Abstract
Bacterial biofilms play an important role in chronic infections due to high-level tolerance to antibiotics. Thus, it is important to eradicate bacterial cells that are attached to implanted medical devices of different materials. Phagocytosis is a key process of the innate immunity to eliminate invading pathogens. Previous research demonstrated that the efficiency of phagocytosis is affected by the aspect ratio of polymer beads. Recently, we reported that the stiffness of polydimethylsiloxane (PDMS) influences Escherichia coli biofilm formation, and the biofilm cells on stiff (5:1) PDMS are 46.2% shorter than those on soft (40:1) PDMS. Based on these findings, we hypothesized that E. coli cells attached on stiff PDMS can be more effectively removed via phagocytosis. This hypothesis was tested in the present study using viability assays, flow cytometry and cell tracking. The results revealed that shorter E. coli cells detached from stiff PDMS were easier to be phagocytized than the longer cells from soft PDMS surfaces. Furthermore, macrophage cells were found more motile on stiff PDMS surfaces and more effective at phagocytosis of E. coli cells attached on these surfaces. These results may help the design of better biomaterials to reduce fouling and associated infections.
Keywords: Stiffness, Polydimethylsiloxane, Phagocytosis, Bacteria, Biofilm
Introduction
A total of 60 –70% of hospital-acquired infections are associated with medical devices of different materials (Bryers 2008). These infections are normally persistent when bacteria produce an extracellular matrix and establish multicellular structures known as biofilms (Vergidis et al. 2012). Due to the protection of the extracellular matrix and slow growth, biofilm cells can tolerate up to 1,000 times higher concentrations of antibiotics than the planktonic cells of the same strain (Anwar and Patel 1990; Costerton et al. 1987; Khoury et al. 1992; Olsen 2015; Dubois-Brissonnet et al. 2016; Martens et al. 2017).
As a major mechanism of innate immunity, phagocytosis is a receptor-mediated immune response of macrophage cells against invading pathogens (Aderem and Underhill 1999). It plays an important role in stopping bacterial pathogens before a long-term infection can be established. During phagocytosis, macrophage cells recognize pathogens using surface receptors, engulf the pathogens into plasma membrane-derived phagosomes, and eventually eliminate the pathogens using multiple strategies (Tauber 2003; Underhill and Goodridge 2012). While phagocytosis of free-swimming bacteria is well documented, such defense against biofilm cells is not well understood.
Bacteria adhesion and biofilm formation are influenced by material properties, such as surface charge, hydrophobicity, topography, stiffness and surface chemistry (Song et al. 2015). Recently, we reported that Escherichia coli and Pseudomonas aeruginosa attach more on soft (40:1) polydimethylsiloxane (PDMS) surfaces than stiff (5:1) PDMS; we also demonstrated that cells attached to soft surfaces are longer (2.6 ± 0.07 µm vs 1.4 ± 0.03 µm), and more susceptible to antibiotics (Song and Ren 2014).
Previously, phagocytosis has been shown to be affected by the size and shape of the target particles. Champion et al. (2006; 2009) used polymeric particles with 2 – 3 µm diameters to represent bacterial cells with different sizes and shapes. The results showed that longer cells are less susceptible to phagocytosis. Doshi et al. (2010) also reported that the geometry of particles plays an important role in the recognition and clearance by macrophages; and membrane ruffles of macrophages are important to target recognition. Furthermore, Blakney et al. (2012) reported that stiff poly (ethylene glycol)-based hydrogels promote macrophages to spread and secret a number of molecules involved in inflammation. These findings led to our hypothesis that material stiffness affects phagocytosis of attached bacterial cells. To test this hypothesis, E. coli was used as a model organism to study phagocytosis of attached cells during early stage biofilm formation by the human macrophage cell line U937.
