Since the start of the Human Genome Project 25 years ago, basic discoveries related to genomics and other “-omic” fields have continued to advance exponentially. This progress has facilitated the 2015 launch of the US Precision Medicine Initiative (PMI). The PMI is intended to merge genomic, biological, behavioral, environmental, and other data on individuals to identify drivers of health that might support personalized healthcare decision making. In the cancer domain, for example, recognition of both inherited genetic susceptibility (eg, Lynch syndrome for colorectal cancer, and BRCA1/2 for breast cancer) and cancer genome sequence alterations that can pinpoint therapeutic agents (eg, National Cancer Institute’s MATCH trials) has the potential to make clinical decisions more personalized both in prevention and treatment. The “National Cancer Moonshot Initiative” seeks to rapidly scale up these efforts.1
There has been a parallel increase in the availability of personal devices (eg, smartphones, activity monitors, wearable GPS units) and electronic data capture tools to monitor behavior. Access to such technology has the potential to improve accessibility of behavioral interventions and enable real-time assessment of influences on personal health. If implemented well, the precision medicine approach to health care combining personal biological and behavioral data could have a significant benefit in reducing unmet health care needs in the population. Yet biomedical science faces a considerable challenge in evaluating the clinical usefulness of PMI-related scientific breakthroughs efficiently and at low cost, particularly if modeled on the traditional model of intervention development, then efficacy and effectiveness studies, followed by implementation.
The vision of the learning health care system2 can address this, by repositioning the formal health care delivery sector as a set of nimble organizations that focus on ongoing system improvement by capturing data at the clinical encounter and using those data to inform ongoing clinical and community practice. Learning health care systems are designed to improve care over time, using continuous quality improvement strategies, and seek to integrate a range of scientific methodologies at the point of patient care. If successful, these health systems offer an opportune setting for the findings of the PMI to be incorporated into day-to-day clinical care, provided that precision medicine advances can adapt to and integrate within incredibly diverse clinical and community practice settings.
Implementation science is intended to support exactly this integration. This emerging discipline3 can provide evidence-based strategies (eg, system change interventions, training, supervision, quality monitoring tools) to improve the integration of genomics and other precision medicine interventions within real-world practice. It is defined as “the scientific study of methods to promote the systematic uptake of research findings and other evidence-based practices into routine practice….”4 The National Institutes of Health, Agency for Health-care Research and Quality, Department of Veterans Affairs (VA), and Patient-Centered Outcomes Research Institute sponsor ongoing funding opportunities in this area, focusing on identifying strategies that support the adoption, implementation, sustainability, and ongoing improvement of evidence-based interventions and the optimal application of scientific knowledge to benefit health and health care outcomes.
With the rapidly changing environment of health care delivery and the numerous precision medicine studies across the biomedical enterprise, an opportunity exists to study how data from both observational studies and clinical trials can be used to improve health outcomes in real-world practice settings. Implementation science may be able to help provide the missing ingredient at the intersection of precision medicine and learning health care systems.
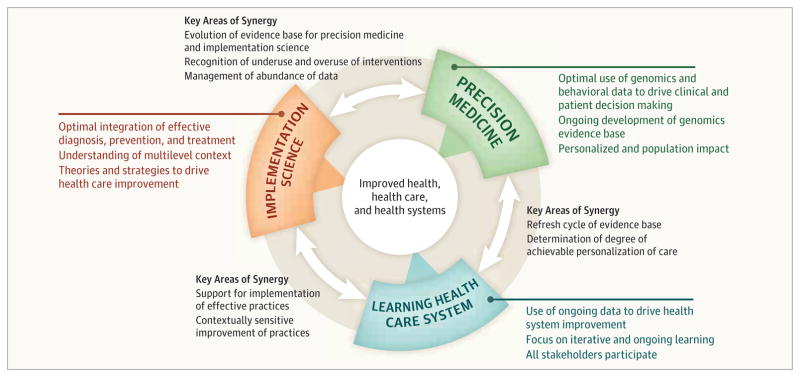
Potential synergies among the 3 concepts—implementation science, precision medicine, and the learning health care system—were explored at a National Academies of Science, Engineering, and Medicine workshop sponsored by the Roundtable on Translating Genomic-Based Research into Health. 5 Two major themes emerge from those discussions (Figure).
Figure.
