Abstract
In spite of accelerating human genome discoveries in a wide variety of diseases of public health significance, the promise of personalized health care and disease prevention based on genomics has lagged behind. In a time of limited resources, public health agencies must continue to focus on implementing programs that can improve health and prevent disease now. Nevertheless, public health has an important and assertive leadership role in addressing the promise and pitfalls of human genomics for population health. Such efforts are needed not only to implement what is known in genomics to improve health but also to reduce potential harm and create the infrastructure needed to derive health benefits in the future.
Introduction
A decade after the completion of the human genome project, we live in an era of unprecedented gene discovery for almost all human diseases.1–3 Discoveries are fueled by declining laboratory research costs and multiplexing platforms.4–6 Promise of the impact of genomics on health care and disease prevention is being heralded by prominent scientists.7 In an online horizon scanning of genomic tests, more than 200 new tests were found that are in transition from bench to bedside in the past year.8 More than 1800 tests for genetic diseases are available9 and an increasing number of genetic conditions are included in newborn screening panels.10 However, there is a currently a wide gap between new discoveries and the realization of their promise for population health.11 The use of human genomics carries the potential for harm, especially from unnecessary or unproven interventions.12 The recent surge of direct-to-consumer (DTC) sales of personal genomic tests exemplifies the premature deployment of genomic technologies without a scientific evidence base.13
The incorporation of human genomic discoveries into public health practice must deal with an apparent paradox. The mission of public health is to improve health from a population perspective14 and its unit of intervention is the “population,” an approach seemingly at odds with the “one person at a time” vision of genomic medicine.15 The debate about the role of genomics in public health practice has been ongoing for quite some time. Some point out that that the applications of human genomics will be made at the clinical level and not through population screening.16 Some have even argued that there is very little role for genomics in public health when the environmental causes of disease are known (i.e., infectious, chemical, behavioral, and social factors).17 Moreover, in a time of diminishing resources, new technologies can divert much needed resources away from what can be done today in delivering basic public health services and addressing social and environmental determinants of disease.18 Finally, a reductionist, individually targeted approach to health may not substantially improve the health of populations. Such is the dilemma of human genomic applications in public health practice.
Increasingly, a public health research agenda is being articulated for the translation of human genomic discoveries into health benefits. This includes multiple population disciplines such as epidemiology, behavioral, social, and communication sciences.19–22 These disciplines assess the impact of genomic factors and their interactions with environmental and social factors on population health. In addition, the use of genomics in public health research is expected to lead to better identification of environmental causes of diseases using studies on gene–environment interaction,23 epigenetics,24 and Mendelian randomization.25
Nevertheless, traditional public health practice represented by programs at federal, state, local, and community levels have not integrated human genomic advances, except in certain well-established programs such as newborn screening.26 Most public health practitioners do not have the training to integrate rapidly emerging genomic information into their programs, although many see future value for using genetic information.27 The contention in this paper is that there is a real, current, and increasing role for human genomics in public health practice, and a more assertive and broad-based approach to human genomics that takes into account multilevel interventions, including policy change, clinical–public health partnerships, and consumer and provider education.
Three priorities are discussed for the integration of genomics into public health practice. The focus is on human genomics, including both genetic diseases and common diseases with strong genetic components, information derived from germline, somatic, and gene products such as expression profiles, and proteomics. Not part of this paper are public health issues related to genetic engineering of crops and their consequences on people (e.g., safety and broader consequences of spread into other parts of the ecosystem); or public health issues related to pathogen genomics in developing new diagnostic tests, tracking infectious diseases in populations,28 and new vaccines.29
Multilevel Interventions in Public Health Practice
The Association for State and Territorial Health Officials recently revisited the well-known framework of three essential public health functions (assessment, policy development, and assurance and evaluation) and 10 essential services for genomics.30 It is not the intent of this paper to discuss these functions and services, as in previous publications,19,31 but to expand on priorities for short-term action within these services. It is recognized that public health is practiced at multiple levels, including federal agencies, state and local health departments, and clinical medicine as well as the private sector.14 Generally, national and state public health roles tend to differ. States are typically in delivery mode, whereas federal roles are more often informational and providing funding, policy, and regulation.
