Abstract
Background
Impairment in prediction and appreciation for choice outcomes could contribute to several core symptoms of ASD. We examined electroencephalography (EEG) oscillations in 27 youth and young adults diagnosed with autism spectrum disorder (ASD) and 22 IQ-matched neurotypical controls while they performed a chance-based reward prediction task.
Method
We re-analyzed our previously published ERP data (Larson et al., 2011) and examined theta band oscillations (4–8 Hz) at frontal midline sites, within a timing window that overlaps with the feedback-related negativity (FRN). We focused on event-related changes after presentation of feedback for reward (WIN) and punitive (LOSE) outcomes, both for spectral power and inter-trial phase coherence.
Results
In our reward prediction task, for both groups, medial frontal theta power and phase coherence were greater following LOSE compared to WIN feedback. However, compared to controls, inter-trial coherence of medial frontal theta was significantly lower overall (across both feedback types) for individuals with ASD. Our results indicate that while individuals with ASD are sensitive to the valence of reward feedback, comparable to their neurotypical peers, they have reduced synchronization of medial frontal theta activity during feedback processing.
Conclusions
This finding are consistent with previous studies showing neural variability in ASD and suggest that the processes underlying decision-making and reinforcement learning may be atypical and less efficient in ASD.
Keywords: autism spectrum disorders (ASD), reward processing, theta oscillations, event-related spectral analysis, inter-trial phase coherence
Introduction
Individuals with Autism Spectrum Disorder (ASD) display an array of social and communication deficits in both verbal and nonverbal domains (Geschwind & Levitt, 2007; Pelphrey, Yang, & McPartland, 2014). These include abnormal behavioral responses when processing social stimuli (Sepeta et al., 2012; Yeung, Han, Sze, & Chan, 2014), such as atypical activation of reward circuitry during the anticipation and processing of monetary incentives and social cues (Dichter et al., 2012; Ingersoll, Schreibman, & Tran, 2003; Panasiti, Puzzo, & Chakrabarti, 2015; Rademacher, Schulte-Ruther, Hanewald, & Lammertz, 2016). Studies using a variety of methods suggest that ASD is characterized, in part, by atypical engagement of frontal and striatal systems (Glerean et al., 2016; Langen et al., 2014).
The interplay between prediction, anticipation, action, and outcome-related feedback is central to reinforcement learning processes (Holroyd & Coles, 2002; Schultz & Dickinson, 2000) that rely on both positive and negative feedback in order to adaptively shape behavior (Sutton & Barto, 1998). It has been shown that medial prefrontal and limbic structures are engaged during feedback processing, serving to mediate reinforcement learning (Frank & Claus, 2006; O’Doherty, Dayan, Friston, Critchley, & Dolan, 2003; Pagnoni, Zink, Montague, & Berns, 2002; Pasupathy & Miller, 2005). Activity in the striatum and medial frontal cortex is sensitive to deviations from expected outcomes (Pagnoni et al., 2002; Pasupathy & Miller, 2005) and is linked to the rapid updating of expectations following prediction errors (Silvetti, Nunez Castellar, Roger, & Verguts, 2014). Abnormal functioning of striatal and frontal systems are also thought to manifest as difficulty in prediction and valuation of reward outcomes (Just, Keller, Malave, Kana, & Varma, 2012; Schmitz et al., 2008), systems found to be atypical in persons with ASD and mice with autistic-like behaviors (Peça et al., 2011; Thakkar et al., 2008).
The feedback related negativity (FRN) is a well-established EEG event-related potential (ERP) measure. Broadly, the FRN is sensitive to choice outcomes and is larger (i.e., more negative) to losses and unexpected outcomes than to gains and expected outcomes (Gehring & Willoughby, 2002; Hajcak, Moser, Holroyd, & Simons, 2006; Holroyd, Nieuwenhuis, Yeung, & Cohen, 2003; Oliveira, McDonald, & Goodman, 2007). The FRN has been localized to medial-frontal cortex (Gehring & Willoughby, 2002; Holroyd & Coles, 2002; Nieuwenhuis, Slagter, von Geusau, Heslenfeld, & Holroyd, 2005; Ruchsow, Grothe, Spitzer, & Kiefer, 2002), as well as posterior cingulate, fusiform gyrus, and superior temporal gyrus (De Pascalis, Varriale, & D’Antuono, 2010). A recent study, using intracranial recordings, provides more conclusive evidence that the medial prefrontal cortex generates the FRN, where larger amplitude and greater theta-band phase coupling are found compared to lateral prefrontal regions (Smith et al., 2015). In the context of reinforcement learning, the FRN signal may reflect brain processes involved in facilitating behavioral adaptation when external outcomes are not consistent with predictions (Cavanagh, Frank, Klein, & Allen, 2010; Hajcak et al., 2006; van de Vijver, Ridderinkhof, & Cohen, 2011). The FRN is also sensitive to social rejection cues and explicit violations in social expectancy (Sun & Yu, 2014), outcomes which are relevant in ASD and may relate to the commonly observed social deficits and atypical processing of reward feedback.
Impairment in appreciation for choice outcomes could contribute to several core symptoms of ASD (Sinha et al., 2014). However, support for this idea using ERPs has been mixed, perhaps due to reward paradigms that differ with respect to probabilistic and learning features as well as whether feedback is concrete or abstract (see Hüpen, Groen, Gaastra, Tucha, & Tucha, 2016). Two studies by Crowley and colleagues (Larson, South, Krauskopf, Clawson, & Crowley, 2011; McPartland et al., 2012), one dataset which is included in the current study, did not find reliable differences in FRN amplitude between age and IQ-matched cohorts of ASD and neurotypical controls. On the other hand, another study documented a reduced FRN among individuals with subthreshold ASD compared to controls (Groen et al., 2008). Given that individuals with ASD show reduced sensitivity to outcomes that inform optimal response strategies (i.e., making errors; Jeste & Nelson, 2009; South, Larson, Krauskopf, & Clawson, 2010), and may be less motivated to attend to and appreciate rewards (Kohls et al., 2013), it is possible that impairment in prediction and atypical reward processing in ASD, not consistently evident in ERPs, may be detected with an oscillatory dynamics approach (Delorme & Makeig, 2004). However, few if any studies have considered rapid oscillatory dynamics of EEG as an indicator of neural synchrony in response to feedback processing.
