ABSTRACT
Pancreatic islet transplantation is an established treatment to restore insulin independence in type 1 diabetic patients. Its success rates have increased lately based on improvements in immunosuppressive therapies and on islet isolation and culture. It is known that the quality and quantity of viable transplanted islets are crucial for the achievement of insulin independence and some studies have shown that a significant number of islets are lost during culture time. Thus, in an effort to improve islet yield during culture period, researchers have tested a variety of additives in culture media as well as alternative culture devices, such as scaffolds. However, due to the use of different categories of additives or devices, it is difficult to draw a conclusion on the benefits of these strategies. Therefore, the aim of this systematic review was to summarize the results of studies that described the use of medium additives, scaffolds or extracellular matrix (ECM) components during human pancreatic islets culture. PubMed and Embase repositories were searched. Of 5083 articles retrieved, a total of 37 articles fulfilled the eligibility criteria and were included in the review. After data extraction, articles were grouped as follows: 1) “antiapoptotic/anti-inflammatory/antioxidant,” 2) “hormone,” 3) “sulphonylureas,” 4) “serum supplements,” and 5) “scaffolds or ECM components.” The effects of the reviewed additives, ECM or scaffolds on islet viability, apoptosis and function (glucose-stimulated insulin secretion - GSIS) were heterogeneous, making any major conclusion hard to sustain. Overall, some “antiapoptotic/anti-inflammatory/antioxidant” additives decreased apoptosis and improved GSIS. Moreover, islet culture with ECM components or scaffolds increased GSIS. More studies are needed to define the real impact of these strategies in improving islet transplantation outcomes.
KEYWORDS: additives in cultures, culture of islets, extracellular matrix, human pancreatic islet, islet isolation, scaffolds
Introduction
Pancreatic islet transplantation is an established treatment for patients with type 1 diabetes mellitus (T1DM) that suffer from hypoglycemia unawareness with frequent episodes of hypoglycemia and marked glycemic lability.1-5 A publication from The Collaborative Islet Transplant Registry (CITR) in 2012 showed a clear improvement in islet transplantation outcomes in the recent era. Insulin independence, 3 y after transplantation, improved from 27% in 1999–2002 (n = 214) to 37% in 2003–2006 (n = 255) and to 44% in the most recent period, 2007–2010 (n = 208).4 Recently, Brennan et al.6 reported the results from a 12 -year follow-up of 7 subjects initially assigned for the Edmonton protocol in 2000. One patient experienced graft failure only 10.9 y after islet transplantation. The other 6 patients continued to have sustained C-peptide and improved glycemic control without episodes of severe hypoglycemia after islet transplantation even in this long follow-up, although all of them have lost insulin independence in different time-points. Hering et al.7 recently published results from the Phase 3 study of Clinical Islet Transplantation (CIT) Consortium. They demonstrated that purified human pancreatic islets transplantation provided good glycemic control, restoration of hypoglycemia awareness, and protection from severe hypoglycemia. Also related to CIT, Ricordi et al.8 demonstrated the feasibility of implementing a harmonized process at multiple facilities for the manufacture of a complex cellular product of human islet for transplantation.
An important criteria for the achievement of long-term insulin independence is the total number of viable islets transplanted per Kg of the recipient´s weight.9 It is generally assumed that a combined implant mass of at least 10,000 islet equivalents (IEQ) per kg is required to routinely achieve insulin-independence.4 Nowadays, most of the islet isolation facilities keep the isolated islets in culture for 24–48 hours prior transplantation, allowing them to recover from the stress generated during the isolation process and also allowing the preparation of the recipient, including the administration of immunosuppressive induction therapy.10 During this culture period, up to 10–20% of the total islet mass is lost, which may, in turn, compromise the success of the transplant.11
Studies have shown that the loss of islets during the culture period is due, in part, to the apoptosis that is triggered along the whole process of procurement (due to brain death catecholamine storm and cold ischemia time) and also during the islet isolation process.12-15 In this context, protective strategies to preserve islets from damage during culture time have been studied as a way to improve islet transplant outcomes. These strategies include the use of different additives in the islet culture media or novel culture methods, such as scaffolds or extracellular matrix (ECM) components.16-20 However, the high variability of additives and culture methods tested makes it difficult to draw a conclusion on the subject. So, the aim of this systematic review was to summarize the research findings on the use of medium additives, scaffolds or ECM components to improve viability and function during human pancreatic islet culture.
Results
Literature search
Fig. 1 is a flow diagram showing the strategy used to identify and select studies for inclusion in this systematic review. All studies that analyzed effects of additives added to the human pancreatic islet culture medium on IEQ, viability/apoptosis and/or function (assessed by glucose-stimulated insulin secretion - GSIS) were selected for inclusion. In addition, studies that cultured islets on different scaffolds or ECM components were also selected for inclusion. A total of 5083 possible relevant citations were retrieved by searching the electronic databases, and 5032 of them were excluded following the reading of titles and abstracts. Fifty articles remained to be fully evaluated. However, after careful analysis of the complete texts, another 14 articles were excluded due to use of islet encapsulation or co-culture. Thirty-seven articles16-19,21-53 fulfilled the eligibility criteria and were included in the systematic review (Fig. 1). After data extraction, the studies were grouped to better describe and summarize the results, as follows: 1) “antiapoptotic/anti-inflammatory/antioxidant” additives, 2) “hormone” additives, 3) “sulphonylurea” agents, 4) “serum supplements,” and 5) “scaffolds or ECM components.”
Figure 1.
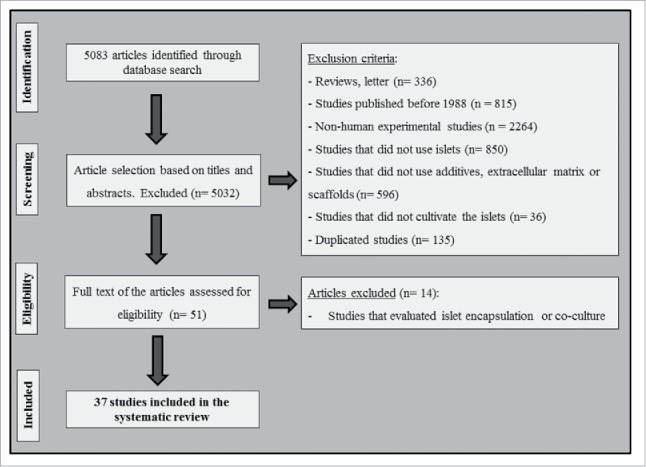
Flowchart illustrating the search strategy used to identify studies for inclusion in the systematic review.
Main characteristics of the eligible studies
Table 1 shows the main characteristics of the 37 studies included in this systematic review. In brief, the number of donors ranged from 2 to 18 and the donor's mean age ranged from 24 y to 70 y among studies. Of note, some studies did not reported donor's characteristics. Purity (%) of the islet preparations were reported by 21 studies, but only in the pre-culture period, and no description was available after the interventions.
Table 1.
Main characteristics of the pancreas donors and purity of the isolated islets described in the included studies.
| Groups of additives | N° of donors | Mean age | Gender (%male) | CIT (h) | Pre-culture purity (%) |
|---|---|---|---|---|---|
| 1st Author (year)Ref | |||||
| “Antiapoptotic/anti-inflammatory/antioxidant” | |||||
| Emamaullee (2008)32 | — | — | — | — | — |
| Mancarella (2008)18 | 8 | 49 | 50 | — | — |
| McCall (2011)33 | — | — | — | — | — |
| Mita (2008)23 | 9 | — | — | — | Pure: >90; Impure: 40–60 |
| Moriscot (2007)24 | — | — | — | — | — |
| Mwangi (2011)34 | 4 | 45.8 | 25 | 89.2 | |
| Nakano (2004)17 | 12 | 41.8 | 50 | — | 70–95 |
| Omori (2010)25 | — | — | — | — | >70 |
| Pepper (2017)35 | 2 | — | — | — | 43.8 |
| Scholz (2009)26 | 5 | 57.6 | 20 | 9.52 | 50–95 |
| Yang (2005)27 | 3 | 46 | 80 | 11 | 50–95 |
| Zhang (2004)19 | 6 | 43 | — | 16 | 57 |
| “Hormones” | |||||
| Farilla (2003)21 | 3 | — | — | — | >90 |
| Liu (2009)28 | — | — | — | — | — |
| Miki (2014)22 | — | — | — | — | — |
| Sakuma (2009)29 | — | — | — | — | — |
| Toso (2010)16 | 14 | 53 | 21.4 | 10 | — |
| Yamamoto (2010)30 | 14 | 48 | 64.3 | 11.5 | — |
| “Sulphonylureas” | |||||
| Del Guerra (2005)31 | 18 | 51 | 55.6 | — | — |
| Maedler (2005)47 | 7 | 38–70 | — | — | >75 |
| “Serum” | |||||
| Avgoustiniatos (2012)48 | — | — | — | — | >70 |
| Bucher (2003)53 | — | — | — | — | 85 |
| Kerr-Conte (2010)49 | — | — | — | — | 50–80 |
| Lee (2008)50 | — | — | — | — | >50 |
| Matsumoto (2003)51 | — | 47.4 | 20 | 35.8 | 62 |
| Nacher (2013)52 | 15 | — | — | — | — |
| “Scaffolds or ECM components” | |||||
| Benti-Barnes (2008)36 | 8 | — | — | — | >70 |
| Buitinga (2013)37 | 4 | — | — | — | — |
| Daoud (2010)38 | — | — | — | <8 | >80 |
| Daoud (2011)39 | — | — | — | <8 | >80 |
| Kitzmann (2014)40 | — | — | — | — | low purity |
| Maillard (2011)41 | 8 | 24–61 | — | 5.5–9 | — |
| Matsushima (2016)42 | — | — | — | — | — |
| Marchioli (2015)43 | — | — | — | — | — |
| Murray (2009)44 | — | — | — | — | — |
| Papas (2005)45 | 3 | — | — | — | >90 |
| Zhang (2012)46 | — | — | — | — | ∼90 |
CIT: Cold ischemia time (in hours); ECM: extracellular matrix.