The primary audience of this paper are those in state and local health departments, voluntary organizations, and others addressing health-related issues on a population basis. Public health uses an array of tools and actions to ensure health and focus more on prevention than clinical management.32 Generally, addressing social determinants of health (reduced poverty, increased education) can have the largest impact on population health.32 Policy-level interventions can make the individual’s default decisions healthy (such as policy interventions to reduce tobacco use, trans-fats and salt in the diet, clean air, seat belt laws, and occupational safety requirements).32 In the context of human genomics, regulatory approaches are needed to control misuse and overuse of new technologies.33 The Food and Drug Administration (FDA) is currently moving to apply its existing regulatory authorities to laboratory-developed tests (LDTs), especially the direct-to-consumer market industry in genetic testing.34
Public health interventions also include clinical interventions that require limited contact but confer long-term health protection (such as immunizations to prevent the spread of infectious diseases and colonoscopy to reduce morbidity from colorectal cancer). Another form of public health interventions involves ongoing direct clinical care (suchasHIVtreatmenttoreducetransmission,treatmentof hypertension and high cholesterol to reduce the risk of heart disease). Public health also includes health education and counseling (such as health education about diet and physical activity). Interventions focusing on policy contexts tend to have greater health impact than health education, because they reach broader segments of society and require less individual effort. Nevertheless, implementing interventions at multiple levels can achieve the most public health benefit. For example, the successful reduction in the number of folic acid–preventable neural tube defects was achieved by interventions using multiple levels. The most effective intervention was FDA’s mandated folic acid food fortification efforts, followed by clinical and educational interventions to encourage women to include more folic acid in their diet.35,36
How can human genome discoveries fit into public health practice and provide new tools to achieve important public health goals? There are currently a few applications for which there is potential public health benefit. Family health history and some genetic tests provide mechanisms for early detection and intervention for a number of conditions, mostly in newborn screening10 and genetic disorders with high individual risk.37–39 Recent evidence, for example, suggests that cascade screening of first-degree relatives for selected conditions such as familial hypercholesterolemia (FH), a main cause of premature heart disease38 and Lynch syndrome,39 a main cause of early-onset colorectal cancer, can reduce morbidity and mortality outcomes in relatives of affected people.
The implementation of such applications could save thousands of lives every year.37–39 Implementation can be achieved using multiple levels, primarily working with clinicians and conducting health education. Policies to enhance the oversight of genomic testing by ensuring the use of evidentiary standards of validity and utility as well as quality-testing process are crucial.33
Current Priorities for Public Health Practice in Genomics
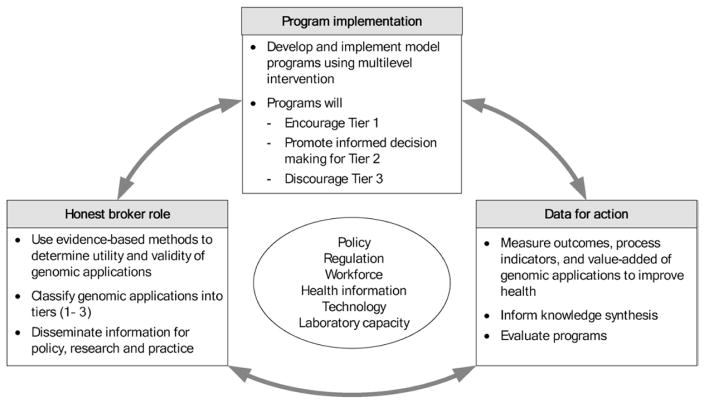
Table 1 shows three immediate priorities for public health action that are essential to reap the population health benefits of emerging human genomic discoveries: (1) serving as the honest broker for emerging genomic applications in practice; (2) implementing current evidence-based genomic applications to improve health and prevent disease, while discouraging premature use, misuse, and overuse of genomic applications; and (3) using genomics tools to evaluate the health impact of public health interventions regardless of whether they currently use genomics. These actions are highly synergistic as shown in Figure 1.
Table 1.
Current priorities for public health practice to address human genomic information to improve population health
| Priorities | Actions | Magnitude and timeline of population health impact |
|---|---|---|
| Serving as the honest broker for emerging genomic applications to consumers, providers, and policymakers | Conducting and sponsoring knowledge synthesis and evidence recommendations on appropriate use (see examples in Table 2) | Small but increasing impact in saving unnecessary healthcare costs and preventing harm while illuminating what is ready for implementation |
| Implementing evidence-based genomic applications and discouraging use of unvalidated applications | Implementing multilevel interventions (see examples in Table 2) | Small but increasing impact for improving health and reducing unnecessary healthcare costs |
| Evaluating impact of public health interventions that currently do not use genomics (e.g., smoking cessation) | Evaluating benefits and harm of public health programs in subgroups of population using genomic tools | Small but increasing impact in evaluating current public health programs and informing next generation of programs |
Figure 1.