Materials and Methods
Macrophage
The human monocyte macrophage cell line U937 (ATCC CRL1593.2. Manassas, VA, USA) was cultured in RPMI medium (ATCC, Manassas, VA, USA) supplemented with 10% fetal bovine serum (FBS) and 5% CO2 at 37°C. To activate the macrophages, 100 ng/mL phorbol 12-myristate 13-acetate (PMA) (Cayman chemical, Ann Arbor, Michigan, USA) was added to the cultures at least 15 h before experiments. The activated macrophages were released from the surface of T-flasks (Falcon, Corning, NY, USA) with ethylenediaminetetraacetic acid (EDTA) (Thermo Fisher Scientific, Waltham, MA, USA) before being tested on biofilm cells.
Bacterial strains and growth conditions
E. coli RP437 (CGSC# 12122) (Parkinson et al. 1982), E. coli RP437/pBAD-mPlum and E. coli RP437/pGLO (Hou et al. 2007) were used in this study. The plasmids of pBAD-mPlum (Addgene, Plasmid #54565) and pGLO (Bio-rad) allow the cells to produce purple and green fluorescence induced by arabinose. Ampicillin and arabinose were supplemented at a concentration of 100 µg/mL and 2 mg/L, respectively, to keep plasmids and induce the fluorescence genes. All strains were routinely grown at 37°C in Lysogeny Broth (Hou et al. 2011) which contains 10 g/L tryptone, 5 g/L yeast extract, and 10 g/L NaCl.
Preparation of PDMS surfaces
PDMS surfaces were prepared using SYLGARD184 Silicone Elastomer Kit (Dow Corning Corporation, Midland, MI). The stiffness was adjusted by varying the mass ratio of base to curing agent as we described previously (Evans et al. 2009; Fuard et al. 2008; Wang 2011). Specifically the base: curing agent ratios (w/w) of 5:1, 20:1 and 40:1 were tested in this study. To prepare PDMS, the elastomer base and curing agent were thoroughly mixed and degassed under vacuum for 40 min. Then the mixture was added into 24 well plates (0.2 mL each), or 35 mm×10 mm petri dishes (0.5 mL each) (Thermo Fisher Scientific, Waltham, MA, USA), cured at 50°C for 24 h, and incubated at room temperature for another 24 h to fully polymerize. Finally, the PDMS surfaces were sterilized by soaking in 200 proof ethanol for 20 min and dried at 50°C for 1 h. All of the sterilized PDMS substrates were stored at room temperature until use.
Bacterial attachment and biofilm formation
E. coli cells from overnight cultures were harvested by centrifugation at 8000 rpm for 3 min at 4°C, washed with fresh phosphate buffered saline (PBS, pH 7.3) three times, and then resuspended in fresh PBS to desired cell density (Thermo Fisher Scientific, Waltham, MA, USA). Cell suspensions were added to 24 well plates or 35 mm×10 mm petri dishes containing sterilized 5:1, 20:1 or 40:1 PDMS surfaces, after a serial dilution (2×, 4×, 8×, 16× and 32×) from a suspension with an optical density at 600 nm (OD600) of 0.01. The biofilm samples were incubated at 37°C for 2 h without shaking, then the PDMS surfaces with attached cells were gently washed three times with fresh PBS (changed to clean PBS for each step). After washing, the medium was changed from PBS to LB to allow the attached cells to grow for 5 h at 37°C. Using different inoculum cell density allowed us to obtain similar numbers of attached cells on soft (40:1), medium (20:1) and stiff (5:1) PDMS surfaces to start biofilm growth, which were determined using the drop plate assay as described previously (Chen et al. 2003).