All studies included in this systematic review used the Ricordi's semi-automated technique for islet isolation.54 Twenty-three studies (62.2%) used CRML 1066 as the islet culture media after isolation,16,17,19,24,25,27,32-35,37-39,41-43,47-53 4 studies used Miami media,22,23,29,30 3 used M199 media,21,31,44 6 of them did not report the media used,18,26,28,36,40,45 and one study used Ham's/F10 media.46 All studies had an experimental group where modifications were added to the culture and a control group without modifications added to the culture. The culture time varied from 16 hours to 10 d. The seeding density ranged from 30 IEQ/cm2 to 5000 IEQ/cm2, and the volume density varied from 300 IEQ/mL to 1500 IEQ/mL.
Table 2 shows the interventions made to the islet culture, including type of modification, concentrations of additives, ECM components or substances used in scaffolds, and the number of replications per each study. Twelve studies analyzed “antiapoptotic/anti-inflammatory/antioxidant” additives, 6 studies analyzed “hormones,” 2 study investigated “sulphonylurea” agents, 6 studies analyzed “serum supplements,” and 11 studies investigated the use of “scaffolds or ECM components” in islet culture. Concentrations of these substances varied among studies, and the number of experimental replications per study ranged from 2 to 15.
Table 2.
Additives, scaffolds and ECM components used in included studies and their concentrations.
| Groups of additives | Component | Concentration | n experimental |
|---|---|---|---|
| 1st Author (year)Ref | |||
| “Antiapoptotic/anti-inflammatory/antioxidant” | |||
| Emamaullee (2008)32 | EP1013 | 1mg/mL | 3 |
| Mancarella (2008)18 | IAC | 10µM/L | 8 |
| Mc Call (2011)33 | IDN-6556 | 100μM | 3 |
| Mita (2008)23 | Sirolimus | 30ng/mL | 3 |
| Moriscot (2007)24 | MnTMPyP | 25µM/L | 3 |
| Mwangi (2011)34 | GDNF | 100ng/mL | 4 |
| Nakano (2004)17 | Z-DEVD-FMK | 25 and100µM/L | — |
| Omori (2010)25 | SD-282 | 0.1µM and 0.3µM | 3 |
| Pepper (2017)35 | F573 | 100µM | 2 |
| Scholz (2009)26 | GW3965 | 1µM/L | 10 |
| Yang (2005)27 | LSF | 20, 50 e 100µM/L | 3 |
| Zhang (2004)19 | Polyphenol (green tea extract) | 0, 30, 60, 125, 250 and 500µg/mL | — |
| “Hormones” | |||
| Farilla (2003)21 | GLP-1 | 10nM | 3 |
| Liu (2009)28 | β-E2 and α-E2 (17α-estradiol) | 10−8M | 5 |
| Miki (2014)22 | Exendin-4 | 10nM | 3 |
| Sakuma (2009)29 | PACAP | 10−12M/L | — |
| Toso (2010)16 | Liraglutide | 1µM/L | — |
| Yamamoto (2010)30 | Prolactin | 500µg/L | — |
| “Sulphonylureas” | |||
| Del Guerra (2005)31 | Glimepiride, glibenclamide and chlorprapamide | 10µM, 10µM and 600µM; respectively | 10 |
| Maedler (2005)47 | Repaglimide, nateglimide and glibenclamide | 0.01 or 1μM, 10 or 1000μM, 0.1 or 1 or 10 or 100nM; respectively | 3 |
| “Serum” | |||
| Avgoustiniatos (2012)48 | FBS and HSA | 10% and 0.5%; respectively | 10 |
| Bucher (2003)53 | FCS, HSA and human AB serum | 10%, 0.625% and 2.5%; respectively | 5 |
| Kerr-Conte (2010)49 | HSA and human AB serum | 0.625% and 2.5%; respectively | 9–15 |
| Lee (2008)50 | HSA and human AB serum | 0.5% and 10%; respectively | 4 |
| Matsumoto (2003)51 | FBS and HA | 10% and 1.4%; respectively | — |
| Nacher (2013)52 | HSA and HS | — | — |
| “Scaffolds or extracellular matrix” | |||
| Bentsi-Barnes (2008)36 | Gas-permeable membranes | — | 1–7 |
| Buitinga (2013)37 | PEOT/PBT | — | 3 |
| Daoud (2010)38 | Collagen I/IV, fibronectin and laminin | 6.25µg/cm2 | 3 |
| Daoud (2011)39 | Collagen I gel with or without ECM components, micro-fabricated scaffold | 4mg/mL and 100 g/mL; respectively | 3 |
| Kitzmann (2014)40 | Silicone rubber membrane | — | 5–6 |
| Maillard (2011)41 | Fibrinogen, thrombin and PDC | 20mg/mL, 10mU/mL and 10%; respectively | 7–8 |
| Matsushima (2016)42 | Fibroblasts | — | 5–6 |
| Marchioli (2015)43 | Alginate/gelatingel | — | — |
| Murray (2009)44 | Pancreatic duct-derived epithelial cells | — | 6 |
| Papas (2008)45 | Silicone rubber membrane | — | 3 |
| Zhang (2012)46 | CM and FPCM | — | 3 |
EP1013: N-benzyloxycabonyl-Val Asp-fluoromethyl ketone [zVD-FMK]; IAC: bis (1-hydroxy-2,2,6,6-tetramethyl-4-piperidinyl) decantionate; IDN-6556: caspase inhibitor; MnTMPyP: SOD mimeticmanganese (III) tetrakis (1-methyl-4-pyridyl) porphyrin (MnTMPyP); GDNF: glial cell line-derived neurotrophic factor; Z-DEVD-FMK: Z-Asp (OMe)-Glu (OMe)-Val-Asp (OMe)-fluoromethylketone; SD-282: indole-5-carboxamide ATP-competitive inhibitor of p38α MAPK; F573: pan-caspase inhibitor; GW3965: synthetic nonsteroidal liver X receptor (LXR) agonist; LSF: lysofylline; GLP-1: glucagon-like peptide-1; PACAP: pituitary adenylate cyclase-activating peptide; FBS: fetal bovine serum; FCS: fetal calf serum; HSA: human serum albumin; HA: human albumin; HS: human serum; PEOT: poly(ethylene oxide terephthalate); PBT: poly(butylenes terephthalate); ECM: extracellular matrix; PDC: perfluorodecalin; CM: collagen matrix; FPCM: human fibroblast-populated collagen matrix.
The methods used to assess viability, apoptosis and function (GSIS) varied widely among studies, as depicted in Table 3. Twenty out of 37 studies evaluated islet viability,16,19,22-24,26,29,30,32-35,40-42,45,46,48,49,51 but only 3 of them applied the standard technique used to evaluate islet viability for transplantation [fluorescein diacetate (FDA) and propidium iodide (PI)].19,34,40 Apoptosis evaluation was reported in 16 studies, with Terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling (TUNEL),16,25,31,33-35,47,52 and ELISA17,26,31,41,53 being the most used techniques. The majority of studies used ELISA for GSIS evaluation.16,17,19,22,23,25-27,29,34-39,41-44,46,51,52 Some studies analyzed GSIS using radioimmunoassays (RIA)21,24,32,47,49 or immunoradiometric assays (IRMA).18,31 Islet equivalents (IEQ) were calculated using diphenylthiocarbozone dye (Dithizone).16,17,25
Table 3.
