurology (Deutsche Gesellschaft fu¨r Neurologie, DGN), the European Stroke Organization (ESO), and the American Academy of Neurology (AAN) were taken into account throughout the text.
Etiology of young adult arterial ischemic stroke
Classification of strokes according to the underlying etiology is usually based on the TOAST (“Trial of ORG 10172 in Acute Stroke Treatment”) classification, which is divided into five main categories: large-artery atherosclerosis, cardioembolism, small-vessel occlusion, stroke of other determined etiology, and stroke of undetermined or cryptogenic etiology (10).
The TOAST classification of strokes should be interpreted carefully, making sure that both well-known and less-certain cardioembolic sources have been included under the heading of “cardioembolic stroke”; for instance, artial fibrillation (AF) is a well-known embolic source, while patent foramen ovale (PFO) is a more controversial one (table). Further, the category of “cryptogenic” in the TOAST classification is inconsistent, as not only strokes with no clear cause but also strokes with competing causes have been grouped together. The ESUS concept (“embolic stroke of undetermined source”), which in contrast to “cryptogenic stroke” has a positive (operative) definition, is increasingly being used as a subcategory of cryptogenic stroke.
Table. Stroke etiology based on the TOAST classification—Comparison of young adult with older adult stroke patients.
| Macroangiopathy | Cardioembolic | Microangiopathy | Other definite causes | Cryptogenic | |
|
Young adult 18–55 years |
8–28% |
5–25% PFO with/without ASA (5–20%) Arrhythmias (AF) Endocarditis Valve defects Cardiomyopathy Cardiac valve tumors (myxoma / fibroelastoma) |
5–19% |
20–30% Dissection (10–25 %) Vasculitis Thrombophilia RCVS/PRES Moyamoya disease Hereditary microangiopathies MELAS/M. Fabry Paraneoplastic disease |
25–50% |
| >55 years | 15–20% | 25–35% Atrial fibrillation (AF)(20–30%) Myocardial infarct Valve defects Cardiomyopathy | 20–25% | <5% | 20–35% |
AF, atrial fibrillation; ASA, atrial septal aneurysm; MELAS, mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes; PFO, patent foramen ovale; PRES, posterior reversible encephalopathy syndrome; RCVS, reversible cerebral vasoconstriction syndrome; TOAST, Trial of ORG 10172 in Acute Stroke Treatment
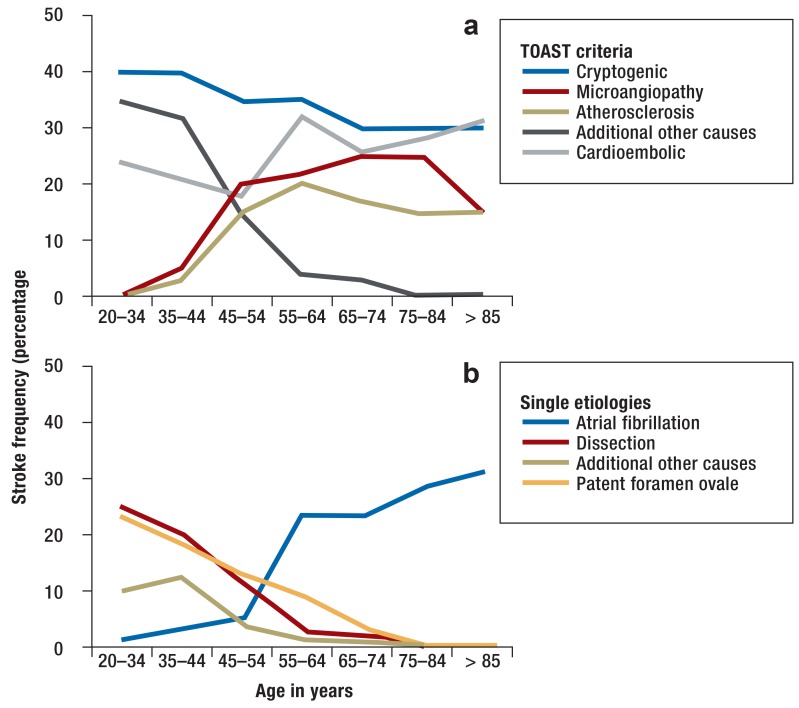
The Table shows the frequency distribution of stroke cases based on the TOAST classification, comparing young and older adults. Cervical artery dissection is a very common cause of stroke in young adults, and PFO is more associated with cryptogenic stroke in young than older adults. In contrast, the classic stroke etiologies, such as macro- and microangiopathy and AF, play a minor role in the younger age group. However, young adult stroke patients show a marked increase in macroangiopathy and acquired microangiopathy in the age group of 40–55 years (figure 1).
Figure 1.
Age-dependent frequency of single stroke causes
Based on the TOAST criteria
Detailed depiction of known etiologies (artial fibrillation, dissection, other definite causes, patent foramen ovale).
TOAST, Trial of Org 10172 in Acute Stroke Treatment
Specific etiology—diagnostics, therapy, and prognosis
Spontaneous cervical artery dissection
Although spontaneous cervical artery dissections are rare overall, with an incidence of about 3 per 100000 per year, they represent one of the most common causes of young adult stroke, of 10–25% (11). The causes of spontaneous dissections are still not conclusively clarified and are presumably multifactorial. In addition to genetic predisposition, environmental factors, such as trivial traumas or infections, play a role (11). Approximately 15% of patients present multiple dissections in which more than one carotid artery is affected.