Effects of E. coli cell size on phagocytosis
Phagocytosis of E. coli cells was studied with two complementary approaches. In the first work flow, macrophages were cultured in petri dishes in RPMI medium supplemented with 10% FBS and 5% CO2 at 37°C; and the attached macrophages were activated by 100 ng/mL PMA for 15 h. E. coli cells after 5-h biofilm growth on PDMS with varying stiffness were released from surfaces by pipetting for 30 s and resuspending in 2 mL PBS. The size of these cells was examined using an inverted fluorescence microscope (AX10, Carl Zeiss, Berlin, Germany). Then, the cells were concentrated using a filter plate (Pall Corporation, Ann Arbor, MI, USA) and resuspended in 300 µL PBS. The E. coli cells were added to activate macrophages and incubated at 37°C for 90 min in 1 mL RPMI medium supplemented with 10% FBS and 5% CO2. After incubation, the cells were harvested by pipetting for 30 s, and the numbers of viable E. coli cells before and after incubation were determined by counting CFU to evaluate the efficiency of phagocytosis. E. coli cells incubated in 1 mL RPMI medium supplemented with 10% FBS but without macrophages were used as control. In the second workflow, each PDMS with 5-h biofilm cells was washed three times with PBS. Then the biofilm cells were challenged with 104 cells/mL macrophages in 3 mL RPMI medium supplemented with 10% FBS and 5% CO2 at 37°C for 90 min to evaluate phagocytosis of surface attached cells. The bacterial cells incubated in 3 mL RPMI medium supplemented with 10% FBS but without macrophages were used as control.
Effects of PDMS stiffness on phagocytosis
Macrophages were cultured on PDMS surfaces with different stiffness in RPMI medium supplemented with 10% FBS and 5% CO2 at 37°C; and the attached macrophages were activated by 100 ng/mL PMA for 15 h. E. coli cells from an overnight culture were added to activated macrophages at 105 cells/mL and incubated in 1 mL RPMI medium supplemented with 10% FBS and 5% CO2 at 37°C for 90 min. After incubation, the cells were harvested by pipetting for 30 s. The numbers of viable E. coli cells before and after incubation were determined by counting CFU to evaluate the efficiency of phagocytosis. E. coli cells incubated in 1 mL RPMI medium supplemented with 10% FBS on different stiffness of PDMS surfaces but without macrophages were used as controls.
Tracking macrophage movement on different PDMS
After activating macrophage for 15 h with PMA, E. coli cells were added to study phagocytosis as described above but the cells were incubated in a custom-made flow cell chamber to allow direct monitoring phagocytosis and the movement of macrophages. The chamber has two compartments: one (7 cm × 4 cm × 1 cm) is filled with water and another (7 cm × 7 cm × 1 cm) is for holding samples under a glass cover. The water temperature was controlled as 37°C using a heating coil; and 5% CO2 was purged into the water before entering the culture compartment. An inverted fluorescence microscope (AX10, Carl Zeiss, Berlin, Germany) was used to monitor phagocytosis and the movement of macrophages in the chamber (Fig. S1). The cell tracks were generated from the acquired images using ImageJ.
Effects of PDMS stiffness on the activity of macrophages
To study the effects on macrophages specifically, U937 cells were cultured in RPMI medium supplemented with 10% FBS and 5% CO2 at 37°C on different PDMS surfaces (5:1, 20:1 and 40:1) for one day first. Then the macrophages cells were activated with 100 ng/mL PMA for 15 h, E. coli cells from an overnight culture were dispersed into 1 mL PBS by pipetting for 30 s, and added to the cultures containing activated macrophages. After incubation for 90 min, the macrophages and bacterial cells on PDMS surfaces were released by pipetting for 30 s. The number of viable E. coli cells after incubation was determined by counting CFU. The macrophages in the co-culture were harvested by centrifugation at 125× g for 5 min, and resuspended in PBS. The number of macrophages that had uptaken bacteria was determined using flow cytometry (FACS Aria II SORP, BD Biosciences). To corroborate the results, phagocytosis on different PDMS surfaces was also directly monitored using an inverted fluorescence microscope (AX10, Carl Zeiss, Berlin, Germany). At least 5 images were randomly taken from each sample to count the total number of macrophages and the number of macrophages that had were associated with E. coli cells.