Methods used to evaluate the outcomes of interest.
| Methods |
|||
|---|---|---|---|
| 1st Author (year)Ref | Viability assesment | Apoptosis assessment | Insulin secretion |
| Emamaullee (2008)32 | Sytogreen/ethidium bromide | — | Static incubation/RIA |
| Mancarella (2008)18 | — | — | Static incubation/IRMA |
| Mc Call (2011)33 | Sytogreen/ethidium bromide | TUNEL/DAPI | — |
| Mita (2008)23 | FACS using NG, TMRE and 7AAD | — | Perifusion/ELISA |
| Moriscot (2007)24 | FACS using Live/Dead kit | — | Static incubation/RIA |
| Mwangi (2011)34 | — | TUNEL/DAPI | Static incubation/ELISA |
| Nakano (2004)17 | — | DNA fragment using ELISA | Static incubation/ELISA |
| Omori (2010)25 | — | TUNEL | Perifusion/ELISA |
| Pepper (2017)35 | Sytogreen/ethidium bromide | TUNEL/DAPI | Static incubation/ELISA |
| Scholz (2009)26 | ApoGlow Kit/XTT Assay/CellTiter-Glo | Apo-ONE caspase assay/ELISA | Perifusion/ELISA |
| Yang (2005)27 | — | Apo Percentage apoptosis assay kit | Static incubation/ELISA |
| Zhang (2004)19 | FDA/PI | — | Static incubation/ELISA |
| Farilla (2003)21 | — | DAPI | Static incubation/RIA |
| Liu (2009)28 | — | Hoechst | — |
| Miki (2014)22 | FACS using NG, TMRE and 7AAD | — | Perifusion/ELISA |
| Sakuma (2009)29 | FACS using NG, TMRE and 7AAD | — | Perifusion/ELISA |
| Toso C (2010)16 | SYTO_13 Kit/ethidium bromide | TUNEL | Static incubation/ELISA |
| Yamamoto T (2010)30 | FACS using NG, TMRE and 7AAD | — | — |
| Del Guerra (2005)31 | — | TUNEL/ELISA | Static incubation/IRMA |
| Maedler (2005)47 | — | TUNEL | RIA |
| Avgoustiniatos (2012)48 | OCR | — | — |
| Bucher (2003)53 | — | Cell Death Detection ELISA | Static incubation / - |
| Kerr-Conte (2010)49 | Tripan-blue | Cell Death Detection Kit | Static incubation/RIA |
| Lee (2008)50 | — | — | Static incubation/Immulite immunometric assay |
| Matsumoto (2013)51 | AO/PI | — | Static incubation/ELISA |
| Nacher (2013)52 | — | TUNEL/DAPI | Static incubation/ELISA |
| Bentsi-Barnes (2008)36 | — | — | Perifusion/ELISA |
| Buitinga (2013)37 | — | — | Static incubation/ELISA |
| Daoud (2010)38 | — | — | Static incubation/ELISA |
| Daoud (2011)39 | — | — | Static incubation/ELISA |
| Kitzmann (2014)40 | OCR/FDA/PI | — | — |
| Maillard (2011)41 | FDA/ethidium bromide | Caspase 3/ELISA | Static incubation/ELISA |
| Matsushima (2016)42 | AM/PI | — | Static incubation/ELISA |
| Marchioli (2015)43 | — | — | Static incubation/ELISA |
| Murray (2009)44 | — | — | Static incubation/ELISA |
| Papas (2008)45 | OCR | — | — |
| Zhang (2012)46 | Viability/cytotoxicity assay kit | — | Static incubation/ELISA |
FACS: Fluorescence-activated cell sorting; NG: Newport green; TMRE: Tetramethyl rhodamine ethylester; 7AAD: 7-Aminoactinomycin D; FDA: Fluorescein diacetate; PtdIns: Propidium iodide; OCR: oxygen consumption rate; AO: acridine orange; AM: calcein-acetoxymethyl; TUNEL: Terminal deoxynucleotidyl transferase dUTP nickend labeling; DAPI: 4′,6-diamidino-2-phenylindole; ELISA: enzyme-linked immunosorbent assay; RIA: Radioimmunoassay; IRMA: Immunoradiometric assay.
Results of studies that evaluated “antiapoptotic/anti-inflammatory/antioxidant” additives
Results of the studies that evaluated the effect of “antiapoptotic/anti-inflammatory/antioxidant” additives added to the culture media on islet viability, apoptosis, GSIS or IEQs are summarized in Table 4. A total of 12 studies analyzed the effects of this group of additives on the islet outcomes of interest. Among them, 7 studies analyzed islet viability. Three of them showed an increased in viability using the pan-caspase inhibitor F573,35 the caspase inhibitors EP101332 and IDN-655633 additives. Four studies did not observe any difference in viability between additive-treated and non-treated islets. The additives tested in these 4 studies were: polyphenol (green tea extract);19 sirolimus;23 SOD mimetic manganese (III) tetrakis (1-methyl-4-pyridyl) porphyrin (MnTMPyP),24 and synthetic nonsteroidal liver X receptor (LXR) agonist (GW3965).26 Regarding apoptosis, P38 inhibitor SD-28225 and GW396526 additives had no significant effect on this outcome, while caspase-3 inhibitor Z-DEVD-FMK,17 lysofylline (LSF),27 IDN-6556,33 F573,35 and glial cell line-derived neurotrophic factor (GDNF)34 additives were associated with decreased apoptosis rates compared with control condition.
Table 4.
Summary of the effects of the additives, scaffolds and ECM components on islet outcomes of interest.
| Groups of additives | N of studies | Results |
|---|---|---|
| Viability | ||
| “Antiapoptotic/anti-inflammatory/antioxidant” | 7 | ↑ 3 studies found increased viability in treated groups (EP1013;32 IDN-6556;33 F57335) ↔ 4 studies found no differences between groups.19,23,24,26 |
| “Hormones” | 3 | ↑ 2 studies found increased viability in treated groups |
| (exendin-4;22 PACAP29) | ||
| ↔ 1 study found no differences between groups16 | ||
| “Serum” | 3 | ↑ 2 studies found increased viability in treated groups (FBS vs HSA;48 AB serum vs HSA49) |
| ↔ 1 study found no differences between groups51 | ||
| “Scaffolds or ECM components” | 6 | ↑ 4 studies found increased viability in treated groups (SRM;40 collagen I, IV, fibronectin and laminin;38 fibroblasts;42 CM and FPCM46) |
| ↔ 2 studies found no differences between groups41,45 | ||
| Apoptosis | ||
| “Antiapoptotic/anti-inflammatory/antioxidant” | 7 | ↔ 2 studies found no differences between groups25,26 ↓ 5 studies found decreased apoptosis in treated groups |
| (Z-DEVD-FMK;17 LSF;27 IDN-6556;33 F573;35 GDNF34) | ||
| “Hormones” | 3 | ↓ 2 studies found decreased apoptosis in treated groups |
| (GLP-1;21 β-E2 or α-E2 estradiol28) | ||
| ↔ 1 study found no difference between groups16 | ||
| “Sulphonylureas” | 2 | ↔ 1 study found no difference between groups31 ↑ 1 study found increased apoptosis in treated groups (repaglimide, nateglimide and glibenclamide47) |
| “Serum” | 3 | ↓ 3 studies found decreased apoptosis in treated group (AB serum vs HSA,49 and vs FCS;53 HS vs HSA52) |
| “Scaffolds or ECM components” | 1 | ↓ 1 study found decreased apoptosis in treated groups (fibrinogen, thrombin and PDC41) |
| GSIS | ||
| “Antiapoptotic/anti- | 11 | ↑ 7 studies found increased insulin secretion in treated groups |
| inflammatory/antioxidant” | (Z-DEVD-FMK;17 IAC;18 Sirolimus;23 GW3965;26 LSF;27 F573;35 GDNF34) | |
| ↔ 3 studies found no differences between groups19,25,32 | ||
| ↓ 1 study found decreased insulin secretion in treated group (MnTMPyP24) | ||
| “Hormones” | 4 | ↑ 2 studies found increased insulin secretion in treated groups (GLP-1;21 exendina-422) |
| ↔ 2 studies found no differences between groups16,29 | ||
| “Sulphonylureas” | 2 | ↓ 1 study found decreased insulin secretion in treated group |
| (glimepiride, glibenclamide and chlorprapamide31) | ||
| ↑ 1 study found increased insulin secretion in treated group (Glibenclamide47) | ||
| “Serum” | 5 | ↑ 3 studies found increased insulin secretion in treated groups (AB serum vs HSA,49,53 and vs FCS;53 HS vs HSA52) |
| ↔ 2 studies found no differences between groups50,51 | ||
| “Scaffolds or ECM components” | 9 | ↑ 6 studies found increased insulin secretion in treated groups (gas-permeable membrane;36 collagen I gel with or without ECM components, micro-fabricated scaffold;39 fibrinogen, thrombin and PDC;41 fibroblasts;42 pancreatic duct-derived epithelial cells;44 CM and FPCM46) |
| ↔ 2 studies found no differences between groups37,43 | ||
| ↓ 1 study found decreased insulin secretion in treated group (collagen I, IV, fibronectin and laminin38) | ||
| IEQ | ||
| “Antiapoptotic/anti-inflammatory/antioxidant” | 2 | ↑ 1 study found increased IEQ in treated group (Z-DEVD-FMK17) ↔ 1 study found no differences between groups25 |
| “Hormone” | 1 | ↑ 1 study found increased IEQ in treated group (liraglutide16) |
↔: No difference in outcome between treated and non-treated islets; ↑: increase of the outcome in treated islets; ↓: decrease of the outcome in the treated islets. ECM: extracellular matrix components; GSIS: glucose stimulation insulin secretion; IEQ: islets equivalents. EP1013: N-benzyloxycabonyl-Val Asp-fluoromethyl ketone [zVD-FMK]; IDN-6556: caspase inhibitor; F573: pan-caspase inhibitor; PACAP: pituitary adenylate cyclase-activating peptide; FBS: fetal bovine serum; HSA: human serum albumin; SRM: silicone rubber membrane; CM: collagen matrix; FPCM: human fibro-blast-populated collagen matrix; Z-DEVD-FMK: Z-Asp (OMe)-Glu (OMe)-Val-Asp (OMe)-fluoromethylketone; LSF: lysofylline; GDNF: glial cell line-derived neurotrophic factor; FCS: fetal calf serum; GLP-1: glucagon-like peptide-1; HS: human serum; PDC: perfluorodecalin; IAC: bis (1-hydroxy-2,2,6,6-tetramethyl-4-piperidinyl) decantionate; GW3965: synthetic nonsteroidal liver X receptor (LXR) agonist; MnTMPyP: SOD mimetic manganese (III) tetrakis (1-methyl-4-pyridyl) porphyrin.