The most frequent clinical symptoms are headache and sore throat (30–70%), Horner’s syndrome (15–35%), cerebrospinal nerve disruption—especially of hypoglossal and vagal nerves (up to 10%), and tinnitus (up to 10%) (12, 13, e1). Stroke occurs in clinical cohorts in up to 90% of cases, although this figure does not adequately reflect reality due to the high number of undiagnosed asymptomatic dissections (11, 14, e2).
The gold standard for diagnostics is an MRI scan of the neck with T1 sequences after fat suppression. The vessel wall hematoma can be directly visualized in nearly all cases using this method, from 2 to 4 days to several weeks following the event. Multislice spiral CT angiography and color-coded duplex sonography also allow dissection to be diagnosed in 90 to 100% of the cases (15, 16, e3).
Randomized studies on acute therapy are not available. Case series suggest that, similar to ischemic stroke events of other etiologies, systemic thrombolysis therapy as well as mechanical thrombectomy can be performed (14, 17). For secondary prophylaxis, the one randomized study that was found showed no comparative differences between antiplatelet drugs (APD) and oral anticoagulation with vitamin K antagonists, in the context of a very low recurrence rate of ischemic stroke events of 1 to 2% per year (18). Ischemic patients should be treated with APD for life. Patients who had only local symptoms without cerebral ischemia can usually finish taking secondary prophylaxis after 6 to 12 months (19).
Cardioembolic causes and patent foramen ovale (PFO)
Between 5 to 25% of strokes in young adults are attributed to cardiac embolism (1, 2). Atrial fibrillation (AF) is one of the most common causes of stroke in the elderly, accounting for 25 to 35% of cases. An intensive search for AF is also recommended for young adults with stroke. However, in young adults, an AF is found relatively rarely, in only about 5% of cases (e4). The relationship between AF and stroke is currently undergoing a fundamental reinterpretation. On the one hand, AF is a strong independent risk factor for the onset of stroke, and oral anticoagulation is a very effective method for stroke prevention (20, e5). On the other hand, recent studies have given rise to doubts about a simple relationship between AF and stroke. For instance, there is only a weak temporal relationship between AF and stroke (21). Additionally, intensive long-term cardiac monitoring has led to more frequent detection of AF, yet AF is detected not only in patients after stroke but also in completely asymptomatic patients (22, 23, e6).
In younger stroke patients, transesophageal echocardiography (TEE) is usually performed in case of unclear etiology. Valvular heart disease, infectious and non-infectious endocarditis, or (in very rare cases) tumors (atrial myxomas, fibroelastomas) can be the cause of cardioembolism (1).
The role of PFO is controversial. This relic from the embryonic stage is present in about 25% of people. In young adult stroke, however, it is detected in 30 to 50% of patients (24– 26). PFO as the cause of a cardiac right-to-left shunt appears to be pathophysiologically plausible: thrombus material would pass from the venous system into the arterial system as a paradoxical embolism (for example, for deep venous thrombosis). Alternative causes that have been discussed are the direct formation of intracardiac thrombi with additional atrial septal aneurysm (ASA), a low-flow situation within a PFO, or thrombus formation by paroxysmal arrhythmias (1).
No previous study that has investigated an interventional closure of PFO as compared to drug treatment for relapse prevention has shown any advantage of the intervention (27, e7, e8). An important result of these studies, however, was that the recurrence rate for stroke was extremely low, at 1% per year. However, the number needed to treat by PFO closure to prevent another stroke is 67 (e9). It should also be noted that the rate of newly diagnosed AF in the intervention group was significantly higher, although it is not known whether this has a causal relationship. Further, the consequences of PFO for stroke risk are also unclear, as long-term data is lacking (8). Antiplatelet drugs (APD), such as acetylsalicylic acid, are currently recommended for secondary prophylaxis in the presence of a PFO. In case of a second event while using APD or if a paradoxical (venous) embolism has been reliably demonstrated, oral anticoagulant therapy with phenprocoumone is recommended (box) (28). PFO closure can be discussed as a treatment at the individual level, especially if the patient is young and the risk of paradoxical embolism (RoPE) is likely (due to a previous Valsalva maneuver and the detection of deep venous thrombosis). The individual risk of repeated stroke in PFO can be assessed using the so-called RoPE score (29).
BOX. The most important facts about dissection, PFO, and cryptogenic stroke.
-
Spontaneous cervical artery dissection:
Incidence: 2–3/100 000
Accounts for 10–25% of young adult stroke
Etiology / pathogenesis: for the most part, unclear; presumably multifactorial (genetics, environmental factors, trivial traumas, infections)
Clinical: ischemic stroke (up to 90%), headache (30–70%), Horner’s syndrome (15–35%), pulsatile tinnitus (up to 10%), cranial nerve palsy (up to 10%)
Diagnostics: Magnetic resonance angiography with T1 sequences after fat suppression to detect vessel wall hematoma
Prophylaxis: Antiplatelet inhibitors or oral anticoagulation (Phenprocoumon) for 6 to 12 months for patients without prior stroke, lifelong secondary prophylaxis with acetylsalicylic acid (ASA) for patients after stroke; no authorizations for new oral anticoagulants
Prognosis: Accurate rates of recurrence of stroke or dissection are unclear, but both are presumably low-to-moderate.