Synergy of public health actions in addressing the role of genomics in population health
Priority 1: Serving as the Honest Broker for Genomic Applications in Practice
The most important priority for public health practice today in genomics is to be the honest broker to inform providers, the public, and policymakers whether the deployment of a particular technology for a particular intended use can have a net positive health impact on the population.40 Because of the potential for conflicts of interests among various groups, public health can serve as an unbiased convener of stakeholders.
Although such a role is not well established in the genomic arena at present, for other areas of health care and disease prevention, some precedents exist, for example, the U.S. Preventive Services Task Force (USPSTF), which makes evidence-based recommendations on clinical practices to improve prevention.41 Similarly, the Community Guide makes evidence-based recommendations for community prevention efforts.42 NIH sponsors consensus development panels around specific health issues and applications (e.g., the recent panel on family history in primary care).43
Newborn screening provides an important model for the integration of genomic modalities into the public health sphere. For example, state-specific panels have made recommendations on which genetic conditions should be included in the screening panels, and because of state-by-state variation, a federal advisory panel now makes nationwide recommendations for which conditions to include in newborn screening, incorporating a rigorous process for assessing evidence. Such a change will enhance the uniformity and evidentiary basis for newborn screening programs.10
In 2005, the CDC launched an independent multidisciplinary panel to address specifically the issue of genetic applications in clinical practice and disease prevention (www.egappreviews.org/default.htm). The Evaluation of Genomic Applications for Practice and Prevention (EGAPP) working group systematically reviews and updates evidence of validity and utility of genomic applications and makes recommendations for appropriate use. EGAPP has filled a void in genomic medicine technology assessment. Several recommendations have been issued to date and more are under way.
A transparent, independent, and continuously updated assessment of emerging genomic applications is crucial to save unnecessary healthcare expenditures from unwarranted tests, minimize harm to individuals and groups from the unintended consequences of premature use of technologies, and maximize the potential population health benefits of rapidly emerging technologies.
The EGAPP working group has established methods for reviewing the evidence for different types of genomic tests, including those for screening, early detection, risk assessment and prognosis, as well as therapeutic choice (pharmacogenomics). As shown in Table 2, in collaboration with the EGAPP working group and various stakeholders, the CDC is establishing an evidence-based triaging of emerging genomic applications with a three-tier classification schema that would be based on the EGAPP working group methods paper.44 Tier-1 applications include those with sufficient evidence for validity and clinical utility for the test to be implemented into practice (e.g., Lynch syndrome). Tier-2 applications are those with sufficient evidence of validity and promising evidence of utility but insufficient to support a recommendation for use. For such applications, outcomes research is needed in clinical trials and/or comparative effectiveness studies. In addition, public health and clinical practitioners can provide individuals with information needed for them to make informed decisions on use. Tier-3 applications are those with either sufficient evidence for a lack of utility or overt harm, or insufficient evidence on validity and utility. Public health would discourage using Tier-3 applications until further studies establish validity and utility (e.g., personal genomic tests sold directly to consumers).
Evidence-based triaging of genomic applications would be an iterative process, constantly changing based on newly acquired evidence.45,46 Finally, it is important to conduct cost-effectiveness analyses as part of this priority to assess the preventable burden and associated costs as a potential guide for implementation. This was recently done for Lynch syndrome cascade screening.47
Priority 2: Implementing Evidence-Based Genomic Applications and Discouraging Premature Use of Unvalidated Applications
In addition to being an honest broker, there are important roles for public health programs (Table 2). For Tier-1 applications, exemplified by Lynch syndrome, public health programs can actively encourage implementation of evidence recommendations. For Tier-2 genomic applications, exemplified by the use of family history as a tool for risk assessment and disease prevention (see below), public health programs can promote additional data collection on health impact to assess the added value of using genomic tools in practice. For Tier-3 genomic applications, such as DTC personal genomics tests, public health programs can actively discourage their use while additional research is done.
Table 2.