Eleven of the 12 studies in the “antiapoptotic/anti-inflammatory/antioxidant” group evaluated GSIS. Islets treated with Z-DEVD-FMK,17 radical scavenger IAC,18 sirolimus,23 GW3965,26 LSF,27 GDNF34 and F57335 additives had increased GSIS compared with non-treated islets. In contrast, one study showed that the MnTMPyP24 additive added to the culture media decreased GSIS in comparison to the control group. Three other studies found no significant effects of polyphenol (green tea extract),19 SD-28225 and EP101332 additives on GSIS.
Nakano et al.17 reported that Z-DEVD-FMK also was associated with an increase in IEQ in relation to the non-treated group, which was in agreement with a protective effect of this additive against apoptosis. The other study that evaluated IEQ showed no effect of SD-282 on this outcome.25
Based on above-mentioned studies, it is possible to suggest that some additives with “antiapoptotic/anti-inflammatory/antioxidant” effects added to the islet culture media have the potential to improve GSIS and decrease apoptosis (Table 4).
Results of studies that evaluated “hormones” additives
Six studies reported the effects of hormones added to the culture medium on islet outcomes (Table 4). Viability was evaluated in 3 studies. Exendin-422 and pituitary adenylate cyclase-activating peptide (PACAP)29 additives were associated with increased viability compared with control conditions. In contrast, liraglutide16 did not modify islet viability. Moreover, glucagon-like peptide-1 (GLP-1)21 and estradiol28 were able to decrease apoptosis rates compared with non-treated islets. Liraglutide added to the culture medium did not alter apoptosis rates.16
Four studies analyzed the effects of culture medium additives on GSIS (Table 4). Islet treated with GLP-121 or Exendin-422 had an improvement in GSIS compared with non-treated islets. Liraglutide16 and PACAP29 had no significant effect on this outcome. However, liraglutide in the culture medium of islets was associated with higher IEQ than the control condition.16
Results of studies that evaluated “sulphonylureas” agents
Only 2 studies by Del Guerra et al.31 and Maedler et al.47 added “sulphonylurea” agents to the culture media. Del Guerra et al.31 showed that glimepiride, glibenclamide, and chlorpropamide had no effect on apoptosis rate but decreased GSIS. Maedler et al.47 reported that repaglimide, nateglimide and glibenclamide caused an increase in islet apoptosis, but only glibenclamide was able to increase GSIS. Viability was not assessed in these studies.
Results of studies that evaluated “serum supplements”
Six studies analyzed “serum supplements” added to the islet culture media: 3 evaluated viability, 3 evaluated apoptosis and 5 evaluated GSIS. Regarding viability, 2 studies found an increase in viability when using fetal bovine serum (FBS)48 or AB serum49 while one study found no difference between islet cultured with FBS or human albumin.51 The 3 studies that analyzed apoptosis showed that AB serum,49,53 and human serum52 decreased apoptosis. These 3 supplements were also able to improve GSIS.49,52,53 Two other studies reported no difference on GSIS when comparing human albumin vs. AB serum50 or FBS vs. human albumin.51
Results of studies that evaluated “scaffolds or extracellular matrix components”
Eleven studies were included in the group “scaffolds or ECM components.” Of them, 6 evaluated viability, one analyzed apoptosis, and 9 evaluated GSIS. In relation to viability, 4 studies demonstrated an improvement in this outcome when culturing islets with collagen I/IV, fibronectin and laminin,38 collagen human fibroblast-populated collagen matrix (FPCM),46 on a silicon rubber membrane40 or on a fibroblast matrix.42 Two other studies found no differences in viability between groups.41,45 Only one study in this group evaluated apoptosis, showing a reduction in this outcome when using a perfluorodecalin (PDC)-enriched fibrin matrix.41
Regarding GSIS, 6 studies demonstrated an improvement in insulin secretion when islets were cultured on gas-permeable membranes,36 collagen I with or without ECM components or micro-fabricated scaffold with ECM,39 PDC-enriched fibrin matrix,41 fibroblasts,42 pancreatic duct-derived epithelial cells,44 and collagen or FPCM.46 Two studies were not able to find any differences between experimental groups,37,43 and one study observed a decrease in GSIS when islets were cultured on collagen I/IV, fibronectin and laminin matrixes.38
Discussion
Since the initial era of islet isolation and transplantation, many advances have been achieved in respect to the islet isolation process and its standardization, and also a better knowledge of the handling and implantation (transplantation) of the islets was acquired. Despite these improvements, islet loss during isolation, culture period and right after implantation still represent a barrier for a widespread utilization of this therapy. There is evidence to link early graft loss following islet transplantation to isolation-induced β-cell apoptosis.15 It has been established, in vitro, that apoptosis participates in the death of freshly isolated islets cultured under standard conditions and it might be related, in part, to anoikis and lack of growth factors.55-58
The ability to maintain isolated islets in culture have been essential for the improvements of islet transplantation outcomes.59 Factors that may augment or even preserve β-cell mass are of particular interest in the field of islet transplantation because, not unfrequently, the number of viable islets isolated from one pancreas is not sufficient to perform the transplant.3,60
As shown in this systematic review, many additives, ECM components and scaffolds have been investigated as potential agents to increase or preserve islet mass before and after transplantation.61-68 For a better analysis in this systematic review, we classified the additives, ECM components and scaffolds used during culture of human islets in groups according to its main mechanism of action. Regarding “antiapoptotic/anti-inflammatory/antioxidant” additives, 3 studies32,33,35 were able to show an improvement in viability, 5 studies17,27,33-35 demonstrated a reduction in apoptosis rate and 7 studies showed an increase in GSIS.17,18,23,26,27,34,35
The research group from University of Alberta (Edmonton, AB, Canada) has shown, in 3 different studies,32,33,35 the improvement of viability when adding anti-apoptotic additives in culture media of human islets. Especially during culture time, it seems that the use of caspase inhibitor additives has the ability to distress human islets leading to substantial reduction in cell death; thereby, improving viability and reducing islet mass required for transplantation. Nakano et al.17 showed that caspase-3 has a crucial role in apoptosis of human islets immediately after isolation and that its inhibitor ameliorates the function of isolated islets. Moreover, the caspase-3 inhibitor Z-DEVD-FMK prevented apoptosis in a dose-dependent manner and also improved islet yield.17 According to these findings, Yang et al.27 showed that in vitro short-term treatment with LSF enhanced human islet metabolism and β-cell insulin secretion, also reducing apoptosis as compared with the control group. These effects were associated with promotion of mitochondrial metabolism since mitochondrial function regulates β-cell insulin secretion and controls the end point of apoptosis. Unexpectedly, this occurred through inhibition of TNF, which induces apoptosis in β-cells through suppression of caspase-8 pathway but not through caspase-3, contradicting the results of Nakano et al. In addition to an antiapoptotic effect, studies that used other “antiapoptotic/anti-inflammatory/antioxidant” additives in the human islets culture also have shown an improvement in GSIS.17,18,23,26,27,34,35 However, the study that used sirolimus in islet culture showed no improvement in viability or GSIS.23 Sirolimus is an immunosuppressive drug that inhibits IL-2 pro-inflammatory cytokine and, consequently, inhibits the activation and proliferation of T lymphocytes through mTOR.23 This result should be expected by the authors since sirolimus is anti-proliferative and decreases insulin secretion.69
In relation to “hormones,” some of them seem to enhance islet “health” Sakuma et al.29 observed a significant increase of viability (4.2%) in islets cultured with PACAP. Miki et al.22 verified that exendin-4 increased islet viability up to 1.85 fold in relation to the control group. However, it is uncertain to what extent these increases in viability are clinically relevant. It is known that viability above 80% is a release criterion to perform islet transplantation and that the majority of the isolations reach viability post-culture above 80% with the standard culture media. So, we still do not have the answer if, above 80%, small increments in viability may impact outcomes.