Comment: The rate of asymptomatic dissections may be high, which would mean that the risk of stroke is overrated.
-
Patent foramen ovale (PFO):
Prevalence: 25 to 30% (30 to 50% for cryptogenic strokes) of the strokes in young adults
Etiology / pathogenesis: Transition of a venous thrombosis due to a right-to-left shunt, local formation of embolus due to atrial septal aneurysm
Clinical: ischemic stroke
Diagnostics: transesophageal echocardiography (TEE) to detect a right-to-left shunt
Prophylaxis: almost always ASA; for secondary prevention, anticoagulation with vitamin K antagonists; interventional closure in individual cases
Prognosis: recurrence rate of about 1% per year; risk of paradoxical embolism (RoPE) score estimates individual risks
-
Cryptogenic stroke:
Prevalence: up to 25 to 50% of young adult stroke
The proposed stepwise diagnostics (figure 2) implies that terminological distinctions should be made between cryptogenic strokes after performing basic, advanced, or specialized diagnostics
Practical procedure for atrial fibrillation (AF) detection: 12-channel electrocardiogram (ECG), with monitoring over 24 to 72 h, supplemented with long-term ECGs, and if necessary, implantation of an event recorder
Problem: It is unclear which stroke patients should receive long-term cardiac monitoring. The importance of AF episodes with a great temporal distance to the stroke, as well as very short-duration AF episodes, is likewise unclear.
New clinical construct: ESUS (embolic stroke of undetermined source); ongoing clinical trials compare new oral anticoagulants (NOAC) versus standard treatment with ASA.
Comment: The large number of cryptogenic strokes is due, among other things, to the large number of different causes in young adult stroke. It is unclear how much diagnostic effort the treating physician should pursue for which patients.
PFO, patent foramen ovale
Classical vascular risk factors
The importance of classical cardiovascular risk factors increases significantly with age. Young adult stroke patients are also more likely to have cases of macro- and microangiopathy starting from the age of 40, even though these played almost no role in the previous years (1, 2). The main risk factors are: arterial hypertension (25–50%), cigarette smoking (35–50%), fat metabolism disorders (40–70%), and diabetes mellitus (5–20%) (e10). Frequently, several risk factors exist in parallel, which increases the risk exponentially (e10).
Other rare and very rare causes
A number of other rare causes must also be taken into account in young adult stroke, which cause at least 10% of these strokes.
Pregnancy is associated with an increased rate of stroke, especially in late pregnancy and the weeks after birth (puerperium) (e11, e12). In addition to immediate stroke, there are a number of neurological complications that are indirectly associated with stroke: pre-eclampsia, reversible cerebral vasoconstriction syndrome (RCVS), posterior reversible encephalopathy syndrome (PRES), and cerebral venous sinus thrombosis. However, experiencing a stroke during pregnancy is not a fundamental reason to refrain from further pregnancies (30).
Migraine, and in particular migraine with aura, increases the risk of stroke by a factor of 2. This is especially true for women younger than 55 years. The risk of stroke increases with the number of migraine attacks (hazard ratio: 4.25; 95% confidence interval [1.36; 13.29]) (e13). There is currently no clear strategy for primary prevention (31, e14).
The influence of oral contraceptives and hormone replacement therapy on the development of stroke is unclear. Observational studies suggest a link between estrogen and stroke incidence (e15). The route of administration also appears to play a role: transdermal administration is less associated with vascular events than the oral dosage form (32). Guidelines recommend that women with additional risk factors (for example, migraine with aura, cigarette smoking) do not use oral contraceptives that contain estrogens (33).
The use of illicit drugs can cause stroke: sympathomimetic drugs carry a risk of hypertensive crises, cerebral vasospasm, vasculitis, and a disturbed rheology; intravenous substance abuse is associated with an increased risk of thromboembolic events, for instance in the case of endocarditis (34, e16).
Other rare to very rare causes of young adult stroke are presented in the eTable.
eTable. Overview of rare and very rare causes.