ERP studies used a fixed-latency approach to mark specific time windows, often averaging signal across trials and/or subjects. This approach ignores other useful information in the EEG signal including spectral information (distribution in the strength of variation across frequencies) and event-related oscillatory dynamics (neural rhythms) (Le Van Quyen & Bragin, 2007; Sauseng et al., 2007). Measures of spectral power (from event-related spectral perturbations, ERSPs) and phase (inter-trial coherence, ITC) offer a somewhat more nuanced interpretation of the EEG signal that can target specific frequency ranges. ERSPs are a temporally sensitive index of the relative change of mean EEG power from baseline associated with stimulus presentation or response execution that may not show temporal stability. The ITC reflects the temporal and spectral synchronization within EEG and captures the consistency in phase alignment of neuronal activity that is elicited by task events. ITC is direct measure of trial-to-trial cortical synchrony, not available in the ERP waveform (Delorme & Makeig, 2004) and, together with ERSP, could help clarify whether feedback processing is atypical in ASD.
In particular, ITC can provide information on alignment or synchrony of brain activity in response to feedback, such as that provided by an experimental task or in everyday social situations. ITC is sensitive to rapid neural changes across time in response to a stimulus, including a feedback event. Previous research has suggested that that intra-individual variability may be higher in ASD (Dinstein et al., 2012; Haigh et al., 2015). Some evidence suggest that within-subject variability in the amplitude and timing of early visual P1 ERPs is greater in ASD compared to neuro-typical matched controls (Milne, 2011). Others have examined neural variability in youth and young adults with ASD and found low coherence across multiple frequency bands during resting states (Dinstein et al., 2011; Lushchekina, Khaerdinova, Novototskii-vlasov, & Lushchekin, 2016), stimulus processing (Catarino et al., 2013), and cognitive tasks (Lushchekina et al., 2016), leading some to suggest that a lack of synchrony in neural oscillations reflects an endophenotype of ASD (David et al., 2016; Schwartz, Kessler, Gaughan, & Buckley, 2016). Thus, we consider whether this variability could also be present in the FRN and reflect a lack of synchronization in medial frontal neural systems that mediate feedback processing.
In the present study we assess the spectral power shown by ERSP and phase coherence shown by the ITC, in ASD participants vis-à-vis a typical comparison group during a reward feedback processing task. We focus on the theta EEG frequency range (4–8 Hz) because a growing body of evidence shows that several medial frontal negativities, including the FRN, oscillate in this range and reflect changes in both spectral power and phase coherence (Cavanagh et al., 2010; Hajihosseini & Holroyd, 2013; Luft, Nolte, & Bhattacharya, 2013; Nigbur, Ivanova, & Stürmer, 2011; Trujillo & Allen, 2007; van Noordt, Campopiano, & Segalowitz, 2016). This study expands on our previous report of FRN data for the same participants (Larson et al., 2011), but employs time-frequency analysis of event-related EEG dynamics to quantify medial frontal theta ESRP and ITC across desirable and punitive outcomes in the task.
Materials and Methods
Participants
Enrollment and data acquisition were conducted at Brigham Young University as approved by the Institutional Review Board. The initial study enrollment included sixty-five participants, ranging from youth to young adults. However, EEG assessment was not performed on nine participants. In addition, seven participants (four neurotypical controls, three ASD) were excluded from subsequent analysis due to having fewer than 15 artifact-free EEG trials when using the large segmentation window (+/− 1000 ms) for spectral analyses. Thus, the current sample included 27 individuals with an ASD diagnosis (mean age = 14.1, SD = 2.57, range 9 to 21, 24 male), and 22 neurotypical controls (mean age = 13.98, SD = 2.80, 8 to 18, 20 male), matched on the range and distribution of age and IQ scores.
ASD participants all scored above the recommended cutoff of 7 on the Autism Diagnostic Observation Schedule (Lord et al., 2000) administered by a clinician trained to research reliability, and also scored above the recommended cutoff of 12 on the parent-report Social Communication Questionnaire (Corsello et al., 2007). Demographic information for study variables are listed in Table 1.
Table 1.
Demographics for Study Variables
| ASD | Control | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| M | SD | M | SD | t | p | |
| Age | 14.08 | 2.57 | 13.98 | 2.80 | .13 | .99 |
| FSIQ | 107.78 | 14.28 | 110.27 | 14.88 | −.60 | .55 |
| ADOS | 12.15 | 3.93 | – | – | ||
| SCQ | 21.17 | 5.49 | – | – | ||
| Theta 4–8 Hz | ||||||
| ERSP lose | 3.21 | 1.43 | 3.63 | 1.78 | −.91 | .37 |
| ERSP win | 2.68 | 1.63 | 2.45 | 1.11 | .57 | .57 |
| .02* | ||||||
| ITC lose | 0.37 | 0.13 | 0.46 | 0.14 | −2.37 | |
| ITC win | 0.30 | 0.12 | 0.37 | 0.11 | −2.34 | .02* |
FSIQ = Full Scale IQ, ADOS = Autism Diagnostic Observation Schedule, SCQ = Social Communication Questionnaire, ERSP= event-related spectral perterbation, ITC = intertrial coherence;
p<.05
Feedback-Reward Paradigm
Participants played a variation of the balloon gain context task, originally developed by Holroyd (Holroyd et al., 2003), and subsequently adapted for children by Crowley and colleagues (Crowley et al., 2009). Four colored balloon images (blue, green, red, and orange) appeared horizontally, in random order, in the center of the screen. Participants began the task with zero coins and were instructed on each trial to select a balloon to try to gain a coin worth a gain of 25 cents (see Figure 1). Incorrect choices were punished with a loss of 25 cents. Win feedback consisted of a green “$” on a black background, whereas Lose feedback was presented as a red “X” on a black background. The mean luminance for pixels that contained color values (i.e., non-black pixels) for Win and Lose feedback was .84 and .86, respectively. As a proxy for contrast, we calculated ratio of luminance range to mean luminance to be 1.09 and 1.05 for Win and Lose feedback, respectively. Unknown to the participants, feedback was rigged with a random 50% chance of winning (WIN) and a 50% chance of losing (LOSE) money. There was no pattern for certain colors or orders predicting specific outcomes. The balloons remained on-screen until the participant made a selection. Feedback appeared 1000ms after balloon selection and lasted 800ms, with a 700ms inter-trial interval. Participants played 2 blocks of 72 consecutive trials, (144 outcomes total). Each block began with 10–12 WIN trials to avoid frustration. All participants were debriefed and compensated the same fixed amount at the conclusion of the experiment.
Figure 1.