Statistical analysis
All data are presented as mean ± standard deviation. Statistical significance was assessed with one-way ANOVA followed by Tukey test, or Pearson correlation analysis. Statistical significance was set as P<0.05. All the analyses were performed using SAS 9.4 software (SAS Institute, Cary, NC, USA).
Results
E. coli cells attached on stiff PDMS are more susceptible to phagocytosis
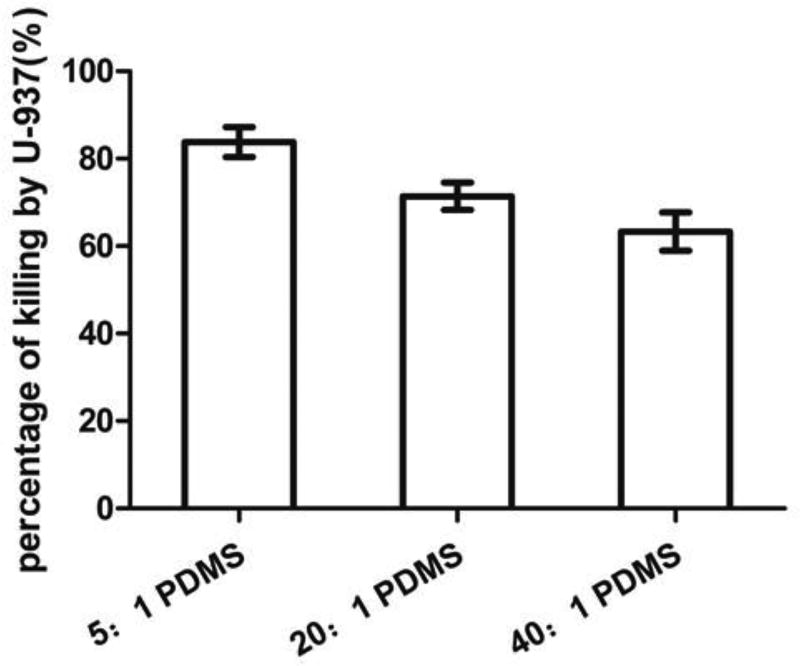
This experiment was conducted by adding U937 cells to E. coli RP437/pGLO biofilms on different PDMS surfaces. The results show that 94.0 ± 2.5% E. coli biofilm cells on stiff (5:1) PDMS were killed by macrophages based on CFU data, while only 59.0 ± 1.3% E. coli biofilm cells on soft (40:1) PDMS were killed (p=0.007, one-way ANOVA; Fig. S1). Although the initial cell density was the same on soft and stiff surfaces, the number of 5-h biofilm cells on soft surfaces was much higher than that on stiff surfaces (107 vs 105 cells/mL), likely due to faster growth on soft PDMS, as we reported previously (Song and Ren 2014). Thus, the ratio of biofilm cells to macrophages on stiff surfaces was lower than that on soft surfaces. To further evaluate the effects of PDMS stiffness without the confounding factor of E. coli cell density, the cell densities of 5-h biofilm cells on soft and stiff PDMS surfaces were controlled to be the same by adjusting the initial cell density in the inocula. We found that inoculum densities of 1.0×107, 1.3×106 and 3.1×105 cells/mL E. coli RP437/pGLO led to similar biofilm cell density after 5 h of incubation on 5:1, 20:1 and 40:1 PDMS surfaces (all around 1.0×104 cells/cm2). As shown in Fig. 1, the number of viable E. coli RP437/pGLO cells decreased due to phagocytosis and the killing effects increased when PDMS got stiffer; e.g. (83.8 ± 3.4) % on 5:1 PDMS, (71.4 ± 3.1) % on 20:1 PDMS, and (63.3 ± 4.4) % on 40:1 PDMS (r=0.94, p<0.001, Pearson correlation analysis).
Fig. 1.