| Disease / syndrome | Symptoms and findings | Diagnostics |
| Large and medium vessels | ||
| Reversible cerebral vasoconstriction syndrome (RCVS), posterior reversible encephalopathy syndrome (PRES) | (Thunderclap) headache/hypertensive crisis | Catheter angiography: multilevel segment stenoses |
| Moyamoya disease | Asian descent, multiple ischemic strokes, cognitive impairment | Catheter angiography: rete mirabile and distal stenoses of the internal carotid artery |
| Polyarteritis nodosa | Increased C-reactive protein (CRP) levels, erythrocyte sedimentation rate, presence of B-symptoms, involvement of multiple organ systems | Catheter angiography: renal and abdominal vessel stenosis |
| Takayasu’s disease | Female sex, Asian descent, different blood pressure between upper arms | Computed tomography angiography (CTA), magnetic resonance angiography (MRA): subclavian artery stenosis, etc. FDG-positron emission tomography of the aorta: hypermetabolism of the aorta wall |
| Central nervous system vasculitis of medium-sized arteries (primary and secondary) | Headaches, multiple ischemic strokes in multiple territories, encephalopathy, cognitive impairment, psychiatric disorders | Inflammatory cerebrospinal fluid (differential diagnosis of secondary vasculitis due to herpes simplex virus, varicella zoster virus, bacteria, tuberculosis, fungi) MRA with black-blood sequences: vessel wall contrast imaging Angiography: segmental stenosis Brain biopsy (leptomeningeal/parenchymal in the area of ischemia): panmural inflammatory infiltrate of vessel wall |
| Small and very small vessels | ||
| Susac’s syndrome | Visual impairment, visual field defect, hearing loss, ischemic microangiopathy, encephalopathy | Fluorescein angiography of retina: vascular stenosis, occlusion Cranial magnetic resonance imaging (MRI): snowball-ike lesions |
| CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy) | Positive family history, migraines, subcortical dementia, lacunar stroke, leukoencephalopathy (medullary and temporal lobe), microbleeding | Genetic (NOTCH 3) |
| HERNS (hereditary endotheliopathy with retinopathy, nephropathy, and stroke) | Visual impairment, retinopathy, renal dysfunction, leukoencephalopathy, intracerebral hemorrhage | Genetic (TREX 1) |
| HANAC (hereditary angiopathy with nephropathy, aneurysms, and muscle cramps) | Eye involvement, conspicuous retinal vessels, muscle cramps, renal dysfunction, leukoencephalopathy, porencephaly (internal/external), intracerebral aneurysms | Genetic (COL4A1 and COL4A2) |
| Eales disease | Visual disturbances, leukoencephalopathy | Ophthalmologist: retinopathy with neovascularization |
| Acute posterior multifocal placoid pigment epitheliopathy | Visual disturbances, leukoencephalopathy | Ophthalmologist: multiple lesions of the retinal pigment epithelium |
| Ehrmann–Sneddon syndrome | Livedo racemosa of the skin, headache, ischemia, transient ischemic attacks | Skin biopsy: subintimal smooth muscle cell proliferation / thrombotic, non-inflammatory vessel occlusion |
| Post-radiation vasculopathy | Condition after brain radiation, lacunar stroke, leukoencephalopathy, microbleeding | Cranial MRI: typical microangiopathic changes |
| Central nervous system vasculitis of the small and very small vessels (primary and secondary) | Headaches, multiple microangiopathic ischemias in multiple territories, encephalopathy, cognitive impairment | Cerebrospinal fluid: inflammatory changes p-ANCA/cANCA (granulomatous polyangiitis, microscopic polyangiitis, eosinophilic polyangiitis) Brain biopsy (leptomeningeal/parenchymal in the ischemia area): panmural inflammatory infiltrate of vessel wall Diagnosis of borrelia, syphilis, varicella zoster virus, HIV, hepatitis C and B, cryoglobulins, tuberculosis Cryoglobulins (for hepatitis C) Brain biopsy (leptomeningeal/parenchymal): panmural inflammatory infiltrate of vessel wall |
| Cardioembolism and pulmonary embolism after AV shunts | ||
| Endocarditis | B symptoms, inflammation values, valve defects, heart murmurs, multiple embolic ischemic strokes (which possibly bled), Osler nodes | Transthoracic echocardiography (TTE), transesophageal echocardiography (TEE): valvular insufficiency and direct detection of vegetationBlood culture: pathogen detection |
| Myocarditis | Influenza infections, tachycardia, cardiac insufficiency, embolic ischemic stroke | Electrocardiogram: ST changes Transthoracic echocardiography: reduced pump function Cardiac MRI: myocardial changes |
| M. Osler | Signs of chronic hypoxia, frequent nose bleeds, multiple ischemic strokes, brain abscesses | CTA of the thorax: pulmonary AV shunt ENT doctor: nosebleeds Genetics |
| Heart tumors (myxoma, fibroelastoma) | Embolic ischemic strokes | TTE, TEE, CT of thorax: direct detection of tumor |
| Coagulopathies and rheological disorders | ||
| Antiphospholipid syndrome (APS, primary and secondary) | Abortions, venous thrombosis, clinical signs of collagenosis (for example, butterfly erythema) | Laboratory: elevated antiphospholipid antibodies (cardiolipin, ß2-glycoprotein-IgG / IgM antibodies, lupus anticoagulant) (2 × at 8-week interval) Laboratory: increased dsDNA antibodies, increased ANA / ANA differentiation / ENA |
| Hyperviscosity syndrome | Lymphadenopathy, B symptoms, thrombocytosis, polycythemia, polyglobulia | Positron emission tomography (PET)/CT analysis: malignant lymph nodes Lymph node biopsy: non-Hodgkin lymphoma Bone marrow biopsy / immunofixation: M. Waldenström Laboratory: increased viscosity Janus kinase mutation: essential thrombocythemia, polycythemia vera |
| Paraneoplastic coagulopathy | Tumor disease, multiple ischemias in multiple territories | Tumor screening: sonography, CT, PET/CT, differential blood count, bone marrow biopsy, gastroscopy, colonoscopy Exclusion of other etiologies |
| Hereditary coagulopathy | Venous thrombosis without triggers | Laboratory: factor V Leiden mutation (activated protein C resistance), prothrombin gene mutation, protein C deficiency, protein S deficiency |
| Other causes | ||
| M. Fabry | Renal insufficiency, cardiomyopathy, vitreous opacity, painful acral polyneuropathy, dilated angiopathy of vertebral and basilar arteries | Genetic: α-galactosidase gene |
| Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) | Myopathy, epilepsy, encephalopathy, ischemia (parieto-occipital cortical), lactic acid in serum, cerebrospinal fluid | Genetic: MELAS |
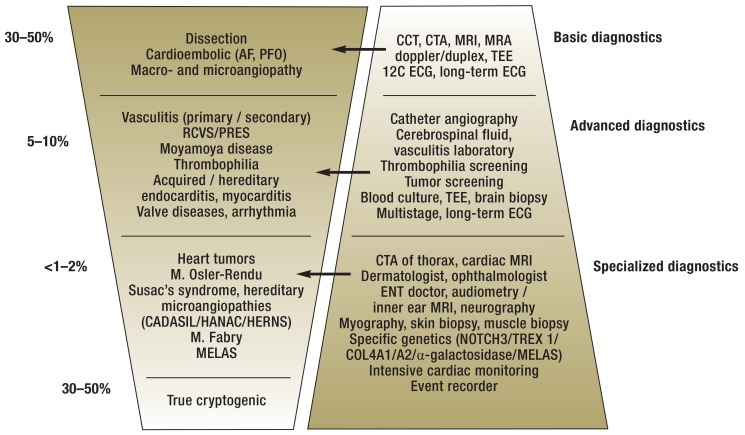
Based on the cause spectrum and the frequency distribution, a stepwise diagnosis is recommended for young adult stroke, consisting of basic diagnostics, advanced diagnostics, and ultimately, specialized diagnostics (figure 2). It must be emphasized, however, that this is a guide for orientation rather than a standardized algorithm. Especially for the detection of very rare causes, targeted specialized diagnostics that are based on a clinical-anamnestic suspicion are sensible and clinically feasible.