Besides viability, some “hormone” additives enhanced GSIS and reduced apoptosis. We ought to highlight results from studies using GLP-1 analogs, a 30-amino-acid peptide hormone secreted from the L-cells of the intestinal epithelium in response to meals. Its analogous (exendin-4 and liraglutide) were approved as a therapy for type 2 diabetes, since they enhance glucose-stimulated postprandial insulin release, and inhibit inadequate glucagon secretion and gastrointestinal motility.70,71 Lately, anti-inflammatory, antiapoptotic and cytoprotective properties of the GLP-1 analogues have been revealed, opening new therapeutic perspectives for this class of drugs.72-75
Farilla et al.21 demonstrated that GLP-1 analogues delayed the morphological changes that occurs in human islets in culture, as indicated by a longer-lasting preservation of their 3D structure. GLP-1 analogs also promoted an increase in expression of the antiapoptotic protein Bcl-2 and a downregulation of the active form of caspase-3.21 Moreover, these authors verified that GLP1-treated islets contained more insulin and were capable of a greater glucose-dependent insulin secretion.21 Miki et al.22 showed that exendin-4 supplementation in the culture media significantly reduced pro-inflammatory cytokine/chemokine production from human islet preparations and improved β-cell survival through increased Erk2 phosphorylation, which may be helpful for possible β-cell proliferation after islet transplantation.
Regarding “sulphonylurea” additives, 2 studies were found.31,47 These oral hypoglycemic agents reduce blood glucose levels by stimulating insulin release from β-cells.76 Their actions occur through ATP-sensitive potassium (K-ATP) channel, fundamental to the control of β-cell function.76,77 Based on their mechanism of action, it is expected that the use of sulfonylureas in islet culture media would improve, at least, insulin secretion, as seen in Maedler et al.47 study but not by Del Guerra et al.31
On the topic of “serum supplements,” the use of AB serum,49,53 and human serum52 decreased apoptosis and increased GSIS when compared with HSA, the most widely used serum supplement. AB serum has its rational based on serum derived from AB blood donors, making it less immunoreactive. AB serum49 and FBS48 improved viability compared with HSA, also suggesting that the quality of clinical islet preparations might be improved when culture is performed in media supplemented with serum instead of albumin.
Scaffolds and ECM components had positive effects on islets in many studies, especially related to viability,38,40,42,46 and function.34,37,39,40,42,44 In general, this group of cell culture modifications transmits a variety of chemical and mechanical signals to the islets, mediating key aspects of cellular physiology, such as adhesion, migration, proliferation, differentiation, and death.78 Probably for these reasons the “scaffolds and ECM components” group showed a good performance on the evaluated outcomes.
An emerging strategy to improve islet viability and function and, thus, graft survival, involves the co-culture of pancreatic islets with mesenchymal stem cells (MSCs). This topic was not included in this study because our group has recently published a systematic review and meta-analysis on it.79 Souza et al.79 evaluated 20 studies of co-culture of human islets with MSCs, showing that the co-culture with MSCs improved both islet viability and GSIS compared with islets cultured alone. Thus, this co-culture system has the potential for protecting islets from injury after isolation and during culture period.
The results of this systematic review should be interpreted in the context of some limitations. First, the fact that studies included in the systematic review were experimental studies. Second, most additives and scaffolds/ECM components were tested only once, not been replicated in other studies, making difficult to draw firm conclusions. Third, despite the increased number of human isolation facilities inaugurated around the world in the last decade, allowing studies in human islets, most of the studies with additives, ECM components and scaffolds were performed in murine islets, which was not the scope of our systematic review. These facts limited the number of included studies. Fourth, small variation in the composition of the standard culture media used in different clinical centers might have influenced the results of the analyzed studies; however, this information was not described in the articles.
This systematic review results allowed us to draw a better picture on the effect of additives and scaffolds/ECM components in culture of human islets. Overall some “antiapoptotic/anti-inflammatory/antioxidant” additives appear to offer an increment in islets outcomes after culture period by improving GSIS and reducing apoptosis. Moreover, culture of islets on scaffolds or ECM components is able to improve GSIS. More studies, especially with human islets, are needed to define the real impact of these therapeutic strategies in improving islet transplantation, as well as the combination of more than one approach.
Material and methods
Selection criteria and search strategy
PubMed and Embase repositories were searched to identify all articles that analyzed the effect of additives added to the culture medium of human pancreatic islets on IEQ, viability, apoptosis and/or GSIS outcomes. In addition, studies that cultured islets on different scaffolds or with ECM components were also selected for inclusion. The following medical subject headings (MeSH) were used for this search: (“Cell Culture Techniques” OR “Primary Cell Culture” OR “Batch Cell Culture Techniques”) OR (“Culture Media” OR “Culture Media, Conditioned” OR “Culture Media, Serum-Free”) OR (“Tissue Scaffolds”) OR (“Extracellular Matrix” OR “Extracellular Matrix Proteins”) AND (“Islets of Langerhans Transplantation” OR “Islets of Langerhans”). The search was restricted to human islet studies and it was completed on January, 2017. All articles identified were also searched manually to detect other relevant citations. This systematic literature search was designed and described in agreement with current guidelines.80
Study selection and data extraction
Eligibility evaluation was made by 2 pairs of independent investigators (A.C.B. and N.E.L.; A.P.B. and J.R.), through title and abstracts reviews. When abstracts did not provide sufficient data, the full text of the paper was retrieved for analysis. Disagreements were resolved by discussion between the investigators and, when required, a third reviewer (DC) was consulted. Articles were excluded from the systematic review if: 1) were published before 1988 (data of publication of Ricordi's semi-automated technique);54 2) did not use human pancreatic islets; or 3) were review articles, letter or abstracts without description of results (Fig. 1). If data were duplicated and had been published more than once, the most complete study was chosen.
Information of interest from each study was independently extracted by 2 investigators (N.E.L. and L.A.B.) using a standardized extraction form and consensus was sought in all extracted items. When consensus could not be achieved, differences in data extraction were decided by reading the original publication. The data extracted from each study were as follows: (1) general characteristics of the studies, including name of first author and publication year; (2) brain-dead donor characteristics, such as age, gender, cold ischemia time (CIT) of the pancreas; (3) pre-culture islet isolation characteristics as purity and total IEQ; (4) additives or scaffolds/ECM components used and their concentrations (5) post-culture outcomes of interest: number of IEQ, purity, viability, apoptosis and function (assessed by GSIS).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was partially supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Fundo de Incentivo à Pesquisa e Eventos (FIPE) at the Hospital de Clínicas de Porto Alegre and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).D.C and C.B.L. were recipientes of a scholarship (PQ grant) from CNPq.