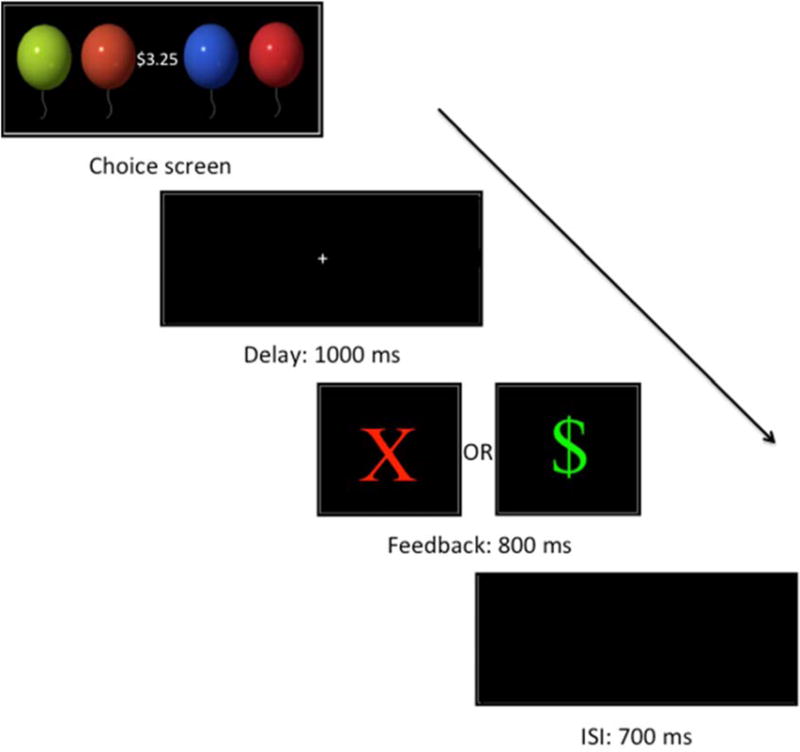
Balloon Feedback Prediction Task. A red “X” indicates a 25¢ loss; a “$” indicates a 25¢ win.
Data Collection
EEG recordings were acquired on a high-density Electrical Geodesics, Inc. (EGI; Eugene, OR) system using 128 channel hydrocel sensor nets and NetStation software (v4.4). The data were initially referenced to Cz and sampled at 250 Hz. All impedances were maintained below 50 kΩ. Offline, the data were re-referenced to a standard average (Junghöfer, Elbert, T ucker, & Braun) and processed sequentially through a 0.1 Hz high-pass filter and a 30 Hz low-pass filter. The continuous recordings were segmented into 2-second epochs, corresponding to a 1000 ms pre-stimulus and a 1000 ms post-stimulus interval. Pre-processing of the data and artifact rejection was performed using NetStation (v4.4) to remove segments containing extreme voltage fluctuations (threshold 200 μV) or muscle activity association with saccades and eye blinks (threshold 150 μV). Epochs with any eye blink or eye movement (threshold 150 μV) were rejected. Epochs with more than 10 bad channels were rejected. A channel was interpolated from surrounding sites if it was marked bad on more than 40% of trials. The single trial data were re-referenced to an average reference of all electrodes because the latter is thought to be a better representation of a true zero (Junghofer, Elbert, Tucker, & Braun, 1999). The data were baseline corrected using the 200 ms pre-stimulus interval.
EEG Oscillations
ERSP and ITC were computed with EEGLab version 11.0.4.3 in MATLAB version R2012b; subsequent statistical analysis was performed in SPSS version 19. Given that theta oscillations have been linked to medial frontal sources (Asada, Fukuda, Tsunoda, Yamaguchi, & Tonoike, 1999), and are typically maximal at frontal midline sites during the FRN (site Fz in the 10–20 system; Cavanagh, Figueroa, Cohen, & Frank, 2012; Cavanagh et al., 2010; Gehring & Willoughby, 2002; van den Bos, Cohen, Kahnt, & Crone, 2012) we extracted the average signal across four electrodes (5, 6 (Fz), 11, and 12) in the frontal midline region (see Figure 2).
Figure 2.
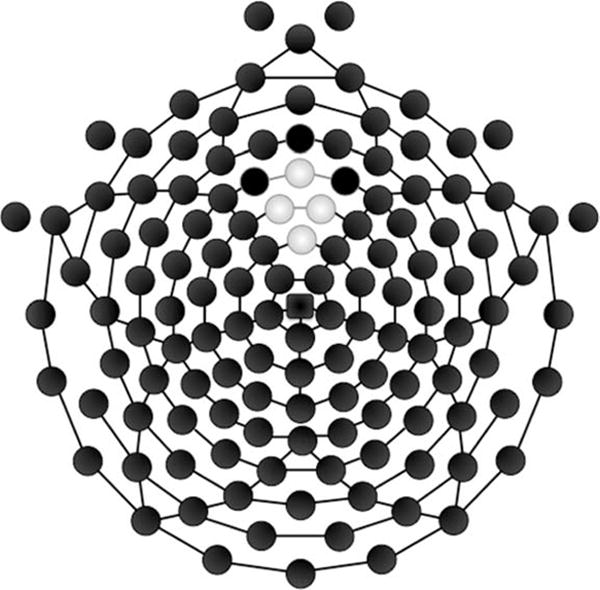
128 channel Hydrocel geodesic sensor net. The electrodes shaded in gray were used in the present analysis corresponding to channels 11 (Fz), 12, 5, and 6.
The mean number of available, artifact free trials for the LOSE and WIN conditions were comparable between the neurotypical control group (LOSE mean = 53.18, SD = 13.12; WIN mean = 53.09, SD = 13.27) and the ASD group (LOSE mean = 53.72, SD = 16.20; WIN mean = 54.59, SD = 15.82). A repeated measures ANOVA for trial numbers revealed no reliable differences for condition (F(1, 47) = 0.39, p = 0.54), group (F(1, 47) = 0.11, p = 0.74), or their interaction (F(1, 47) = .60, p = 0.44). Following the work of (Crowley et al., 2014), the EEGLab function “newtimef” was used with default parameter settings (Delorme & Makeig, 2004) to compute ERSP and ITC in the medial frontal channel cluster across 28 linearly spaced frequencies ranging from 3–30 Hz and 220 linearly time points spanning −440 to 440 ms around feedback onset. We focused on the theta frequency range (4 to 8 Hz) and during the 200–400 ms time range as this corresponds to the time window commonly used to capture the FRN. The ERSP and ITC values were averaged for each subject and exported to SPSS for hypothesis testing.
Results
Group by condition ERSP and ITC values are depicted in Figure 3 and Figure 4, respectively, which show that much of the oscillatory activity occurs in the theta band (4–8 Hz) in a temporal range overlaps with the window commonly associated with the FRN. A 2 (condition: LOSE vs. WIN) by 2 (group: Control vs. ASD) repeated measures analysis of variance (ANOVA) was carried out for both ERSP and ITC measures. Given that age related to FRN amplitude in our previous study using this sample, we included age as a covariate in each of the ANOVAs. A Fisher r-to-z transformation to the ITC values was performed prior to the statistical analysis. As with correlations, ITC values are not linearly distributed across their potential range of 0.0–1.0 (e.g., Liu, Woltering, & Lewis, 2014; Roach & Mathalon, 2008). The transformation puts each individual’s ITC value on the same metric. Levene’s test for homogeneity of variances across groups, by condition, indicated the homoscedasticity assumption was met for all comparisons across ERSP and transformed ITC measures (ERSP: LOSE p =.57, WIN p =.16; ITC: LOSE p =.64, WIN p =.76). Table 1 summarizes the means and standard deviations for ERSP and ITC values.