Figure 2.
Stage diagnostics and frequency distribution of stroke etiologies of young adult stroke
AF, artial fibrillation; CADASIL, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (due to mutation in the NOTCH3 gene); CCT, cranial computed tomography; CTA, CT angiography; HANAC, hereditary angiopathy, nephropathy, aneurysms, and muscle cramps (due to COL4A1 / A2 gene mutations); HERNS, hereditary endotheliopathy, retinopathy, nephropathy, and stroke (due to TREX1 gene mutation); MELAS, mitochondrial encephalopathy, lactic acidosis and stroke-like episodes; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging; PFO, patent foramen ovale; PRES, posterior reversible encephalopathy syndrome; RCVS, reversible cerebral vasoconstriction syndrome; TEE, transesophageal echocardiography; 12C ECG, 12-channel electrocardiogram
The cryptogenic stroke
In up to 50% of strokes in young adults, no definitive cause can be determined (1, 35). However, the term “cryptogenic” is also unspecific: strokes can be classified as cryptogenic if no clear etiology is revealed after either the basic, advanced, or specialized diagnostic step (figure 2). To date, it is unclear which anamnestic, clinical, or diagnostic combinations trigger further diagnostics, or how much non-informative diagnostic results are necessary to define a “true” cryptogenic stroke (36).
In this context, the importance of non-diagnosed AF has been discussed intensively. Recent studies have shown that in patients with cryptogenic stroke, an increased rate of AF can be detected by intensive long-term cardiac monitoring (for example, by an implantable event recorder) (22, 23). Until now, however, it is unclear which patients benefit from long-term cardiac monitoring—all patients with cryptogenic stroke or only patients selected after risk assessment? The latest findings indicate that, for patients with a vascular risk profile but without prior stroke, an event recorder detects clinically silent AF in up to one-third of cases (e6). Thus, the question of causality between undiagnosed AF and cryptogenic stroke remains unanswered.
To address the difficulties not only in the definition, but also in the diagnosis and therapy, of cryptogenic stroke, the so-called ESUS concept (ESUS, embolic stroke of undetermined source) was developed in 2014. This is a “positively defined” term, requiring:
Presence of an embolic stroke pattern (in contrast to lacunar ischemia)
Absence of a high-grade stenosis of the vessel supplying the area of ischaemia
No AF detected in long-term ECG
No other specific alternative etiology.
ESUS accounts for 80 to 90% of cryptogenic strokes. Currently, clinical trials are being conducted to compare the safety and efficacy of new oral anticoagulants (NOAC) versus standard acetylsalicylic acid (ASA) therapy in ESUS patients with respect to recurrence rates (37). If NOAC proves to be superior for ESUS, the urgency of the search for undiagnosed AF might be relativized.
Health and psychosocial effects
Overall, the mortality and recurrence rate of arterial ischemic stroke in young adults is significantly lower than that for older adults (e17). For instance, the 1-year mortality rate for young adult stroke is 4.5%, and the 1-year recurrence rate, 1.5% (e17). In contrast, the 1-year mortality in older adults is 15 to 35%, and the 1-year recurrence rate, 2 to 15% (38, 39). Also the functional outcome, as measured by the modified Rankin scale (mRS), is significantly better on average for young adults than for older adult stroke patients (40). Nonetheless, 11% of young adult patients will still have severe impairment (mRS 4 to 5), and 59%, mild-to-moderate impairment (mRS 1 to 3). A complete recovery without symptoms (mRS 0) occurs in 30% of affected patients (40). However, the psychosocial effects are usually more far-reaching and are not sufficiently represented by the purely medical outcome parameters; for instance, only 40% of young adult stroke patients return to their original workplace, 27% have to change workplace, and 33% remain permanently unable to work (40).
Key Messages.