Examples of evidence-based triaging of human genomic applications and integration into public health programs using multilevel interventions
| Evidence-based triaging
|
|||
|---|---|---|---|
| Tier 1
|
Tier 2
|
Tier 3
|
|
| Genomic application | Evidence-based recommendation for implementation | Established validity/promising utility | Unclear benefits and harm; or evidence recommendation against use |
| Examples | Lynch syndrome | Family history for risk assessment in primary care | Personal genomic tests sold directly to consumers |
|
| |||
| Public health issues | 3%–5% of all cases of colorectal cancer are due to this genetic disorder. Cancer has early age of onset | Family history is a risk factor for common chronic diseases. It can be used to target public health messages (diet, physical activity, screening) | Several companies selling to the public personal genomic test to inform consumers about risks for common diseases |
|
| |||
| Evidence recommendation | EGAPP working group recommended in 2009 that all new cases of colorectal cancer be screened for Lynch syndrome. Cascade testing of relatives can reduce mortality from colorectal cancer39 | NIH conference in 2009 concluded that while “Family history interventions can motivate behavior change, the data are not sufficiently robust to conclude that routine family history can lead to improved health outcomes”a | CDC–NIH conference in 2009 concluded that there is insufficient evidence on clinical validity and utility of these tests13 |
|
| |||
| Public health actions | Promote implementation in practice by educating providers and patients; setting screening programs, and developing clinical decision support tools; encourage coverage evidence recommendations by health insurance | Provide information for decision making about family history as disease risk factor; educating providers and consumers | Discourage use in practice by educating consumers, providers, and policymakers |
|
| |||
| Public health surveillance and epidemiology | Measure impact of actions on implementation and outcomes | Measure impact of information on use and outcomes | Measure impact of discouraging implementation on use and outcomes |
|
| |||
| Assess added value in public health interventions that do not use genomics to inform future public health actions | Assess added value in public health interventions that do not use genomics to inform future public health actions | ||
EGAPP, Evaluation of Genomic Applications for Practice and Prevention
Public health actions can operate at multiple levels, as discussed earlier, for example, by developing health education campaigns targeted to at-risk individuals, clinicians, policymakers, and payers. Implementation could also be integrated into various disease-specific or categoric public health programs. As noted earlier, there are also genomic applications for which organized efforts of public health programs, namely, newborn screening, represent the largest public health genetics program today. It remains to be seen whether or not an expansion of such a program to other conditions outside the newborn period is warranted, but the general principles of evidence assessment set a worthy precedent for the process of determining the desirability of expansion.
The example of Lynch syndrome discussed in Table 2 illustrates another form of screening in a different setting and life stage (i.e., screening all newly diagnosed cases of colorectal cancer for Lynch syndrome). It clearly shows the challenges that public health faces in implementing such evidence-based applications population-wide. At a time when half of adults do not get colorectal cancer screening, one may wonder whether or not state public health cancer programs should emphasize the small proportion of colorectal cancer that have Lynch syndrome or would this be best left to the clinical care system alone? In a CDC multi-disciplinary workshop held in September 2010 to discuss the implementation of EGAPP Lynch syndrome recommendations, data were presented that currently less than 10% of cases of Lynch syndrome are ascertained by the healthcare system, and several stakeholders asserted that public health should take on the responsibility for ensuring that effective Lynch syndrome services are delivered and tracked using approaches embedded in state cancer registries.
Many challenges remain to make this first example of cascade screening a reality in public health practice. Other examples could follow, including familial hypercholesterolemia, hereditary breast/ovarian cancer, and others, affecting larger and larger segments of the population. Prioritization will obviously depend on level of evidence, the burden of morbidity and mortality that can be prevented in the population, and cost effectiveness of interventions. Finally, public health can use family history to reach relatives of individuals with common diseases and to implement nongenetic evidence-based recommendations (such as screenings and primary prevention in diabetes, cancer, and heart disease), therefore achieving larger health impact than by targeting genetic conditions alone.
Priority 3: Using Population Data to Guide Implementation of Genomic Applications and to Evaluate the Impact of Interventions That Currently Do Not Use Genomics
One important public health function is surveillance and epidemiology that provide “data for action” to guide implementation and evaluation of the impact of interventions. Examples include population-based disease registries,48,49 surveys of risk factors,50 and vaccine adverse effects.51 The challenge is to assess implementation (e.g., use of genomic tests), the appropriateness of implementation (e.g., in comparison with recommended use), health outcomes, as well as disparities in use and outcomes.52
In addition, human genomics may enhance surveillance and epidemiology assessments of existing or planned public health interventions that are not genome-based. Current population-level approaches to prevention have not been fully realized. For example, most Americans do not get enough physical activity, an increasing proportion is overweight, and many do not adhere to evidence-based screening recommendations (such as colorectal cancer screening). Could family history or genomic information be used to identify subsets of the population that merit more (or less) intensive or different types of interventions?