Figure 3.
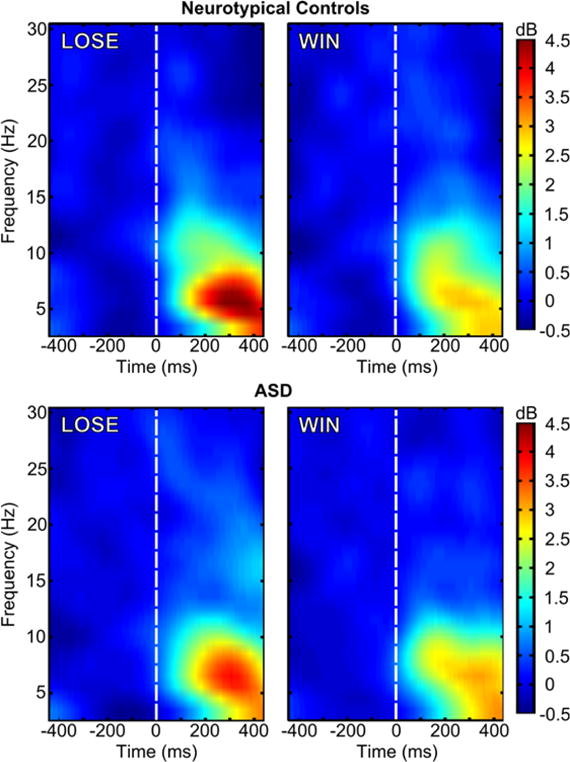
ERSP values for the LOSE (left) and WIN (right) conditions in neurotypical controls (top) and ASD. Feedback stimulus onset is marked by the dashed white line, t=0 ms.
Figure 4.
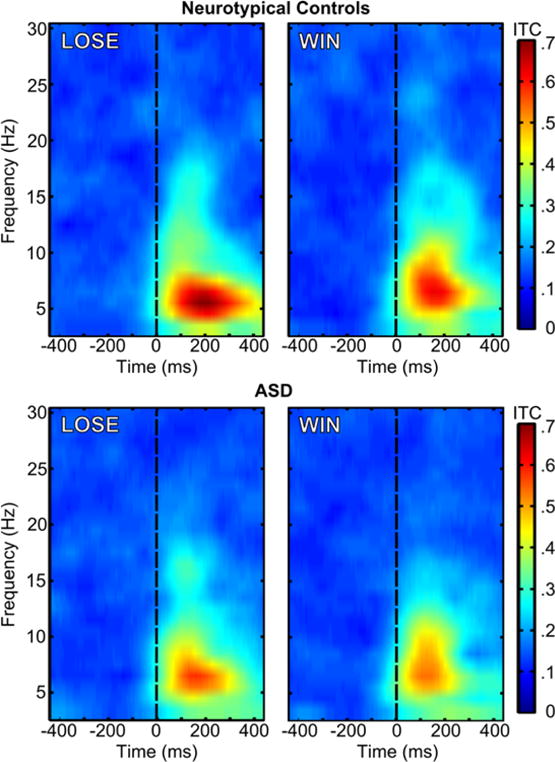
ITC values for the LOSE (left) and WIN (right) conditions for neurotypical controls (top) and ASD (bottom). Feedback stimulus onset is marked by the dashed black line, t=0 ms.
Table 1.
Means and Standard Deviations for EEG Spectral Measures by Group and Condition
| Group | |||
|---|---|---|---|
| ASD | Control | ||
| ERSP | |||
| Win | 2.45 (1.12) | 2.68 (1.63) | |
| Lose | 3.21 (1.43) | 3.63 (1.78) | |
|
| |||
| ITC | |||
| Win | .29 (.12) | .36 (.11) | |
| Lose | .35 (.13) | .43 (.14) | |
There was a significant condition effect for ESRP (F(1, 47) = 14.39, p <0.001, partial η2 = .23), such that theta power in the 200–400 ms time range was greater in the LOSE compared to the WIN condition (Figure 3). There was no evidence of a reliable group effect, irrespective of condition (F(1, 47) = 0.06, p = 0.80, partial η2 = 001), nor a condition-by-group interaction with respect to medial frontal theta power (F(1, 47) = 2.12, p = 0.15, partial η2 = 04).
Next we examined ITC, as a measure of coherence of neural oscillations across time (trial-to-trial cortical synchrony). We observed a significant main effect for condition (F(1, 47) = 11.00, p = 0.002, partial η2 = .19) similar to the results for theta power, indicating that ITC was higher in the LOSE compared to the WIN condition between 200–400 ms post-feedback. There was a reliable between-groups difference in ITC between the groups (F(1, 47) = 9.24, p = 0.004, partial η2 = .16). Specifically, the TYP group showed more consistent trial-to-trial phase locking of theta rhythms (greater overall ITC), in response to feedback across trials (Figure 4). The condition by group interaction was not significant (F(1, 47) = 0.11, p = .74, partial η2 = .002).
Given the potential for developmental changes in reward processing, we tested whether theta power and coherence varied as a function of age. We found no evidence that age is reliably correlated with theta ERSP or ITC in either condition in our sample (p’s > .08). Examining the association between age and medial frontal theta separately for each group revealed a significant negative correlation for ITC during the 200–400 ms window, specifically for win feedback in persons with ASD. Thus, we entered age as a covariate in a repeated measures ANOVA model, and found that ITC in persons with ASD was still significantly lower than neurotypical controls (F (1, 46) = 9.09, p < .004, partial η2 = .17). There were no significant associations between age and theta ERSP or ITC in the neurotypical controls (p’s > .51).
We also performed repeated measures ANOVAs as exploratory analysis of alpha (8–12 Hz) and beta (13–20 Hz) frequency. For alpha ITC in the 200–400 ms window, we did not observe any reliable differences between conditions (F(1,47) = 1.32, p > .05, partial η2 = .03), groups (F(1,47) = 0.24, p > .05, partial η2 = .01), or the interaction between feedback type and group (F(1,47) = 0.19, p > .05, partial η2 = .04). Similar results were observed for beta ITC such that there were no reliable differences between conditions (F(1,47) = 1.40, p > .05, partial η2 = .03), groups (F(1,47) = 0.03, p > .05, partial η2 = .01), and their interaction (F(1,47) = 1.24, p > .05, partial η2 = .03).