Young adult stroke should be defined as that in patients aged 18 to 39 years, according to this analysis. Patients aged 40 to 55 years are an intermediate group, since the relevance of classic cardiovascular risk factors increases dramatically in this age bracket.
Both the spectrum of causes as well as the frequency distribution of strokes in young adults differ from those in older adults, and this must be taken into account during diagnosis.
Acute care in arterial ischemic stroke for young adults does not differ from that for older adults and is based on systemic thrombolysis and/or mechanical thrombectomy.
Young adult stroke patients have a mortality rate of 4.5%, and a recurrence rate of 1.5%, during the first year after stroke.
Acknowledgments
Translated from the original German by Veronica A. Raker, PhD.
Footnotes
Conflict of interest statement
Prof. Ringleb has received consultant fees from Boehringer Ingelheim; congress and training course fee reimbursement from Boehringer Ingelheim and Bayer; travel expenses from Boehringer Ingelheim, Bayer, and Pfizer; speaking honoraria from Boehringer Ingelheim; and funding for a research project (initiated by him) from Boehringer Ingelheim.
PD Wakili has received consultant fees and travel expenses from Daiichi Sankyo, Bayer, and Boston Scientific; consultant fees from Biotronik; funding for research projects (initiated by him) from Bristol-Myers Squibb, Pfizer, and Boston Scientific; and speaking honoraria from Boehringer-Ingelheim, Boston Scientific, Bristol-Myers Squibb, Pfizer, and Daiichi Sankyo.
Dr. Poli has received consultant fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, and Daiichi Sankyo; travel expenses and congress participation fee reimbursement and speaking honoraria from Bayer and Boehringer Ingelheim; and speaking honoraria from Bristol-Myers Squibb, Pfizer, and Daiichi Sankyo.
PD Wollenweber has received consultant fees from Boehringer Ingelheim, Bayer, and Daiichi Sankyo; speaking honoraria from Pfizer; and funding for a clinical study commissioned by Boehringer Ingelheim.
PD Kellert has received consultant fees from Bayer, Boehringer Ingelheim, and Daiichi Sankyo; travel expenses and congress participation fee reimbursement from Bayer, Daiichi Sankyo, and Pfizer; and speaking honoraria from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, and Pfizer.
Dr. Schöberl declares that no conflict of interest exists.
References
- 1.Ferro JM, Massaro AR, Mas JL. Aetiological diagnosis of ischaemic stroke in young adults. Lancet Neurol. 2010;9:1085–1096. doi: 10.1016/S1474-4422(10)70251-9. [DOI] [PubMed] [Google Scholar]
- 2.Putaala J, Metso AJ, Metso TM, et al. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: the Helsinki Young Stroke Registry. Stroke. 2009;40:1195–1203. doi: 10.1161/STROKEAHA.108.529883. [DOI] [PubMed] [Google Scholar]
- 3.Kolominsky-Rabas PL, Sarti C, Heuschmann PU, et al. A prospective community-based study of stroke in Germany—the Erlangen Stroke Project (ESPro): incidence and case fatality at 1, 3, and 12 months. Stroke. 1998;29:2501–2506. doi: 10.1161/01.str.29.12.2501. [DOI] [PubMed] [Google Scholar]
- 4.Michel P, Odier C, Rutgers M, et al. The Acute Stroke Registry and Analysis of Lausanne (ASTRAL): design and baseline analysis of an ischemic stroke registry including acute multimodal imaging. Stroke. 2010;41:2491–2498. doi: 10.1161/STROKEAHA.110.596189. [DOI] [PubMed] [Google Scholar]
- 5.Nedeltchev K, der Maur TA, Georgiadis D, et al. Ischaemic stroke in young adults: predictors of outcome and recurrence. J Neurol Neurosurg Psychiatry. 2005;76:191–195. doi: 10.1136/jnnp.2004.040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobs BS, Boden-Albala B, Lin IF, Sacco RL. Stroke in the young in the Northern Manhattan Stroke Study. Stroke. 2002;33:2789–2793. doi: 10.1161/01.str.0000038988.64376.3a. [DOI] [PubMed] [Google Scholar]
- 7.Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007;6:162–170. doi: 10.1016/S1474-4422(07)70029-7. [DOI] [PubMed] [Google Scholar]
- 8.European Stroke Organisation (ESO) Executive Committee, ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25:457–507. doi: 10.1159/000131083. [DOI] [PubMed] [Google Scholar]
- 9.Fiehler J, Gerloff C. Mechanical thrombectomy in stroke. Dtsch Arztebl Int. 2015;112:830–836. doi: 10.3238/arztebl.2015.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams HP Jr., Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 11.Debette S, Leys D. Cervical-artery dissections: predisposing factors, diagnosis, and outcome. Lancet Neurol. 2009;8:668–678. doi: 10.1016/S1474-4422(09)70084-5. [DOI] [PubMed] [Google Scholar]
- 12.Leys D, Lucas C, Gobert M, Deklunder G, Pruvo JP. Cervical artery dissections. Eur Neurol. 1997;37:3–12. doi: 10.1159/000117396. [DOI] [PubMed] [Google Scholar]