For example, family health history can be used as a stratifying tool for measuring health impact in surveillance systems. Family history is a simple and inexpensive application available in clinical practice today.53 Family history reflects not only genetics but also environmental and socioeconomic factors. Furthermore, clinical decisions that can readily be made on the basis of such history have little or nothing to gain by expensive genomic testing. Family history is a risk factor for most diseases of public health significance, especially common chronic diseases. Only occasionally does family history of a condition point to a classical genetic disorder. Most often, the presence of a family history reflects unmeasured genetic and environmental effects and is an indicator of higher risk for the same disease in comparison with the average population risk.53
Although there has been considerable debate as to whether family history can prevent common disease, it can be used as a surveillance tool to examine the impact of population-level interventions. For example, population-based family studies in Utah have shown that 14% of Utah families have a positive family history of coronary heart disease; these families account for 72% of early coronary events (aged <50 years) and 48% of coronary heart disease events at any age.54 Such data suggest that the implementation of family-centered interventions could lead to overall population health benefits. A recent evaluation of a Utah nationwide program to identify familial hyper-cholesterolemia patients showed that participants in the treatment program achieved greater cholesterol reductions than the comparison group.55
Another condition where family history may play a role is diabetes. Given the large number of undiagnosed diabetics, and the proven effectiveness of primary prevention measures (physical activity and medications), it is important to assess all approaches to help find undiagnosed diabetics and prediabetes. A recent analysis56 of the National Health and Nutrition Examination Survey (NHANES; see description below) found that by adding family history to risk prediction analysis of undiagnosed cases, more than 600,000 such cases are projected to be found in the U.S. population. Although early intervention among people with prediabetes can delay progression to diabetes, the effectiveness of population screening to identify people at risk has not been completely resolved. Such population data can provide a scientific foundation for extending evidence-based recommendation for screening for diabetes in the U.S. Because of the large number of diabetics in the U.S., a family-based intervention to target their relatives for primary prevention and early detection could have a substantially large impact on disease burden compared to screening programs for rare genetic conditions.
Genomic tools are increasingly available to evaluate the health impact of interventions on different segments of the population. For example, genomics could be used as a means to help target specific environmental and occupational exposures by setting the maximum allowed exposure levels based on the most susceptible people in the population.57,58 The declining costs of genomewide analysis are allowing us to add multiple genetic variations to existing surveillance systems for population health.
The NHANES is such an example at the national level.59 The CDC and others have measured an increasing number of genetic variants for numerous diseases to this uniquely representative, large, population-based survey of the U.S. population.60 This survey evaluates the health status of various ethnic/racial subgroups; to monitor the impact of different interventions (e.g., removing lead from the gasoline resulting in dramatic drop in serum lead levels in the U.S.)61; and generate data to inform health policies and interventions. More than 10,000 health-related variables are available in NHANES, including clinical, physiologic, risk factor, biochemical, and epidemiologic data.
The addition of hundreds of genetic variants to this survey is allowing public health epidemiologists to assess the potential added value of genetic information on the health impact of public health interventions. Building on the folic acid story mentioned above is one example. A genetic variant in the MTHFR gene (TT variant), affecting 5% of the population, was found to be associated with a thermolabile enzyme, leading to a relative functional deficiency in folic acid, a known risk factor for neural tube defects.62 An important question is whether or not the effectiveness of folate supplementation extends to this subgroup of the population. Using NHANES analyses, one study63 recently estimated that the current RDC allowance of folic acid (400 μg/day) should be adequate for individuals with the TT genotype. Another study,64 also using NHANES data, showed that folic acid supplementation increased folate levels among all studied alleles of the MTHFR gene, confirming current policies for folic acid supplementation.
Genomic analyses can be readily extended to the evaluation of many public health interventions (such as salt reduction, smoking campaigns, fat reduction, control of drug or vaccine side effects) on health outcomes and indicators in subsets of the population. Such analyses are not merely of academic interest but could inform us whether population interventions (including prevention or therapeutic interventions) need to be adjusted to reach the most susceptible in the U.S. population, which may include testing individuals before an intervention or designing a population strategy that addresses the most vulnerable segments of the population. Similar epidemiologic analyses can be done to explore potentially harmful effects of interventions in subgroups of the population (e.g., vaccine or drug side effects) and protection from harm of environmental and occupational exposures.
Conclusion
Three immediate priorities for action in human genomics are presented that can be integrated into model public health programs using multilevel interventions. The implementation of these priorities can have both near-term and long-term effects in realizing the promise and addressing the limits of human genomic discoveries in population health. Albeit small, the current health impact of genomics is likely to increase considerably over time as more applications become ready for implementation. Importantly, with minimal resources, public health programs can today serve an important honest broker role and use their convening function to accelerate progress, minimize potential harm and reduce unnecessary health-care costs, while setting a strong scientific and ethical foundation based on population-specific data, for future applications of genomics. The balanced approach presented here requires minimal investment compared to the massive investment in basic genomic sciences and technologies, and need not take away precious resources from public health programs in implementing current interventions that can reduce the burden of illness and death from diseases of public health significance.
Acknowledgments
The authors thank Dr. Katie Kolor for helpful comments on an earlier draft of the manuscript.