Contributions of Implementation Science, Learning Health Care System, and Precision Medicine
One, clinical research is not complete prior to implementation. The consistent evolution of genomics suggests that even though a number of actionable genomic tests exist, many more are being discovered and evaluated, and the analytic and clinical validity as well as understanding of the clinical utility of such tests are consistently improving. Therefore, the model for genomics implementation must account for the need for ongoing learning and evidence development.
Two, research and practice can coexist. Some research efforts within health care systems (eg, Geisinger, VA, Kaiser Permanente) embed research data collection within ongoing health care delivery settings and show the joint benefit to science and health care practice through initiatives to aggregate data; improve mechanisms for rapid referral of patients to needed services; and implement strategies to improve disease management, monitoring, and clinician follow-up.
Implementation science, with its focus on identification of all major contributions to improvement of health care, from individual factors up to policy and public health interventions, can serve as a framework for considering the future of precision health care. A number of lessons from implementation science may be particularly helpful in considering the future of a learning health care system incorporating precision medicine data.
First, context matters and is multilevel. A plethora of theoretical models from implementation science identifies fundamental elements of health care and community settings that influence successful implementation.6
Second, it’s not just whether a practice works, but whether that practice can be delivered in many real-world settings. Implementation science has identified many influences on use of evidence-based practices, including intervention characteristics, clinical and administrative staff, health system factors, and the broader environment (eg, policy, financing).
Third, there are effective strategies to implement evidence-based practices. These 68 strategies,7 grouped into 6 different categories—planning, education, financing, restructuring, quality management, and attention to policy contexts—offer promise to support genomics implementation in a range of health care systems.
Fourth, implementation science is a team sport. Implementation studies have demonstrated the need to partner with a range of stakeholders, including patients, clinicians, administrators, researchers, and policy makers.
Achieving high-quality, efficient care based on a foundation of precision medicine will require that researchers, physicians and other health care professionals, and health care systems embrace the learning health care system concept and invest in infrastructure, intellectual as well as physical, to make it a reality. Although precision medicine remains a story to be written, implementation science can substantially add value to learning health care systems, and in turn, the evolutionary nature of precision medicine can reshape current thinking about and approaches to research-practice translation.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Disclaimer: The ideas presented are those of the authors and do not necessarily represent the official position of the National Cancer Institute, National Institutes of Health, or Centers for Disease Control and Prevention.
Contributor Information
David A. Chambers, Division of Cancer Control and Population Sciences, National Cancer Institute, Rockville, Maryland.
W. Gregory Feero, Maine Dartmouth Family Medicine Residency, Augusta; and Associate Editor, JAMA.
Muin J. Khoury, Division of Cancer Control and Population Sciences, National Cancer Institute, Rockville, Maryland; and Office of Public Health Genomics, Centers for Disease Control and Prevention, Atlanta, Georgia.
References
- 1.National Cancer Moonshot Initiative. National Cancer Institute; [Accessed February 25, 2016]. http://www.cancer.gov/research/key-initiatives/biden-cancer-initiative. [Google Scholar]
- 2.Committee on the Learning Health Care System in America. Institute of Medicine; [Accessed April 4, 2016]. Best care at lower cost: the path to continuously learning health care in America. http://www.nationalacademies.org/hmd/Reports/2012/Best-Care-at-Lower-Cost-The-Path-to-Continuously-Learning-Health-Care-in-America.aspx. [PubMed] [Google Scholar]
- 3.Fisher ES, Shortell SM, Savitz LA. Implementation science: a potential catalyst for delivery system reform. JAMA. 2016;315(4):339–340. doi: 10.1001/jama.2015.17949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eccles MP, Mittman BS. Welcome to implementation science. Implement Sci. 2006;1(1) doi: 10.1186/1748-5908-1-1. [DOI] [Google Scholar]
- 5.Roundtable on translating genomic-based research for health. Institute of Medicine; [Accessed February 10, 2016]. http://www.nationalacademies.org/hmd/Activities/Research/GenomicBasedResearch.aspx. [Google Scholar]
- 6.Tabak RG, Khoong EC, Chambers DA, Brownson RC. Bridging research and practice: models for dissemination and implementation research. Am J Prev Med. 2012;43(3):337–350. doi: 10.1016/j.amepre.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powell BJ, McMillen JC, Proctor EK, et al. A compilation of strategies for implementing clinical innovations in health and mental health. Med Care Res Rev. 2012;69(2):123–157. doi: 10.1177/1077558711430690. [DOI] [PMC free article] [PubMed] [Google Scholar]