References
- [1].Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes 2005; 54:2060-9; PMID:15983207; https://doi.org/ 10.2337/diabetes.54.7.2060 [DOI] [PubMed] [Google Scholar]
- [2].Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000; 343:230-8; PMID:10911004; https://doi.org/ 10.1056/NEJM200007273430401 [DOI] [PubMed] [Google Scholar]
- [3].Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, et al.. International trial of the edmonton protocol for islet transplantation. N Engl J Med 2006; 355:1318-30; PMID:17005949; https://doi.org/ 10.1056/NEJMoa061267 [DOI] [PubMed] [Google Scholar]
- [4].Barton FB, Rickels MR, Alejandro R, Hering BJ, Wease S, Naziruddin B, Oberholzer J, Odorico JS, Garfinkel MR, Levy M, et al.. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care 2012; 35:1436-45; PMID:22723582; https://doi.org/ 10.2337/dc12-0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rheinheimer J, Bauer AC, Silveiro SP, Estivalet AA, Boucas AP, Rosa AR, Souza BM, Oliveira FS, Cruz LA, Brondani LA, et al.. Human pancreatic islet transplantation: An update and description of the establishment of a pancreatic islet isolation laboratory. Arch Endocrinol Metab 2015; 59:161-70; PMID:25993680; https://doi.org/ 10.1590/2359-3997000000030 [DOI] [PubMed] [Google Scholar]
- [6].Brennan DC, Kopetskie HA, Sayre PH, Alejandro R, Cagliero E, Shapiro AM, Goldstein JS, DesMarais MR, Booher S, Bianchine PJ. Long-term follow-up of the edmonton protocol of islet transplantation in the United States. Am J Transplant 2016; 16:509-17; PMID:26433206; https://doi.org/ 10.1111/ajt.13458 [DOI] [PubMed] [Google Scholar]
- [7].Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD, Chaloner K, Czarniecki CW, Goldstein JS, Hunsicker LG, et al.. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care 2016; 39:1230-40; PMID:27208344; https://doi.org/ 10.2337/dc15-1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ricordi C, Goldstein JS, Balamurugan AN, Szot GL, Kin T, Liu C, Czarniecki CW, Barbaro B, Bridges ND, Cano J, et al.. National institutes of health-sponsored clinical islet transplantation consortium phase 3 trial: Manufacture of a complex cellular product at eight processing facilities. Diabetes 2016; 65:3418-28; PMID:27465220; https://doi.org/ 10.2337/db16-0234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mineo D, Pileggi A, Alejandro R, Ricordi C. Point: Steady progress and current challenges in clinical islet transplantation. Diabetes Care 2009; 32:1563-9; PMID:19638527; https://doi.org/ 10.2337/dc09-0490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kempf MC, Andres A, Morel P, Benhamou PY, Bayle F, Kessler L, Badet L, Thivolet C, Penfornis A, Renoult E, et al.. Logistics and transplant coordination activity in the GRAGIL Swiss-French multicenter network of islet transplantation. Transplantation 2005; 79:1200-5; PMID:15880070; https://doi.org/ 10.1097/01.TP.0000161224.67535.41 [DOI] [PubMed] [Google Scholar]
- [11].Kin T, Senior P, O'Gorman D, Richer B, Salam A, Shapiro AM. Risk factors for islet loss during culture prior to transplantation. Transpl Int 2008; 21:1029-35; PMID:18564983; https://doi.org/ 10.1111/j.1432-2277.2008.00719.x [DOI] [PubMed] [Google Scholar]
- [12].Contreras JL, Eckstein C, Smyth CA, Sellers MT, Vilatoba M, Bilbao G, Rahemtulla FG, Young CJ, Thompson JA, Chaudry IH, et al.. Brain death significantly reduces isolated pancreatic islet yields and functionality in vitro and in vivo after transplantation in rats. Diabetes 2003; 52:2935-42; PMID:14633854; https://doi.org/ 10.2337/diabetes.52.12.2935 [DOI] [PubMed] [Google Scholar]
- [13].Korsgren O, Lundgren T, Felldin M, Foss A, Isaksson B, Permert J, Persson NH, Rafael E, Ryden M, Salmela K, et al.. Optimising islet engraftment is critical for successful clinical islet transplantation. Diabetologia 2008; 51:227-32; PMID:18040664; https://doi.org/ 10.1007/s00125-007-0868-9 [DOI] [PubMed] [Google Scholar]
- [14].Biarnes M, Montolio M, Nacher V, Raurell M, Soler J, Montanya E. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes 2002; 51:66-72; PMID:11756324; https://doi.org/ 10.2337/diabetes.51.1.66 [DOI] [PubMed] [Google Scholar]
- [15].Cattan P, Berney T, Schena S, Molano RD, Pileggi A, Vizzardelli C, Ricordi C, Inverardi L. Early assessment of apoptosis in isolated islets of Langerhans. Transplant Proc 2001; 33:264-5; PMID:11266811; https://doi.org/ 10.1016/S0041-1345(00)02006-6 [DOI] [PubMed] [Google Scholar]
- [16].Toso C, McCall M, Emamaullee J, Merani S, Davis J, Edgar R, Pawlick R, Kin T, Knudsen LB, Shapiro AM. Liraglutide, a long-acting human glucagon-like peptide 1 analogue, improves human islet survival in culture. Transpl Int 2010; 23:259-65; PMID:19821955; https://doi.org/ 10.1111/j.1432-2277.2009.00984.x [DOI] [PubMed] [Google Scholar]
- [17].Nakano M, Matsumoto I, Sawada T, Ansite J, Oberbroeckling J, Zhang HJ, Kirchhof N, Shearer J, Sutherland DE, Hering BJ. Caspase-3 inhibitor prevents apoptosis of human islets immediately after isolation and improves islet graft function. Pancreas 2004; 29:104-9; PMID:15257101; https://doi.org/ 10.1097/00006676-200408000-00004 [DOI] [PubMed] [Google Scholar]
- [18].Mancarella R, Del Guerra S, Masini M, Bugliani M, Valgimigli L, Pedulli GF, Paolini M, Canistro D, Armando A, Soleti A, et al.. Beneficial effect of the nonpeptidyl low molecular weight radical scavenger IAC on cultured human islet function. Cell Transplant 2008; 17:1271-6; PMID:19181220; https://doi.org/ 10.3727/096368908787236639 [DOI] [PubMed] [Google Scholar]
- [19].Zhang G, Matsumoto S, Hyon SH, Qualley SA, Upshaw L, Strong DM, Reems JA. Polyphenol, an extract of green tea, increases culture recovery rates of isolated islets from nonhuman primate pancreata and marginal grade human pancreata. Cell Transplant 2004; 13:145-52; PMID:15129760; https://doi.org/ 10.3727/000000004773301825 [DOI] [PubMed] [Google Scholar]
- [20].Riopel M, Trinder M, Wang R. Fibrin, a scaffold material for islet transplantation and pancreatic endocrine tissue engineering. Tissue Eng Part B Rev 2015; 21:34-44; PMID:24947304; https://doi.org/ 10.1089/ten.teb.2014.0188 [DOI] [PubMed] [Google Scholar]
- [21].Farilla L, Bulotta A, Hirshberg B, Li Calzi S, Khoury N, Noushmehr H, Bertolotto C, Di Mario U, Harlan DM, Perfetti R. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology 2003; 144:5149-58; PMID:12960095; https://doi.org/ 10.1210/en.2003-0323 [DOI] [PubMed] [Google Scholar]
- [22].Miki A, Ricordi C, Yamamoto T, Sakuma Y, Misawa R, Mita A, Inverardi L, Alejandro R, Ichii H. Improved human islet preparations using glucocorticoid and exendin-4. Pancreas 2014; 43:1317-22; PMID:25036907; https://doi.org/ 10.1097/MPA.0000000000000184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mita A, Ricordi C, Miki A, Barker S, Haertter R, Hashikura Y, Miyagawa S, Burke GW 3rd, Inverardi L, Ichii H. Anti-proinflammatory effects of sirolimus on human islet preparations. Transplantation 2008; 86:46-53; PMID:18622277; https://doi.org/ 10.1097/TP.0b013e31817c79c0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Moriscot C, Candel S, Sauret V, Kerr-Conte J, Richard MJ, Favrot MC, Benhamou PY. MnTMPyP, a metalloporphyrin-based superoxide dismutase/catalase mimetic, protects INS-1 cells and human pancreatic islets from an in vitro oxidative challenge. Diabetes Metab 2007; 33:44-53; PMID:17258921; https://doi.org/ 10.1016/j.diabet.2006.09.004 [DOI] [PubMed] [Google Scholar]
- [25].Omori K, Todorov I, Shintaku J, Rawson J, Al-Abdullah IH, Higgins LS, Medicherla S, Kandeel F, Mullen Y. P38alpha-selective mitogen-activated protein kinase inhibitor for improvement of cultured human islet recovery. Pancreas 2010; 39:436-43; PMID:20084046; https://doi.org/ 10.1097/MPA.0b013e3181c0dd8f [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Scholz H, Lund T, Dahle MK, Collins JL, Korsgren O, Wang JE, Foss A. The synthetic liver X receptor agonist GW3965 reduces tissue factor production and inflammatory responses in human islets in vitro. Diabetologia 2009; 52:1352-62; PMID:19415233; https://doi.org/ 10.1007/s00125-009-1366-z [DOI] [PubMed] [Google Scholar]