- 13.Kellert L, Kloss M, Pezzini A, et al. Prognostic significance of pulsatile tinnitus in cervical artery dissection. Eur J Neurol. 2016;23:1183–1187. doi: 10.1111/ene.13031. [DOI] [PubMed] [Google Scholar]
- 14.Lyrer PA, Brandt T, Metso TM, et al. Clinical import of Horner syndrome in internal carotid and vertebral artery dissection. Neurology. 2014;82:1653–1659. doi: 10.1212/WNL.0000000000000381. [DOI] [PubMed] [Google Scholar]
- 15.Benninger DH, Baumgartner RW. Ultrasound diagnosis of cervical artery dissection. Front Neurol Neurosci. 2006;21:70–84. doi: 10.1159/000092386. [DOI] [PubMed] [Google Scholar]
- 16.Dittrich R, Draeger B, Nassenstein I, et al. Dissection of the common and external carotid artery. Cerebrovasc Dis. 2006;21:208–210. doi: 10.1159/000090793. [DOI] [PubMed] [Google Scholar]
- 17.Engelter ST, Rutgers MP, Hatz F, et al. Intravenous thrombolysis in stroke attributable to cervical artery dissection. Stroke. 2009;40:3772–3776. doi: 10.1161/STROKEAHA.109.555953. [DOI] [PubMed] [Google Scholar]
- 18.Markus HS, Hayter E, Levi C, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14:361–367. doi: 10.1016/S1474-4422(15)70018-9. [DOI] [PubMed] [Google Scholar]
- 19.Ringelstein E, Dittrich R, et al. S1-Leitlinie Spontane Dissektionen der extra- und intrakraniellen hirnversorgenden Arterien 2016 Deutsche Gesellschaft für Neurologie, Hrsg. Leitlinien für Diagnostik und Therapie in der Neurologie. www.dgn.org/leitlinien/3264-030-005-spontane-dissektionen-der-extrakraniellen-und-intrakraniellen-hirnversorgenden-arterien-2016 (last accessed on 22 April 2017) [Google Scholar]
- 20.Hart RG, Pearce LA, Aguilar MI. Adjusted-dose warfarin versus aspirin for preventing stroke in patients with atrial fibrillation. Ann Intern Med. 2007;147:590–592. doi: 10.7326/0003-4819-147-8-200710160-00018. [DOI] [PubMed] [Google Scholar]
- 21.Brambatti M, Connolly SJ, Gold MR, et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129:2094–2099. doi: 10.1161/CIRCULATIONAHA.113.007825. [DOI] [PubMed] [Google Scholar]
- 22.Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–2486. doi: 10.1056/NEJMoa1313600. [DOI] [PubMed] [Google Scholar]
- 23.Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467–2477. doi: 10.1056/NEJMoa1311376. [DOI] [PubMed] [Google Scholar]
- 24.Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc. 1984;59:17–20. doi: 10.1016/s0025-6196(12)60336-x. [DOI] [PubMed] [Google Scholar]
- 25.Lamy C, Giannesini C, Zuber M, et al. Clinical and imaging findings in cryptogenic stroke patients with and without patent foramen ovale: the PFO-ASA Study Atrial septal aneurysm. Stroke. 2002;33:706–711. doi: 10.1161/hs0302.104543. [DOI] [PubMed] [Google Scholar]
- 26.Nouh A, Hussain M, Mehta T, Yaghi S. Embolic strokes of unknown source and cryptogenic stroke: implications in clinical practice. Front Neurol. 2016;7 doi: 10.3389/fneur.2016.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991–999. doi: 10.1056/NEJMoa1009639. [DOI] [PubMed] [Google Scholar]
- 28.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 29.Kent DM, Thaler DE, Di Angelantonio E, et al. The Risk of Paradoxical Embolism (RoPE) Study: developing risk models for application to ongoing randomized trials of percutaneous patent foramen ovale closure for cryptogenic stroke. Trials. 2011;12:185. doi: 10.1186/1745-6215-12-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamy C, Hamon JB, Coste J, Mas JL. Ischemic stroke in young women: risk of recurrence during subsequent pregnancies French Study Group on Stroke in Pregnancy. Neurology. 2000;55:269–274. doi: 10.1212/wnl.55.2.269. [DOI] [PubMed] [Google Scholar]
- 31.Schurks M, Buring JE, Kurth T. Migraine, migraine features, and cardiovascular disease. Headache. 2010;50:1031–1040. doi: 10.1111/j.1526-4610.2009.01609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Canonico M, Carcaillon L, Plu-Bureau G, et al. Postmenopausal hormone therapy and risk of stroke: impact of the route of estrogen administration and type of progestogen. Stroke. 2016;47:1734–1741. doi: 10.1161/STROKEAHA.116.013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:3754–3832. doi: 10.1161/STR.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westover AN, McBride S, Haley RW. Stroke in young adults who abuse amphetamines or cocaine: a population-based study of hospitalized patients. Arch Gen Psychiatry. 2007;64:495–502. doi: 10.1001/archpsyc.64.4.495. [DOI] [PubMed] [Google Scholar]
- 35.Leys D, Bandu L, Henon H, et al. Clinical outcome in 287 consecutive young adults (15 to 45 years) with ischemic stroke. Neurology. 2002;59:26–33. doi: 10.1212/wnl.59.1.26. [DOI] [PubMed] [Google Scholar]