Discussion
We examined medial frontal theta ERSP and ITC during feedback processing in an ASD cohort and matched controls. We found that, compared to WIN outcomes, LOSE outcomes were associated with greater event-related spectral power (ERSP) and phase coherence (ITC) across trials in the theta range. Our findings for ERSP are consistent with prior studies of FRN-related tasks, which have documented greater theta power for loss compared to gain feedback during the time window of the FRN (Cavanagh, Zambrano-Vazquez, & Allen, 2012; Cohen, Elger, & Ranganath, 2007; Cohen, Elger, & Fell, 2009). We did not observe a group-by-condition interaction for spectral power or ITC. However, compared to neurotypical individuals, those with ASD showed less trial-to-trial phase locking of theta rhythms (lower overall ITC, less consistency in response) irrespective of feedback type. Given that some studies show that ASD patients and controls have similar FRN amplitudes to reward and punishment feedback (Larson et al., 2011; McPartland et al., 2012), these data underscore the importance of also measuring the consistency of trial-to-trial phase alignment as a measure of neural synchrony. In a reward prediction task we show a unique difference, lower trial-to-trial phase locking in ASD, consistent with several studies highlighting a lack of neural synchrony as an endophenotype in ASD (Catarino et al., 2013; David et al., 2016; Dinstein et al., 2011; Lushchekina et al., 2016; Schwartz et al., 2016). Our findings point to further evidence for reduced ITC in ASD and the benefit of examining more nuanced measures in EEG studies that can differentiate ASD from neurotypical controls.
An emerging body of evidence suggests that ASD is linked to inconsistency in neural responses. Functional neuroimaging studies find that, compared to controls, sensory evoked responses are more variable in ASD across visual, somatosensory, and auditory domains (Dinstein et al., 2012; Haigh & Heeger, 2015). Other work reports that ASD is related to reduced coherence, as measured by functional connectivity, between posterior and frontal systems when individuals with ASD perform tasks related to executive function (Just, Cherkassky, Keller, Kana, & Minshew, 2007), inhibition (Kana, Keller, Minshew, & Just, 2006; Solomon et al., 2009), learning (Schipul & Adam, 2016), language (Just, Cherkassky, Keller, & Minshew, 2004; Mizuno et al., 2011), and social processing (Kana et al., 2009). Furthermore, Milne (2011) found that ASD is associated with greater variability in P1 ERP responses and reduced synchrony in the phase locking of alpha oscillations during visual processing of Gabor patches. Although focused on alpha and gamma bands, others have shown reduced ITC in persons with ASD (Edgar et al. 2015; Gandal et al. 2010; Milne, 2011; Rojas et al. 2008; Sun et al. 2012). Our work extends the findings of neural variability from the sensory and cognitive domains to the area of reward feedback prediction, highlighting reduced medial frontal theta trial-to-trial synchrony in persons with ASD.
Much evidence implicates reduced neural synchronization in ASD (Catarino et al., 2013; Dinstein et al., 2011; Lushchekina et al., 2016). The variability in theta we observed in persons with ASD aligns with recent work by Sinha and colleagues (Sinha et al., 2014), who proposed that a common underlying deficit within the autism phenotype is poor predictive abilities. Extracting and refining accurate conditional probabilities is influenced by the strength and temporal displacement of successive events (e.g., choice-outcome). Although our task removes the opportunity for individuals to learn predictive associations, persons with ASD have been shown to show smaller FRN ERPs, regardless of outcome valence, in both observational and active conditions of a probabilistic reward-learning task (Bellebaum, Brodmann, & Thoma, 2014), suggesting a global reduction in neural synchrony during reward feedback processing. Our findings suggest a specific deficit in theta ITC rather than the FRN which was comparable across ASD and neurotypical individuals (Larson et al., 2011). Importantly, accumulating evidence suggests atypical functioning in reward circuitry in humans and animals models of ASD, including the medial frontal cortex and the striatum (Peça et al., 2011; Thakkar et al., 2008). At a physiological level, our findings show that feedback stimuli do not induce phase re-setting of ongoing theta oscillations to the same extent in persons with ASD compared to neurotypical controls, suggesting a specific deficit in theta ITC rather than the FRN which was comparable across ASD and neurotypical individuals (Larson et al., 2011). Although our results do not speak directly to an explicit impairment in prediction in a learning context among persons with ASD per se, it is possible that, in the context of reward processing, a lack of neural synchronization could impact reinforcement learning and manifest as a limited capacity to predict, and adapt to, stimulus-outcome contingencies in the environment. However, reduced theta ITC in ASD we observed here may also reflect a more general effect of lower coherence in ASD as reviewed earlier. It will be useful for future studies to include paradigms that examine whether neural variability during feedback processing contributes to difficulties in reinforcement learning.
Implications
Although participants with ASD in this sample did show typical sensitivity to the valence of the feedback, as reflected by increased coherence for LOSS more than WIN trials, they showed an overall reduction in phase consistency of theta oscillations following feedback. Greater variability in medial frontal activity to feedback aligns with a recent proposal suggesting that individuals with ASD suffer from a limited capacity to accurately anticipate and predict behavioral outcomes and the timing of events (see Sinha et al., 2014; Pellicano & Burr, 2012). Given the chance-based nature of our task, it is possible that our findings underscore a general impairment in prediction that is reflected in reduced synchronization of medial frontal theta when individuals with ASD are presented feedback about their choices. In this context, ITC to feedback warrants further examination, as reduced coherence in frontal theta could provide a possible link to common factors in ASD, such as the propensity for insistence on sameness. In addition, future studies would benefit from a thorough examination of ASD comorbidity with other disorders that could impact feedback processing, including anxiety, ADHD, OCD, and ODD.
Our focus on theta oscillations during positive and negative feedback outcomes was guided by a large-sample child study (n = 108, Crowley et al., 2014) and adult studies applying win-loss feedback tasks (Cavanagh et al., 2010). Although Figures 3 and 4 illustrate a clear increase in theta power and coherence, these effects also taper off into the alpha frequency range (9–13 Hz). Prior work has shown that reward processing might elicit midfrontal beta activity (Marco-Pallares et al., 2008), although this was not evident from the event-related ESRP and ITC measures in our study. In addition to theta (Yeung, Han, Sze, & Chan, 2016), other studies on ASD have implicated oscillatory activity in both alpha and gamma ranges (Buard et al., 2013; Milne, 2011;Rojas & Wilson, 2014), suggesting that oscillatory measures hold particular relevance to characterization of ASD. Our results provide an important starting point for future studies on medial frontal theta dynamics in prediction, feedback processing, and reinforcement learning in ASD.
Highlights.
EEG oscillations to feedback processing examined in ASD and controls
Power and phase coherence were measured to WIN/LOSE feedback
Phase alignment in medial frontal theta rhythms to feedback was lower in ASD
Trial-to-trial cortical synchrony linked to atypical feedback processing in ASD
Acknowledgments
This research was supported in part by NARSAD Young Investigator Award (MJC), NIDA grant K01 DA034125 (MJC), and R01 NS035193 (AV).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest.