The opinions in this article reflect those of the authors and do not necessarily reflect the official position of the USDHHS.
Footnotes
No financial disclosures were reported by the authors of this paper.
References
- 1.Collins FS. Has the revolution arrived? Nature. 2010;464:674–5. doi: 10.1038/464674a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venter JC. Multiple personal genomes await. Nature. 2010;464:676–7. doi: 10.1038/464676a. [DOI] [PubMed] [Google Scholar]
- 3.Feero WG, Guttmacher AE, Collins FS. Genomic medicine: an updated primer. N Engl J Med. 2010;362:2001–11. doi: 10.1056/NEJMra0907175. [DOI] [PubMed] [Google Scholar]
- 4.Manolio TA. Genomewide association studies and assessment of the risk of disease. N Engl J Med. 2010;363:166–76. doi: 10.1056/NEJMra0905980. [DOI] [PubMed] [Google Scholar]
- 5.Ashley EA, Butte AJ, Wheeler MT, et al. Clinical assessment incorporating a personal genome. Lancet. 2010;375:1525–35. doi: 10.1016/S0140-6736(10)60452-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biesecker LG, Mullikin JC, Facio FM, et al. The ClinSeq Project: piloting large-scale genome sequencing for research in genomic medicine. Genome Res. 2009;119(9):1665–74. doi: 10.1101/gr.092841.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins FS. The language of life: DNA and the revolution in personalized medicine. New York: Harper Collins; 2010. [Google Scholar]
- 8.Office of Public Health Genomics. The Genomic Applications in Practice and Prevention Knowledge Base (GAPP Kb) http://www.hugenavigator.net/GAPPKB/home.do.
- 9.National Center on Biotechnology Information. Gene tests. www.ncbi.nlm.nih.gov/sites/GeneTests/?db=GeneTests.
- 10.Advisory Committee on Heritable Disorders in Newborns and Children. www.hrsa.gov/heritabledisorderscommittee/
- 11.Khoury MJ, Berg A, Coates RC, Evans J, Teutsch SM, Bradley LA. The evidence dilemma in genomic medicine. Health Aff. 2008;27:1600–11. doi: 10.1377/hlthaff.27.6.1600. [DOI] [PubMed] [Google Scholar]
- 12.Ransohoff DF, Khoury MJ. Personal genomics: information can causeharm. Eur J Clin Invest. 2010;40:64–8. doi: 10.1111/j.1365-2362.2009.02232.x. [DOI] [PubMed] [Google Scholar]
- 13.Khoury MJ, McBride CM, Schully SD, et al. The scientific foundation for personal genomics: recommendations from an NIH–CDC multidisciplinary workshop. Genet Med. 2009;11(8):559–67. doi: 10.1097/GIM.0b013e3181b13a6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.IOM. The future of the public’s health. Washington DC: National Academy Press; 2003. [Google Scholar]
- 15.Rose G. Sick individuals and sick populations. Int J Epidemiol. 2001;30:427–32. doi: 10.1093/ije/30.3.427. [DOI] [PubMed] [Google Scholar]
- 16.Khoury MJ, Burke W, Bowen MS, Zimmern RL. Will genomics widen or heal the schism between medicine and public health? AmJ Prev Med. 2007;33:310–7. doi: 10.1016/j.amepre.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Merikangas KR, Risch N. Genomic priorities and public health. Science. 2003;302(5645):599–601. doi: 10.1126/science.1091468. [DOI] [PubMed] [Google Scholar]
- 18.Buchanan AV, Weiss KM, Fullerton SM. Dissecting complex disease: the quest for the philosopher’s stone? Int J Epidemiol. 2006;35:562–71. doi: 10.1093/ije/dyl001. [DOI] [PubMed] [Google Scholar]
- 19.Beskow L, Khoury MJ, Baker TJ, Thrasher J. The integration of genomic into public health research, policy and practice in the U. S Community Genet. 2001;4(1):2–11. doi: 10.1159/000051150. [DOI] [PubMed] [Google Scholar]
- 20.Khoury MJ, Gwinn M, Yoon PW, Dowling N, Moore CA, Bradley L. The continuum of translation research in genomic medicine: how can we accelerate the appropriate integration of human genome discoveries into health care and disease prevention? Genet Med. 2007;9:665–74. doi: 10.1097/GIM.0b013e31815699d0. [DOI] [PubMed] [Google Scholar]
- 21.McBride CM, Bowen D, Brody LC, et al. Future health applications for genomics: priorities for communication, behavioral, and social sciences research. Am J Prev Med. 2010;38(5):556–61. doi: 10.1016/j.amepre.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khoury MJ, Bedrosian S, Gwinn M, et al., editors. Human genome epidemiology (second edition): building the evidence for using genetic information to improve health and prevent disease. New York NY: Oxford University Press; 2010. [Google Scholar]
- 23.Khoury MJ, Davis R, Gwinn M. Do we need genomic research for the prevention of common diseases with environmental causes? Am J Epidemiol. 2005;161(9):799–805. doi: 10.1093/aje/kwi113. [DOI] [PubMed] [Google Scholar]
- 24.Foley DL, Craig JM, Morley R, et al. Prospects for epigenetic epidemiology. Am J Epidemiol. 2009;169(4):389–400. doi: 10.1093/aje/kwn380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davey Smith G, Ebrahim S. What can Mendelian randomization tell us about modifiable behavioral and environmental exposures? BMJ. 2005;330:1076–9. doi: 10.1136/bmj.330.7499.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piper MA, Lindenmayer JM, Lengerich EJ, et al. The role of state public health agencies in genetics and disease prevention: results of a national survey. Public Health Rep. 2001;116(1):22–31. doi: 10.1016/S0033-3549(04)50019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Secretary’s Advisory Committee on Genetics, health and Society. Genetics education and training for healthcare professionals, public health providers and consumers (draft report) oba.od.nih.gov/SACGHS/sacghs_home.html.