Phagocytosis of 5-h E. coli RP437/pGLO biofilm cells. The graph shows the percentage of killing after incubating with U-937 for 90 min in RPMI medium supplemented with 10% FBS at 37°C and 5% CO2 on 5:1, 20:1 or 40:1 PDMS surfaces (r=0.94, p<0.001, Pearson correlation analysis).
Corroborating the CFU results with biofilm imaging
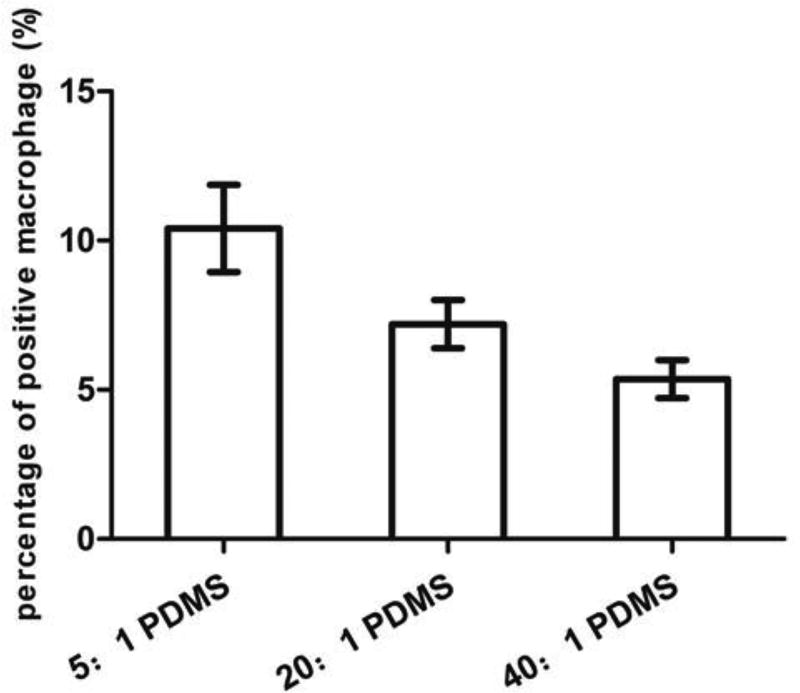
As shown in Fig. 2, the percentage of positive macrophage cells (associated with E. coli cells) based on microscopy was (10.4 ± 1.5)%, (7.2 ± 0.8)% and (5.4 ± 0.6)% on 5:1, 20:1 and 40:1 PDMS, respectively, indicating that phagocytosis increased with PDMS stiffness (r=0.92, p<0.0001, Pearson correlation analysis). This is consistent with the CFU results and supports our hypothesis that phagocytosis is more effective on stiff PDMS under our experimental condition.
Fig. 2.
Percentage of positive macrophage cells (associated with E. coli cells). The graph shows the percentage of positive U-937 cells under microscopy after incubating with 5-h E. coli RP437/pGLO cells for 90 min in RPMI medium supplemented with 10% FBS at 37°C and 5% CO2 on 5:1, 20:1 or 40:1 PDMS surfaces (r=0.92, p<0.0001, Pearson correlation analysis).