- [27].Yang Z, Chen M, Ellett JD, Carter JD, Brayman KL, Nadler JL. Inflammatory blockade improves human pancreatic islet function and viability. Am J Transplant 2005; 5:475-83; PMID:15707401; https://doi.org/ 10.1111/j.1600-6143.2005.00707.x [DOI] [PubMed] [Google Scholar]
- [28].Liu S, Mauvais-Jarvis F. Rapid, nongenomic estrogen actions protect pancreatic islet survival. Islets 2009; 1:273-5; PMID:20634925; https://doi.org/ 10.4161/isl.1.3.9781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sakuma Y, Ricordi C, Miki A, Yamamoto T, Mita A, Barker S, Damaris RM, Pileggi A, Yasuda Y, Yada T, et al.. Effect of pituitary adenylate cyclase-activating polypeptide in islet transplantation. Transplant Proc 2009; 41:343-5; PMID:19249552; https://doi.org/ 10.1016/j.transproceed.2008.10.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yamamoto T, Mita A, Ricordi C, Messinger S, Miki A, Sakuma Y, Timoneri F, Barker S, Fornoni A, Molano RD, et al.. Prolactin supplementation to culture medium improves beta-cell survival. Transplantation 2010; 89:1328-35; PMID:20357700; https://doi.org/ 10.1097/TP.0b013e3181d98af1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Del Guerra S, Marselli L, Lupi R, Boggi U, Mosca F, Benzi L, Del Prato S, Marchetti P. Effects of prolonged in vitro exposure to sulphonylureas on the function and survival of human islets. J Diabetes Complications 2005; 19:60-4; PMID:15642492; https://doi.org/ 10.1016/j.jdiacomp.2004.05.001 [DOI] [PubMed] [Google Scholar]
- [32].Emamaullee JA, Davis J, Pawlick R, Toso C, Merani S, Cai SX, Tseng B, Shapiro AM. The caspase selective inhibitor EP1013 augments human islet graft function and longevity in marginal mass islet transplantation in mice. Diabetes 2008; 57:1556-66; PMID:18356409; https://doi.org/ 10.2337/db07-1452 [DOI] [PubMed] [Google Scholar]
- [33].McCall M, Toso C, Emamaullee J, Pawlick R, Edgar R, Davis J, Maciver A, Kin T, Arch R, Shapiro AM. The caspase inhibitor IDN-6556 (PF3491390) improves marginal mass engraftment after islet transplantation in mice. Surgery 2011; 150:48-55; PMID:21596412; https://doi.org/ 10.1016/j.surg.2011.02.023 [DOI] [PubMed] [Google Scholar]
- [34].Mwangi SM, Usta Y, Shahnavaz N, Joseph I, Avila J, Cano J, Chetty VK, Larsen CP, Sitaraman SV, Srinivasan S. Glial cell line-derived neurotrophic factor enhances human islet posttransplantation survival. Transplantation 2011; 92:745-51; PMID:21869742; https://doi.org/ 10.1097/TP.0b013e31822bc95a [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pepper AR, Bruni A, Pawlick R, Wink J, Rafiei Y, Gala-Lopez B, Bral M, Abualhassan N, Kin T, James Shapiro AM. Engraftment site and effectiveness of the pan-caspase inhibitor F573 to improve engraftment in mouse and human islet transplantation in mice. Transplantation 2017; PMID:28072753; https://doi.org/ 10.1097/TP.0000000000001638 [DOI] [PubMed] [Google Scholar]
- [36].Bentsi-Barnes K, Kandeel F, Al-Abdullah IH. Evaluation of human islet-specific functional quality cultured on different gas-permeable membranes. Transplantation Proc 2008; 40:401-2; PMID:18374081; https://doi.org/ 10.1016/j.transproceed.2008.01.036 [DOI] [PubMed] [Google Scholar]
- [37].Buitinga M, Truckenmuller R, Engelse MA, Moroni L, Ten Hoopen HW, van Blitterswijk CA, de Koning EJ, van Apeldoorn AA, Karperien M. Microwell scaffolds for the extrahepatic transplantation of islets of Langerhans. PLoS One 2013; 8:e64772; Print 0062013; PMID:23737999; https://doi.org/ 10.1371/journal.pone.0064772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Daoud J, Petropavlovskaia M, Rosenberg L, Tabrizian M. The effect of extracellular matrix components on the preservation of human islet function in vitro. Biomaterials 2010; 31:1676-82; PMID:20015544; https://doi.org/ 10.1016/j.biomaterials.2009.11.057 [DOI] [PubMed] [Google Scholar]
- [39].Daoud JT, Petropavlovskaia MS, Patapas JM, Degrandpre CE, Diraddo RW, Rosenberg L, Tabrizian M. Long-term in vitro human pancreatic islet culture using three-dimensional microfabricated scaffolds. Biomaterials 2011; 32:1536-42; PMID:21093908; https://doi.org/ 10.1016/j.biomaterials.2010.10.036 [DOI] [PubMed] [Google Scholar]
- [40].Kitzmann JP, Pepper AR, Gala-Lopez B, Pawlick R, Kin T, O'Gorman D, Mueller KR, Gruessner AC, Avgoustiniatos ES, Karatzas T, et al.. Human islet viability and function is maintained during high-density shipment in silicone rubber membrane vessels. Transplant Proc 2014; 46:1989-91; PMID:25131090; https://doi.org/ 10.1016/j.transproceed.2014.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Maillard E, Juszczak MT, Clark A, Hughes SJ, Gray DR, Johnson PR. Perfluorodecalin-enriched fibrin matrix for human islet culture. Biomaterials 2011; 32:9282-9; PMID:21899883; https://doi.org/ 10.1016/j.biomaterials.2011.08.044 [DOI] [PubMed] [Google Scholar]
- [42].Matsushima H, Kuroki T, Adachi T, Kitasato A, Ono S, Tanaka T, Hirabaru M, Kuroshima N, Hirayama T, Sakai Y, et al.. Human fibroblast sheet promotes human pancreatic islet survival and function in vitro. Cell Transplant 2016; 25:1525-37; PMID:26877090; https://doi.org/ 10.3727/096368916X690854 [DOI] [PubMed] [Google Scholar]
- [43].Marchioli G, van Gurp L, van Krieken PP, Stamatialis D, Engelse M, van Blitterswijk CA, Karperien MB, de Koning E, Alblas J, Moroni L, et al.. Fabrication of three-dimensional bioplotted hydrogel scaffolds for islets of Langerhans transplantation. Biofabrication 2015; 7:025009; PMID:26019140; https://doi.org/ 10.1088/1758-5090/7/2/025009 [DOI] [PubMed] [Google Scholar]
- [44].Murray HE, Paget MB, Bailey CJ, Downing R. Sustained insulin secretory response in human islets co-cultured with pancreatic duct-derived epithelial cells within a rotational cell culture system. Diabetologia 2009; 52:477-85; PMID:19130038; https://doi.org/ 10.1007/s00125-008-1247-x [DOI] [PubMed] [Google Scholar]
- [45].Papas KK, Avgoustiniatos ES, Tempelman LA, Weir GC, Colton CK, Pisania A, Rappel MJ, Friberg AS, Bauer AC, Hering BJ. High-density culture of human islets on top of silicone rubber membranes. Transplant Proc 2005; 37:3412-4; PMID:16298611; https://doi.org/ 10.1016/j.transproceed.2005.09.086 [DOI] [PubMed] [Google Scholar]
- [46].Zhang Y, Jalili RB, Warnock GL, Ao Z, Marzban L, Ghahary A. Three-dimensional scaffolds reduce islet amyloid formation and enhance survival and function of cultured human islets. Am J Pathol 2012; 181:1296-305; PMID:22902430; https://doi.org/ 10.1016/j.ajpath.2012.06.032 [DOI] [PubMed] [Google Scholar]
- [47].Maedler K, Carr RD, Bosco D, Zuellig RA, Berney T, Donath MY. Sulfonylurea induced beta-cell apoptosis in cultured human islets. J Clin Endocrinol Metab 2005; 90:501-6; PMID:15483097; https://doi.org/ 10.1210/jc.2004-0699 [DOI] [PubMed] [Google Scholar]
- [48].Avgoustiniatos ES, Scott WE 3rd, Suszynski TM, Schuurman HJ, Nelson RA, Rozak PR, Mueller KR, Balamurugan AN, Ansite JD, Fraga DW, et al.. Supplements in human islet culture: Human serum albumin is inferior to fetal bovine serum. Cell Transplant 2012; 21:2805-14; PMID:22863057; https://doi.org/ 10.3727/096368912X653138 [DOI] [PubMed] [Google Scholar]
- [49].Kerr-Conte J, Vandewalle B, Moerman E, Lukowiak B, Gmyr V, Arnalsteen L, Caiazzo R, Sterkers A, Hubert T, Vantyghem MC, et al.. Upgrading pretransplant human islet culture technology requires human serum combined with media renewal. Transplantation 2010; 89:1154-60; PMID:20098354; https://doi.org/ 10.1097/TP.0b013e3181d154ac [DOI] [PubMed] [Google Scholar]
- [50].Lee RH, Carter J, Szot GL, Posselt A, Stock P. Human albumin preserves islet mass and function better than whole serum during pretransplantation islet culture. Transplant Proc 2008; 40:384-6; PMID:18374076; https://doi.org/ 10.1016/j.transproceed.2008.02.016 [DOI] [PubMed] [Google Scholar]