- 36.Saver JL. Cryptogenic stroke. N Engl J Med. 2016;375 doi: 10.1056/NEJMc1609156. [DOI] [PubMed] [Google Scholar]
- 37.Diener HC, Easton JD, Granger CB, et al. Design of randomized, double-blind, evaluation in secondary stroke prevention comparing the efficacy and safety of the oral thrombin inhibitor dabigatran etexilate vs acetylsalicylic acid in patients with embolic stroke of undetermined source (RE-SPECT ESUS) Int J Stroke. 2015;10:1309–1312. doi: 10.1111/ijs.12630. [DOI] [PubMed] [Google Scholar]
- 38.Amarenco P, Lavallee PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533–1542. doi: 10.1056/NEJMoa1412981. [DOI] [PubMed] [Google Scholar]
- 39.IST-3 collaborative group. Effect of thrombolysis with alteplase within 6 h of acute ischaemic stroke on long-term outcomes (the third international stroke trial [IST-3]): 18-month follow-up of a randomised controlled trial. Lancet Neurol. 2013;12:768–776. doi: 10.1016/S1474-4422(13)70130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Musolino R, La Spina P, Granata A, et al. Ischaemic stroke in young people: a prospective and long-term follow-up study. Cerebrovasc Dis. 2003;15:121–128. doi: 10.1159/000067139. [DOI] [PubMed] [Google Scholar]
- E1.Sturzenegger M, Huber P. Cranial nerve palsies in spontaneous carotid artery dissection. J Neurol Neurosurg Psychiatry. 1993;56:1191–1199. doi: 10.1136/jnnp.56.11.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Traenka C, Dougoud D, Simonetti BG, et al. Cervical artery dissection in patients = 60 years often painless, few mechanical triggers. Neurology. 2017;88:1313–1320. doi: 10.1212/WNL.0000000000003788. [DOI] [PubMed] [Google Scholar]
- E3.Nebelsieck J, Sengelhoff C, Nassenstein I, et al. Sensitivity of neurovascular ultrasound for the detection of spontaneous cervical artery dissection. J Clin Neurosci. 2009;16:79–82. doi: 10.1016/j.jocn.2008.04.005. [DOI] [PubMed] [Google Scholar]
- E4.Liao J, Khalid Z, Scallan C, Morillo C, O’Donnell M. Noninvasive cardiac monitoring for detecting paroxysmal atrial fibrillation or flutter after acute ischemic stroke: a systematic review. Stroke. 2007;38:2935–2940. doi: 10.1161/STROKEAHA.106.478685. [DOI] [PubMed] [Google Scholar]
- E5.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- E6.Healey JS. New Orleans: 2016. ASSERT-II: sub-clinical AF (SCAF) in older asymptomatic patients Paper presented at: American Heart Association Scientific Sessions; November 12-16. [Google Scholar]
- E7.Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092–1100. doi: 10.1056/NEJMoa1301440. [DOI] [PubMed] [Google Scholar]
- E8.Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083–1091. doi: 10.1056/NEJMoa1211716. [DOI] [PubMed] [Google Scholar]
- E9.Kent DM, Dahabreh IJ, Ruthazer R, et al. Device closure of patent foramen ovale after stroke: pooled analysis of completed randomized trials. J Am Coll Cardiol. 2016;67:907–917. doi: 10.1016/j.jacc.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E10.Ji R, Schwamm LH, Pervez MA, Singhal AB. Ischemic stroke and transient ischemic attack in young adults: risk factors, diagnostic yield, neuroimaging, and thrombolysis. JAMA Neurol. 2013;70:51–57. doi: 10.1001/jamaneurol.2013.575. [DOI] [PubMed] [Google Scholar]
- E11.Sharshar T, Lamy C, Mas JL. Incidence and causes of strokes associated with pregnancy and puerperium A study in public hospitals of Ile de France. Stroke in Pregnancy Study Group. Stroke. 1995;26:930–936. doi: 10.1161/01.str.26.6.930. [DOI] [PubMed] [Google Scholar]
- E12.Helms AK, Kittner SJ. Pregnancy and stroke. CNS Spectr. 2005;10:580–587. doi: 10.1017/s1092852900010221. [DOI] [PubMed] [Google Scholar]
- E13.Kurth T, Schürks M, Logroscino G, Buring JE. Migraine frequency and risk of cardiovascular disease in women. Neurology. 2009;73:581–588. doi: 10.1212/WNL.0b013e3181ab2c20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E14.Spector JT, Kahn SR, Jones MR, Jayakumar M, Dalal D, Nazarian S. Migraine headache and ischemic stroke risk: an updated meta-analysis. Am J Med. 2010;123:612–624. doi: 10.1016/j.amjmed.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E15.Chakhtoura Z, Canonico M, Gompel A, Thalabard JC, Scarabin PY, Plu-Bureau G. Progestogen-only contraceptives and the risk of stroke: a meta-analysis. Stroke. 2009;40:1059–1062. doi: 10.1161/STROKEAHA.108.538405. [DOI] [PubMed] [Google Scholar]
- E16.Sloan MA, Kittner SJ, Feeser BR, et al. Illicit drug-associated ischemic stroke in the Baltimore-Washington Young Stroke Study. Neurology. 1998;50:1688–1693. doi: 10.1212/wnl.50.6.1688. [DOI] [PubMed] [Google Scholar]
- E17.Kittner SJ. Stroke in the young: coming of age. Neurology. 2002;59:6–7. doi: 10.1212/wnl.59.1.6. [DOI] [PubMed] [Google Scholar]