Similar results were observed with E. coli RP437/pBAD-mPlum cells
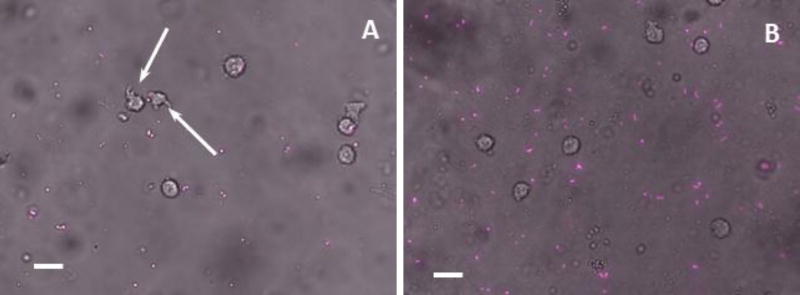
Flow cytometry was used to quantify the number of macrophages involved in phagocytosis. However, U937 macrophage cells were found to have green autofluorescence which interferes with the fluorescence signal from E. coli RP437/pGLO. To address this challenge, we constructed another strain, E. coli RP437/pBAD-mPlum, to detect E. coli cells based on the mPlum, which does not overlap with the GFP spectrum. As shown in Fig. S2, the number of viable E. coli RP437/pBAD-mPlum cells also decreased due to phagocytosis; and the killing effects increased as PDMS got stiffer, e.g. (81.7 ± 9.2)% on 5:1 PDMS, (70.1 ± 5.1)% on 20:1 PDMS and (61.4 ± 18.8)% on 40:1 PDMS (r=0.63, p=0.0019, Pearson correlation analysis). The results of microscopy are shown in Fig. 3. The percentage of positive macrophages (associated with E. coli cells) was (10.9 ± 3.0)%, (7.0 ± 0.8)% and (4.8 ± 1.2)% on 5:1, 20:1 and 40:1 PDMS respectively (r=0.83, p<0.001, Pearson correlation analysis; Figure S3). It was also found that macrophages were more spread on stiff PDMS, and membrane extensions of pseudopods were visible. The E. coli cells on stiff PDMS (5:1) surfaces were smaller than those on soft PDMS (40:1) surfaces, consistent with our previous report (Song and Ren 2014).
Fig. 3.
Microscope images of E. coli RP437/pBAD-mPlum cells and U-937 cells on 5:1 (A) and 40:1 (B) PDMS surfaces. U-937 cells were added to 5-h E. coli RP437/pBAD-mPlum cells on 5:1 or 40:1 PDMS surfaces after 90 min of incubation in RPMI medium supplemented with 10% FBS at 37°C and 5% CO2. Arrows indicate macrophage cell membrane extensions. Bar=20 µm.