- 28.Klint G, Fierro C, Marchal K, et al. Integration of “omics” data: does it lead to new insights into host–microbe interactions? Future Microbiol. 2010;5(2):313–28. doi: 10.2217/fmb.10.1. [DOI] [PubMed] [Google Scholar]
- 29.Mora M, Telford JL. Genome-based approaches to vaccine development. J Mol Med. 2010;88(2):143–7. doi: 10.1007/s00109-009-0574-9. [DOI] [PubMed] [Google Scholar]
- 30.Association for State and Territorial Health Officials. 2010 state public health genomics resource guide. www.astho.org/Programs/Access/Genomics/Genomics/
- 31.Khoury MJ the Genetic Working Group. From genes to public health. Am J Public Health. 1996;86:1717–22. doi: 10.2105/ajph.86.12.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frieden TR. A framework for public health action: the health impact pyramid. Am J Public Health. 2010;100:590–5. doi: 10.2105/AJPH.2009.185652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Secretary’s Advisory Committee on Genetics, Health and Society. U.S. System of oversight of genetic testing. 2008 doi: 10.2217/17410541.5.5.521. oba.od.nih.gov/oba/SACGHS/reports/SACGHS_oversight_report.pdf. [DOI] [PMC free article] [PubMed]
- 34.Food and Drug Administration. Public meeting: oversight of laboratory developed tests. www.fda.gov/MedicalDevices/NewsEvents/WorkshopsConferences/ucm212830.htm.
- 35.CDC. Grand rounds: folic acid in the prevention of birth defects. 2010 www.cdc.gov/about/grand-rounds/archives/2010/02-February.htm.
- 36.Oakley GP. The scientific basis for eliminating folic acid-preventable spina bifida: a modern miracle from epidemiology. Ann Epidemiol. 2009;19(4):226–30. doi: 10.1016/j.annepidem.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 37.U.S. Preventive Services Task Force. Genetic risk assessment andBRCA testing for breast and ovarian cancer susceptibility. 2005 www.ahrq.gov/clinic/uspstf/uspsbrgen.htm.
- 38.Wierzbecki AS. Familial hypercholesterolemia: summary of NICE guidelines. BMJ. 2008;337:a1095. doi: 10.1136/bmj.a1095. [DOI] [PubMed] [Google Scholar]
- 39.Evaluation of Genomic Applications for Practice and PreventionWorking Group. Recommendation from the EGAPP working group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med. 2009;11(1):35–41. doi: 10.1097/GIM.0b013e31818fa2ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Office of Public Health Genomics, Centers for Disease Control and Prevention, Genomic Applications in Practice and Prevention Network (GAPPNet) http://www.cdc.gov/genomics/translation/GAPPNet/
- 41.Agency for Healthcare Research Quality. U.S. Preventive Services Task Force. www.ahrq.gov/clinic/uspstfix.htm.
- 42.CDC. The Guide to Community Preventive Services. www.thecommunityguide.org/index.html.
- 43.NIH state of the science conference. Family history and improving health. consensus.nih.gov/2009/familyhistory.htm.