References
- Asada H, Fukuda Y, Tsunoda S, Yamaguchi M, Tonoike M. Frontal midline theta rhythms reflect alternative activation of prefrontal and anterior cingulate cortex in humans. Neuroscience Letters. 1999;274(1):39–32. doi: 10.1016/s0304-3940(99)00679-5. [DOI] [PubMed] [Google Scholar]
- Bellebaum C, Brodmann K, Thoma P. Active and observational reward learning in adults with autism spectrum disorder: Relationship with empathy in an atypical sample. Cognitive Neuropsychiatry. 2014;19(3):205–225. doi: 10.1080/13546805.2013.823860. [DOI] [PubMed] [Google Scholar]
- Buard I, Rogers SJ, Hepburn S, Kronberg E, Rojas DC. Altered oscillation patterns and connectivity during picture naming in autism. Frontiers in Human Neuroscience. 2013;7(742):1–11. doi: 10.3389/fnhum.2013.00742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catarino A, Andrade A, Churches O, Wagner AP, Baron-cohen S, Ring H. Task-related functional connectivity in autism spectrum conditions: An EEG study using wavelet transform coherence. Molecular Autism. 2013;4(1):1–14. doi: 10.1186/2040-2392-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ, Klein TJ, Allen JJ. Frontal theta links prediction errors to behavioral adaptation in reinforcement learning. NeuroImage. 2010;49(4):3198–3209. doi: 10.1016/j.neuroimage.2009.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Zambrano-Vazquez L, Allen JJ. Theta lingua franca: a common mid-frontal substrate for action monitoring processes. Psychophysiology. 2012;49(2):220–238. doi: 10.1111/j.1469-8986.2011.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Elger CE, Ranganath C. Reward expectation modulates feedback-related negativity and EEG spectra. NeuroImage. 2007;35(2):968–978. doi: 10.1016/j.neuroimage.2006.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Elger Christian E, Fell Juergen. Oscillatory activity and phase-amplitude coupling in the human medial frontal cortex during decision making. Journal of Cognitive Neuroscience. 2009;21(2):390–402. doi: 10.1162/jocn.2008.21020. [DOI] [PubMed] [Google Scholar]
- Corsello C, Hus V, Pickles A, Risi S, Cook EH, Jr, Leventhal BL, Lord C. Between a ROC and a hard place: decision making and making decisions about using the SCQ. Journal of Child Psychology & Psychiatry. 2007;48(9):932–940. doi: 10.1111/j.1469-7610.2007.01762.x. [DOI] [PubMed] [Google Scholar]
- Crowley MJ, van Noordt SJ, Wu J, Hommer RE, South M, Fearon RM, Mayes LC. Reward feedback processing in children and adolescents: medial frontal theta oscillations. Brain and Cognition. 2014;89:79–89. doi: 10.1016/j.bandc.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley MJ, Wu J, Crutcher C, Bailey CA, Lejuez CW, Mayes LC. Risk-taking and the feedback negativity response to loss among at-risk adolescents. Developmental Neuroscience. 2009;31(1–2):137–148. doi: 10.1159/000207501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David N, Schneider T, Peiker I, Al-Jawahiri R, Engel A, Milne E. Variability of cortical oscillation patterns: A possible endophenotype in autism spectrum disorders? Neuroscience and Biobehavioral Reviews. 2016;71:590–600. doi: 10.1016/j.neubiorev.2016.09.031. [DOI] [PubMed] [Google Scholar]
- De Pascalis V, Varriale V, D’Antuono L. Event-related components of the punishment and reward sensitivity. Clinical Neurophysiology. 2010;121(1):60–76. doi: 10.1016/j.clinph.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Green SR, Rittenberg AM, Sasson NJ, Bodfish JW. Reward circuitry function in autism spectrum disorders. Social Cognitive & Affective Neuroscience. 2012;7(2):160–172. doi: 10.1093/scan/nsq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Heeger DJ, Lorenzi L, Minshew NJ, Malach R, Behrmann M. Unreliable evoked responses in Autism. Neuron. 2012;75(6):981–991. doi: 10.1016/j.neuron.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Pierce K, Eyler L, Solso S, Malach R, Behrmann M. Report disrupted neural synchronization in toddlers with Autism. Neuron. 2011;70(6):1218–1225. doi: 10.1016/j.neuron.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar CJ, Roberts TPL. Auditory encoding abnormalities in children with autism spectrum disorder suggest delayed development of auditory cortex. Molecular Autism. 2015;6:69. doi: 10.1186/s13229-015-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank Michael J, Claus Eric D. Anatomy of a decision: striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychol Rev. 2006;113(2):300–326. doi: 10.1037/0033-295X.113.2.300. [DOI] [PubMed] [Google Scholar]
- Gandal MJ, Edgar JC, Ehrlichman RS, Metha M, Roberts TPL, Siegel SJ. Validating γ oscillations and delayed auditory responses as translational biomarkers of autism. Biological Psychiatry. 2010;68:1100–1106. doi: 10.1016/j.biopsych.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring William J, Willoughby Adrian R. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295(5563):2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: Developmental disconnection syndromes. Current Opinions Neurobiology. 2007;17(1):103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Glerean E, Pan RK, Salmi J, Kujala R, Lahnakoski JM, Roine U, Jaaskelainen IP. Reorganization of functionally connected brain subnetworks in high-functioning autism. Human Brain Mapping. 2016;37(3):1066–1079. doi: 10.1002/hbm.23084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen Y, Wijers AA, Mulder LJ, Waggeveld B, Minderaa RB, Althaus M. Error and feedback processing in children with ADHD and children with Autistic Spectrum Disorder: an EEG event-related potential study. Clinical Neurophysiology. 2008;119(11):2476–2493. doi: 10.1016/j.clinph.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Haigh SM, Heeger DJ, Dinstein I, Minshew N, Behrmann N. Cortical variability in the sensory-evoked response in Autism. Journal of Autism and Developmental Disorders. 2015;45(5):1176–1190. doi: 10.1007/s10803-014-2276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biological Psychology. 2006;71(2):148–154. doi: 10.1016/j.biopsycho.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Hajihosseini Azadeh, Holroyd Clay B. Frontal midline theta and N200 amplitude reflect complementary information about expectancy and outcome evaluation. Psychophysiology. 2013;50(6):550–562. doi: 10.1111/psyp.12040. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109(4):679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Nieuwenhuis S, Yeung N, Cohen JD. Errors in reward prediction are reflected in the event-related brain potential. Neuroreport. 2003;14(18):2481–2484. doi: 10.1097/00001756-200312190-00037. [DOI] [PubMed] [Google Scholar]