- [51].Matsumoto S, Goel S, Qualley S, Strong DM, Reems JA. A comparative evaluation of culture conditions for short-term maintenance (< 24 h) of human islets isolated using the edmonton protocol. Cell Tissue Bank 2003; 4:85-93; PMID:15256844; https://doi.org/ 10.1023/B:CATB.0000007043.15164.8a [DOI] [PubMed] [Google Scholar]
- [52].Nacher M, Garcia A, Estilles E, Nadal B, Pairó M, Garcia C, Busquets J, Novials A, Montanya E. Human serum vs human serum albumin for human islet culture. Diabetologia 2013; 56:S224-5; PMID:25955150; https://doi.org/ 10.3727/096368915X688119 [DOI] [PubMed] [Google Scholar]
- [53].Bucher P, Bosco D, Mathe Z, Matthey-Doret D, Andres A, Buhler L, Morel Ph, Berney Th. Human islet culture before transplantation: - Influence of medium on islet apoptosis and function.: 0147. Transplantation 2003; 76:S65-6; https://doi.org/ 10.1097/00007890-200308271-00144 [DOI] [Google Scholar]
- [54].Ricordi C, Lacy PE, Scharp DW. Automated islet isolation from human pancreas. Diabetes 1989; 38(Suppl 1):140-2; PMID:2642838; https://doi.org/ 10.2337/diab.38.1.S140 [DOI] [PubMed] [Google Scholar]
- [55].Paraskevas S, Duguid WP, Maysinger D, Feldman L, Agapitos D, Rosenberg L. Apoptosis occurs in freshly isolated human islets under standard culture conditions. Transplant Proc 1997; 29:750-2; PMID:9123509; https://doi.org/ 10.1016/S0041-1345(96)00452-6 [DOI] [PubMed] [Google Scholar]
- [56].Petrik J, Arany E, McDonald TJ, Hill DJ. Apoptosis in the pancreatic islet cells of the neonatal rat is associated with a reduced expression of insulin-like growth factor II that may act as a survival factor. Endocrinology 1998; 139:2994-3004; PMID:9607811; https://doi.org/ 10.1210/endo.139.6.6042 [DOI] [PubMed] [Google Scholar]
- [57].Thomas FT, Contreras JL, Bilbao G, Ricordi C, Curiel D, Thomas JM. Anoikis, extracellular matrix, and apoptosis factors in isolated cell transplantation. Surgery 1999; 126:299-304; PMID:10455898; https://doi.org/ 10.1016/S0039-6060(99)70169-8 [DOI] [PubMed] [Google Scholar]
- [58].Ilieva A, Yuan S, Wang RN, Agapitos D, Hill DJ, Rosenberg L. Pancreatic islet cell survival following islet isolation: The role of cellular interactions in the pancreas. J Endocrinol 1999; 161:357-64; PMID:10333538; https://doi.org/ 10.1677/joe.0.1610357 [DOI] [PubMed] [Google Scholar]
- [59].Labriola L, Montor WR, Krogh K, Lojudice FH, Genzini T, Goldberg AC, Eliaschewitz FG, Sogayar MC. Beneficial effects of prolactin and laminin on human pancreatic islet-cell cultures. Mol Cell Endocrinol 2007; 263:120-33; PMID:17081683; https://doi.org/ 10.1016/j.mce.2006.09.011 [DOI] [PubMed] [Google Scholar]
- [60].Weir GC, Bonner-Weir S. Islet transplantation as a treatment for diabetes. J Am Optom Assoc 1998; 69:727-32; PMID:9844324 [PubMed] [Google Scholar]
- [61].Gahr S, Merger M, Bollheimer LC, Hammerschmied CG, Scholmerich J, Hugl SR. Hepatocyte growth factor stimulates proliferation of pancreatic beta-cells particularly in the presence of subphysiological glucose concentrations. J Mol Endocrinol 2002; 28:99-110; PMID:11932207; https://doi.org/ 10.1677/jme.0.0280099 [DOI] [PubMed] [Google Scholar]
- [62].Yamaoka T, Yoshino K, Yamada T, Yano M, Matsui T, Yamaguchi T, Moritani M, Hata J, Noji S, Itakura M. Transgenic expression of FGF8 and FGF10 induces transdifferentiation of pancreatic islet cells into hepatocytes and exocrine cells. Biochem Biophys Res Commun 2002; 292:138-43; PMID:11890684; https://doi.org/ 10.1006/bbrc.2002.6601 [DOI] [PubMed] [Google Scholar]
- [63].Edlund H. Factors controlling pancreatic cell differentiation and function. Diabetologia 2001; 44:1071-9; PMID:11596660; https://doi.org/ 10.1007/s001250100623 [DOI] [PubMed] [Google Scholar]
- [64].Garcia-Ocana A, Vasavada RC, Cebrian A, Reddy V, Takane KK, Lopez-Talavera JC, Stewart AF. Transgenic overexpression of hepatocyte growth factor in the beta-cell markedly improves islet function and islet transplant outcomes in mice. Diabetes 2001; 50:2752-62; PMID:11723058; https://doi.org/ 10.2337/diabetes.50.12.2752 [DOI] [PubMed] [Google Scholar]
- [65].Garcia-Ocana A, Vasavada RC, Takane KK, Cebrian A, Lopez-Talavera JC, Stewart AF. Using beta-cell growth factors to enhance human pancreatic Islet transplantation. J Clin Endocrinol Metab 2001; 86:984-8; PMID:11238473; https://doi.org/ 10.1210/jcem.86.3.7315 [DOI] [PubMed] [Google Scholar]
- [66].Lopez-Avalos MD, Tatarkiewicz K, Sharma A, Bonner-Weir S, Weir GC. Enhanced maturation of porcine neonatal pancreatic cell clusters with growth factors fails to improve transplantation outcome. Transplantation 2001; 71:1154-62; PMID:11374418; https://doi.org/ 10.1097/00007890-200104270-00024 [DOI] [PubMed] [Google Scholar]
- [67].Nielsen JH, Galsgaard ED, Moldrup A, Friedrichsen BN, Billestrup N, Hansen JA, Lee YC, Carlsson C. Regulation of beta-cell mass by hormones and growth factors. Diabetes 2001; 50(Suppl 1):S25-9; PMID:11272193; https://doi.org/ 10.2337/diabetes.50.2007.S25 [DOI] [PubMed] [Google Scholar]
- [68].Rhodes CJ. IGF-I and GH post-receptor signaling mechanisms for pancreatic beta-cell replication. J Mol Endocrinol 2000; 24:303-11; PMID:10828823; https://doi.org/ 10.1677/jme.0.0240303 [DOI] [PubMed] [Google Scholar]
- [69].Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest 2007; 117:2553-61; PMID:17786244; https://doi.org/ 10.1172/JCI32959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Drucker DJ, Nauck MA. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368:1696-705; PMID:17098089; https://doi.org/ 10.1016/S0140-6736(06)69705-5 [DOI] [PubMed] [Google Scholar]
- [71].Willard FS, Sloop KW. Physiology and emerging biochemistry of the glucagon-like peptide-1 receptor. Exp Diabetes Res 2012; 2012:470851; PMID:22666230; https://doi.org/ 10.1155/2012/470851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Ranta F, Avram D, Berchtold S, Dufer M, Drews G, Lang F, Ullrich S. Dexamethasone induces cell death in insulin-secreting cells, an effect reversed by exendin-4. Diabetes 2006; 55:1380-90; PMID:16644695; https://doi.org/ 10.2337/db05-1220 [DOI] [PubMed] [Google Scholar]
- [73].Ferdaoussi M, Abdelli S, Yang JY, Cornu M, Niederhauser G, Favre D, Widmann C, Regazzi R, Thorens B, Waeber G, et al.. Exendin-4 protects beta-cells from interleukin-1 beta-induced apoptosis by interfering with the c-Jun NH2-terminal kinase pathway. Diabetes 2008; 57:1205-15; PMID:18252896; https://doi.org/ 10.2337/db07-1214 [DOI] [PubMed] [Google Scholar]
- [74].Shin S, Le Lay J, Everett LJ, Gupta R, Rafiq K, Kaestner KH. CREB mediates the insulinotropic and anti-apoptotic effects of GLP-1 signaling in adult mouse beta-cells. Mol Metab 2014; 3:803-12; PMID:25379405; https://doi.org/ 10.1016/j.molmet.2014.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ravassa S, Zudaire A, Carr RD, Diez J. Antiapoptotic effects of GLP-1 in murine HL-1 cardiomyocytes. Am J Physiol Heart Circ Physiol 2011; 300:H1361-72; PMID:21278133; https://doi.org/ 10.1152/ajpheart.00885.2010 [DOI] [PubMed] [Google Scholar]
- [76].Davies MJ. Insulin secretagogues. Curr Med Res Opin 2002; 18(Suppl 1):s22-30; PMID:12365816; https://doi.org/ 10.1185/030079902125000200 [DOI] [PubMed] [Google Scholar]
- [77].Proks P, Reimann F, Green N, Gribble F, Ashcroft F. Sulfonylurea stimulation of insulin secretion. Diabetes 2002; 51(Suppl 3):S368-76; PMID:12475777; https://doi.org/ 10.2337/diabetes.51.2007.S368 [DOI] [PubMed] [Google Scholar]
- [78].Stendahl JC, Kaufman DB, Stupp SI. Extracellular matrix in pancreatic islets: Relevance to scaffold design and transplantation. Cell Transplant 2009; 18:1-12; PMID:19476204; https://doi.org/ 10.3727/096368909788237195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].de Souza BM, Boucas AP, Oliveira FD, Reis KP, Ziegelmann P, Bauer AC, Crispim D. Effect of co-culture of mesenchymal stem/stromal cells with pancreatic islets on viability and function outcomes: A systematic review and meta-analysis. Islets 2017; 9:30-42; PMID:28151049; https://doi.org/ 10.1080/19382014.2017.1286434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009; 339:b2535; PMID:19622551; https://doi.org/ 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]