- 44.Teutsch SM, Bradley LA, Palomaki GE, et al. The evaluation of genomic applications in practice and prevention initiative: methods of the EGAPP working group. Genet Med. 2009;11(1):3–14. doi: 10.1097/GIM.0b013e318184137c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veenstra DL, Roth JA, Garrison LP, et al. A formal risk–benefit frame-work for genomic tests: facilitating the appropriate translation of genomics into clinical practice. Genet Med. 2010;12:686–93. doi: 10.1097/GIM.0b013e3181eff533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khoury MJ, Coates RJ, Evans JP. Evidence-based classification of genomic applications for use in clinical practice: dealing with insufficient evidence. Genet Med. 2010;12:680–3. doi: 10.1097/GIM.0b013e3181f9ad55. [DOI] [PubMed] [Google Scholar]
- 47.Mvundura M, Grosse SD, Hampel H, Palomaki GE. The cost effectiveness of genetic testing strategies for Lynch syndrome among newly diagnosed colorectal cancer patients. Genet Med. 2010;12(12):93–104. doi: 10.1097/GIM.0b013e3181cd666c. [DOI] [PubMed] [Google Scholar]
- 48.Cancer National Cancer Institute. State Cancer Profiles. statecancerprofiles.cancer.gov/
- 49.CDC. State Birth defects Tracking Systems. www.cdc.gov/ncbddd/bd/state.htm.
- 50.CDC. Behavioral Risk Factor Surveillance System. www.cdc.gov/brfss/
- 51.USDHHS. Vaccine Adverse Event Reporting System. vaers.hhs.gov/index.
- 52.Goddard KAB, Duquette D, Zlot A, et al. Public awareness and se of direct-to-consumer genetic tests: results from three state population-based surveys, 2006. Am J Public Health. 2009;99(3):442–5. doi: 10.2105/AJPH.2007.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valdez R, Yoon PW, Qureishi N, et al. Family history in public health practice: a genomic tool for disease prevention and health promotion. Annu Rev Public Health. 2010;31:69–87. doi: 10.1146/annurev.publhealth.012809.103621. [DOI] [PubMed] [Google Scholar]
- 54.Hunt SC, Gwinn M, Adams TD. Family history assessment: strategies of prevention of cardiovascular disease. Am J Prev Med. 2003;24(2):136–42. doi: 10.1016/s0749-3797(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 55.Stephenson SH, Larinaga-Shum S, Hopkins PN. Benefits of the MEDPED treatment support program for patients with familial hyper-cholesterolemia. J Clin Lipidol. 2009;3:94–100. doi: 10.1016/j.jacl.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 56.Yang Q, Liu T, Valdez R, et al. Improvements in the ability to detect undiagnosed diabetes by using information on family history among adults in the U. S Am J Epidemiol. 2010;171:1079–89. doi: 10.1093/aje/kwq026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Christiani DC, Mehta AJ, Yu CL. Genetic susceptibility to occupational exposures. Occup Environ Med. 2008;65(6):430–6. doi: 10.1136/oem.2007.033977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCunney RJ. Asthma genes and air pollution. J Occup Environ Med. 2005;47(12):1285–91. doi: 10.1097/01.jom.0000188561.75578.bf. [DOI] [PubMed] [Google Scholar]
- 59.National Center for Health Statistics. National Health and Nutrition Examination Surveys (NHANES) www.cdc.gov/nchs/nhanes.htm.
- 60.Chang MH, Lindegren ML, Butler MA, et al. Prevalence in the U.S. of selected candidate gene variants: third National Health and Nutrition Examination Survey. Am J Epidemiol. 2009;169(1):54–66. doi: 10.1093/aje/kwn286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meyer P, Pivert T, Dignam T, et al. Surveillance for elevated blood lead levels among children—U.S. 1997–2001. MMWR Surveill Summ. 2003;52(10):1–21. [PubMed] [Google Scholar]
- 62.Yang Q, Botto LD. 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: a HuGE review. Am J Epidemiol. 2000;151(9):862–77. doi: 10.1093/oxfordjournals.aje.a010290. [DOI] [PubMed] [Google Scholar]
- 63.Robitaille J, Hammer HC, Cogswell E, et al. Does theMTHFR677C→T variant affect the recommended dietary allowance for folate in the U.S. population? Am J Clin Nutr. 2009;89(4):1269–73. doi: 10.3945/ajcn.2008.27282. [DOI] [PubMed] [Google Scholar]
- 64.Yang QH, Botto LD, Gallagher M, et al. Prevalence and effects of gene–gene and gene–nutrient interactions on serum foliate and serum total homocysteine concentrations in the U.S: findings from the third National Health and Nutrition Examination Survey DNA bank. Am J Clin Nutr. 2008;88:232–46. doi: 10.1093/ajcn/88.1.232. [DOI] [PubMed] [Google Scholar]