- Hüpen P, Groen Y, Gaastra GF, Tucha L, Tucha O. Performance monitoring in autism spectrum disorders: A systematic literature review of event-related potential studies. International Journal of Psychophysiology. 2016;102:33–46. doi: 10.1016/j.ijpsycho.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Ingersoll B, Schreibman L, Tran QH. Effect of sensory feedback on immediate object imitation in children with autism. ournal of Autism and Developmental Disorders. 2003;33(6):673–683. doi: 10.1023/b:jadd.0000006003.26667.f8. [DOI] [PubMed] [Google Scholar]
- Jeste SS, Nelson CA., 3rd Event related potentials in the understanding of autism spectrum disorders: an analytical review. Journal of Autism and Developmental Disorders. 2009;39(3):495–510. doi: 10.1007/s10803-008-0652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z. Study on EEG power and coherence in patients with mild cognitive impairment during working memory task. Journal of Zhejiang University Science. 2005;6(12):1213–1219. doi: 10.1631/jzus.2005.B1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghofer M, Elbert T, Tucker DM, Braun C. The polar average reference effect: a bias in estimating the head surface integral in EEG recording. Clinical Neurophysiology. 1999;110(6):1149–1155. doi: 10.1016/s1388-2457(99)00044-9. [DOI] [PubMed] [Google Scholar]
- Junghöfer M, Elbert T, Tucker DM, Braun C. The polar average reference effect: a bias in estimating the head surface integral in EEG recording. Clinical Neurophysiology. 110(6):1149–1155. doi: 10.1016/s1388-2457(99)00044-9. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in Autism: Evidence from an fMRI study of an executive function task and corpus callosum morphometry. Cerebral Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Just Marcel Adam, Keller Timothy A, Malave Vicente L, Kana Rajesh K, Varma Sashank. Autism as a neural systems disorder: A theory of frontal-posterior underconnectivity. Neuroscience and Biobehavioral Reviews. 2012;36(4):1292–1313. doi: 10.1016/j.neubiorev.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G, Schulte-Ruther M, Nehrkorn B, Muller K, Fink GR, Kamp-Becker I, Konrad K. Reward system dysfunction in autism spectrum disorders. Social Cognitive & Affective Neuroscience. 2013;8(5):565–572. doi: 10.1093/scan/nss033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Nancy J, Just MA, Kana RK, Nancy J. Atypical frontal-posterior synchronization of Theory of Mind regions in autism during mental state attribution. Social Neuroscience. 2009;4(2):135–152. doi: 10.1080/17470910802198510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory control in high-functioning Autism: decreased activation and underconnectivity in inhibition networks. Biological Psychiatry. 2006;62:198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen M, Bos D, Noordermeer SD, Nederveen H, van Engeland H, Durston S. Changes in the development of striatum are involved in repetitive behavior in autism. Biological Psychiatry. 2014;76(5):405–411. doi: 10.1016/j.biopsych.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Larson MJ, South M, Krauskopf E, Clawson A, Crowley MJ. Feedback and reward processing in high-functioning autism. Psychiatry Res. 2011;187(1–2):198–203. doi: 10.1016/j.psychres.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Le Van Quyen M, Bragin A. Analysis of dynamic brain oscillations: Methodological advances. Trends in Neurosciences. 2007;30(7):365–373. doi: 10.1016/j.tins.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Liu ZX, Woltering S, Lewis MD. Developmental changes in EEG theta activity in the medial prefrontal cortex during response control. NeuroImage. 2014;85:873–887. doi: 10.1016/j.neuroimage.2013.08.054. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Rutter M. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Luft CD, Nolte G, Bhattacharya J. High-learners present larger mid-frontal theta power and connectivity in response to incorrect performance feedback. The Journal of Neuroscience. 2013;33(5):2029–2038. doi: 10.1523/JNEUROSCI.2565-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lushchekina EA, Khaerdinova OY, Novototskii-vlasov VY, Lushchekin VS. Synchronization of EEG rhythms in baseline conditions and during counting in children with Autism Spectrum Disorders. Neuroscience and Biobehavioral Psychology. 2016;46(4):382–389. [Google Scholar]
- Marco-Pallares J, Cucurell D, Cunillera T, Garcia R, Andres-Pueyo A, Munte TF, Rodriguez-Fornells A. Human oscillatory activity associated to reward processing in a gambling task. Neuropsychologia. 2008;46(1):241–248. doi: 10.1016/j.neuropsychologia.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Makeig S, Debener S, Onton J, Delorme A. Mining event-related brain dynamics. Trends in Cognitive Science. 2004;8(5):204–210. doi: 10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
- McPartland JC, Crowley MJ, Perszyk DR, Mukerji CE, Naples AJ, Wu J, Mayes LC. Preserved reward outcome processing in ASD as revealed by event-related potentials. Journal of Neurodevelopmental Disorders. 2012;4(1):16. doi: 10.1186/1866-1955-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne E. Increased intra-participant variability in children with Autistic Spectrum Disorders: Evidence from single-trial analysis of evoked EEG. Frontiers in Psychology. 2011;2(51):1–12. doi: 10.3389/fpsyg.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno A, Liu Y, Williams DL, Keller TA, Minshew NJ, Just MA. The neural basis of deictic shifting in linguistic perspective-taking in high-functioning autism. Brain. 2011;134(Pt 8):2422–2435. doi: 10.1093/brain/awr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Slagter HA, von Geusau NJ, Heslenfeld DJ, Holroyd CB. Knowing good from bad: differential activation of human cortical areas by positive and negative outcomes. European Journal of Neuroscience. 2005;21(11):3161–3168. doi: 10.1111/j.1460-9568.2005.04152.x. [DOI] [PubMed] [Google Scholar]
- Nigbur Roland, Ivanova Galina, Stürmer Birgit. Theta power as a marker for cognitive interference. Clinical Neurophysiology. 2011;122(11):2185–2194. doi: 10.1016/j.clinph.2011.03.030. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38(2):329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- Oliveira FT, McDonald JJ, Goodman D. Performance monitoring in the anterior cingulate is not all error related: Expectancy deviation and the representation of action-outcome associations. Journal of Cognitive Neuroscience. 2007;19(12):1994–2004. doi: 10.1162/jocn.2007.19.12.1994. [DOI] [PubMed] [Google Scholar]
- Pagnoni G, Zink CF, Montague PR, Berns GS. Activity in human ventral striatum locked to errors of reward prediction. Nature Neuroscience. 2002;5(2):97–98. doi: 10.1038/nn802. [DOI] [PubMed] [Google Scholar]
- Panasiti MS, Puzzo I, Chakrabarti B. Autistic traits moderate the impact of reward learning on social behaviour. Autism Research. 2015 doi: 10.1002/aur.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasupathy A, Miller EK. Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature. 2005;433(7028):873–876. doi: 10.1038/nature03287. [DOI] [PubMed] [Google Scholar]
- Peça J, Ting JT, Wang W, Wells MF, Venkatraman TN, Lascola C, Guoping F. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicano E, Burr D. When the world becomes ‘too real’: A Bayesian explanation of autistic perception. Trends in Cognitive Science. 2012;16(10):504–510. doi: 10.1016/j.tics.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Yang DY, McPartland JC. Building a social neuroscience of autism spectrum disorder. Current Topics in Behavioral Neurosciences. 2014;16:215–233. doi: 10.1007/7854_2013_253. [DOI] [PubMed] [Google Scholar]
- Rademacher L, Schulte-Ruther M, Hanewald B, Lammertz S. Reward: From Basic Reinforcers to Anticipation of Social Cues. Current Topics in Behavioral Neurosciences. 2016 doi: 10.1007/7854_2015_429. [DOI] [PubMed] [Google Scholar]
- Roach BJ, Mathalon DH. Event-related EEG time-frequency analysis: An overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophrenia Bulletin. 2008;34(5):907–926. doi: 10.1093/schbul/sbn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas DC, Maharajh K, Teale P, Rogers SJ. Reduced neural synchronization of gamma-band MEG oscillations in first-degree relatives of children with autism. BMC Psychiatry. 2008;8:66. doi: 10.1186/1471-244X-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas DC, Wilson LB. Gamma-band abnormalities as markers of autism spectrum disorders. Biomarkers in Medicine. 2014;8(3):353–368. doi: 10.2217/bmm.14.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchsow M, Grothe J, Spitzer M, Kiefer M. Human anterior cingulate cortex is activated by negative feedback: evidence from event-related potentials in a guessing task. Neuroscience Letters. 2002;325(3):203–206. doi: 10.1016/s0304-3940(02)00288-4. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Gruber WR, Hanslmayr S, Freunberger R, Doppelmayr M. Are event-related potential components generated by phase resetting of brain oscillations? A critical discussion. Neuroscience. 2007;146(4):1435–1444. doi: 10.1016/j.neuroscience.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Schipul SE, Adam M. Diminished neural adaptation during implicit learning in autism. NeuroImage. 2016;125:332–341. doi: 10.1016/j.neuroimage.2015.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz N, Rubia K, van Amelsvoort T, Daly E, Smith A, Murphy DG. Neural correlates of reward in autism. The British Journal of Psychiatry. 2008;192(1):19–24. doi: 10.1192/bjp.bp.107.036921. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annual Review of Neuroscience. 2000;23(1):473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Kessler R, Gaughan T, Buckley AW. EEG coherence patterns in Autism: An updated review. Pediatric Neurology. 2016 doi: 10.1016/j.pediatrneurol.2016.10.018. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepeta L, Tsuchiya N, Davies MS, Sigman M, Bookheimer SY, Dapretto M. Abnormal social reward processing in autism as indexed by pupillary responses to happy faces. Journal of Neurodevelopmental Disorders. 2012;4(1):17–17. doi: 10.1186/1866-1955-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvetti M, Nunez Castellar E, Roger C, Verguts T. Reward expectation and prediction error in human medial frontal cortex: an EEG study. NeuroImage. 2014;84:376–382. doi: 10.1016/j.neuroimage.2013.08.058. [DOI] [PubMed] [Google Scholar]
- Sinha P, Kjelgaard MM, Gandhi TK, Tsourides K, Cardinaux AL, Pantazis D, Held RM. Autism as a disorder of prediction. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(42):15220–15225. doi: 10.1073/pnas.1416797111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith XEH, Banks XGP, Mikell CB, Cash XSS, Patel SR, Eskandar EN, Sheth SA. Frequency-dependent representation of reinforcement-related information in the human medial and lateral prefrontal cortex. The Journal of Neuroscience. 2015;35(48):15827–15836. doi: 10.1523/JNEUROSCI.1864-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Ozonoff SJ, Ursu S, Ravizza S, Cummings N, Ly S, Carter CS. The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia. 2009;47:2515–2526. doi: 10.1016/j.neuropsychologia.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South M, Larson MJ, Krauskopf E, Clawson A. Error processing in high-functioning Autism Spectrum Disorders. Biological Psychology. 2010;85(2):242–251. doi: 10.1016/j.biopsycho.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Sun L, Grützner C, Bölte S, Wibral M, Tozman T, Schlitt S, Poustka F, Singer W, Freitag CM, Uhlhaas PJ. Impaired gamma-band activity during perceptual organization in adults with autism spectrum disorders: Evidence for dysfunctional network activity in frontal–posterior cortices. Journal of Neuroscience. 2012;32:9596–9573. doi: 10.1523/JNEUROSCI.1073-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Yu R. The feedback related negativity encodes both social rejection and explicit social expectancy violation. Frontiers in Human Neuroscience. 2014;8:556. doi: 10.3389/fnhum.2014.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RS, Barto AG. Reinforcement learning: An introduction. Cambridge, Mass.: MIT Press; 1998. [Google Scholar]
- Trujillo LT, Allen JJB. Theta EEG dynamics of the error-related negativity. Clinical Neurophysiology. 2007;118(3):645–668. doi: 10.1016/j.clinph.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Thakkar KN, Polli FE, Joseph RM, David ST, Barton JJS, Manoach DS. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD) Brain. 2008;131:2464–2478. doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos W, Cohen MX, Kahnt T, Crone EA. Striatum-medial prefrontal cortex connectivity predicts developmental changes in reinforcement learning. Cerebral Cortex. 2012;22(6):1247–1255. doi: 10.1093/cercor/bhr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Vijver I, Ridderinkhof KR, Cohen MX. Frontal oscillatory dynamics predict feedback learning and action adjustment. Journal of Cognitive Neuroscience. 2011;23(12):4106–4121. doi: 10.1162/jocn_a_00110. [DOI] [PubMed] [Google Scholar]
- van Noordt S, Campopiano A, Segalowitz SJ. A functional classification of medial frontal negativity ERPs: Theta oscillations and single subject effects. Psychophysiology. 2016;53:1317–1334. doi: 10.1111/psyp.12689. [DOI] [PubMed] [Google Scholar]
- Yeung MK, Han YMY, Sze SL, Chan AS. Abnormal frontal theta oscillations underlie cognitive flexibility deficits in children with high-functioning autism spectrum disorders. Neuropsychology. 2016;30(3):281–295. doi: 10.1037/neu0000231. [DOI] [PubMed] [Google Scholar]
- Yeung MK, Han UMY, Szem SL, Chan AS. Altered right frontal cortical connectivity during facial emotion recognition in children with autism spectrum disorders. Research in Autism Spectrum Disorders. 2014;8(11):1567–1577. [Google Scholar]