Phagocytosis analyzed with flow cytometry
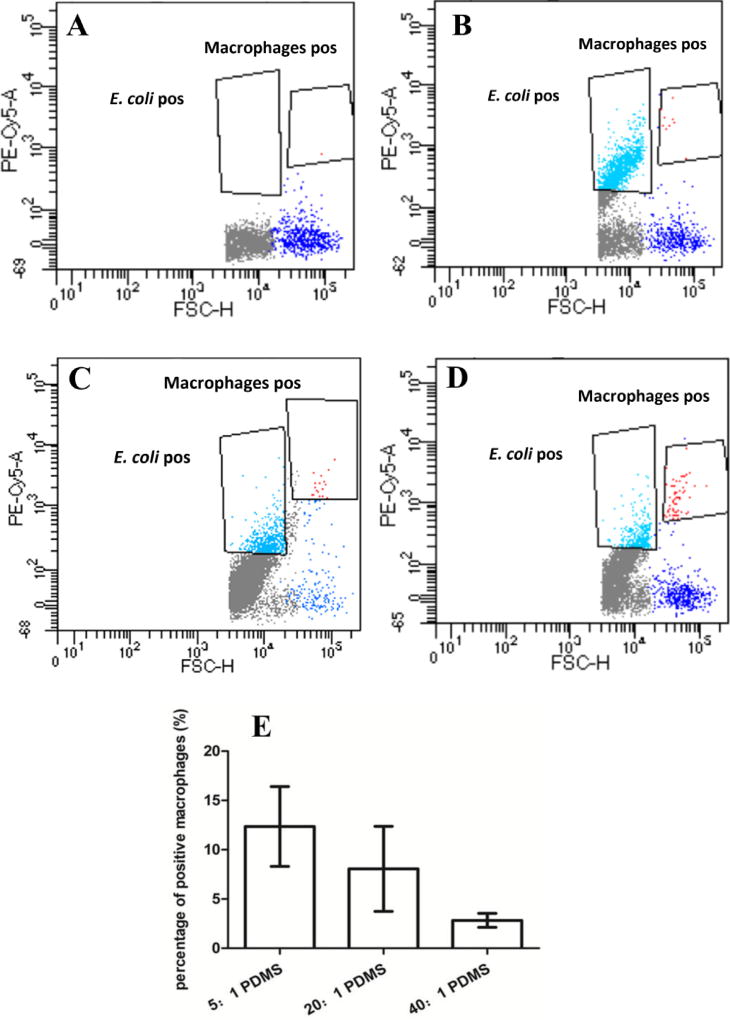
Flow cytometry was used to corroborate the results of CFU and microscopy. In principle, three populations were obtained: E. coli, U937, and U937 associated with E. coli cells. The cells of E. coli RP437/pBAD-mPlum have far-red fluorescence that does not overlap with the autofluorescence of U937 cells. Thus the three population can be differentiated based on size, morphology and fluorescence (Plum from E. coli cells and background green autofluorescence from U937). As shown in Fig. 4, the phagocytosis rate (the amount of macrophages associated with E. coli cells) was (12.4 ± 4.1)%, (8.1 ± 4.3)%, (2.8 ± 0.7)% on 5:1, 20:1, 40:1 PDMS surface, respectively (r=0.64, p<0.001, Pearson correlation analysis). This is consistent with the results of microscopy.
Fig. 4.
Flow cytometry analysis of phagocytosis of 5-h biofilm cells. The plots show the macrophage control (A) and the cells of E. coli and U937 on (40:1) PDMS (B), (20:1) PDMS (C), and (5:1) PDMS (D). Figure E shows the percentage of positive macrophage cells (associated with E. coli cells) after incubating with E. coli RP437/pBAD-mPlum biofilms for 90 min in RPMI medium supplemented with 10% FBS at 37°C and 5% CO2 on 5:1, 20:1 or 40:1 PDMS surfaces (r=0.64, p<0.001, Pearson correlation analysis).
Effects of E. coli cell size on phagocytosis
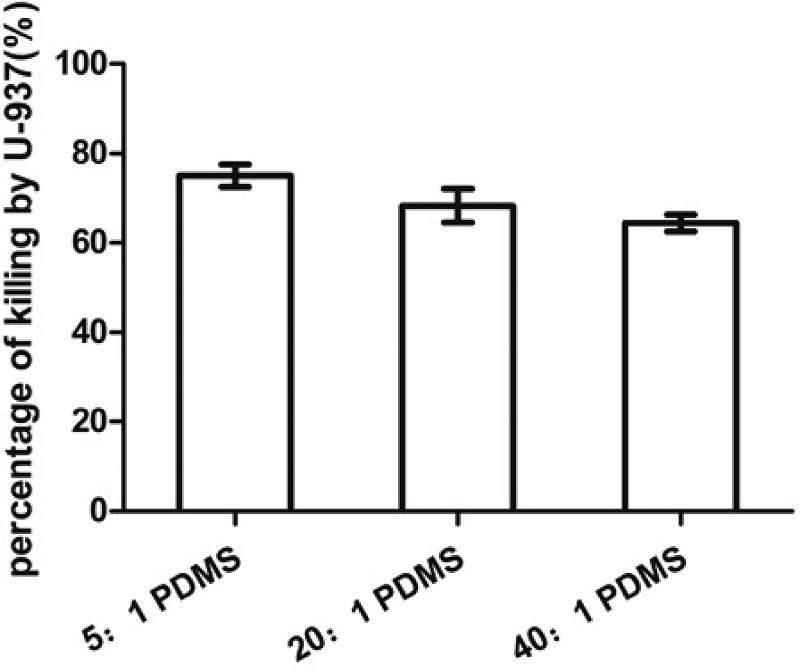
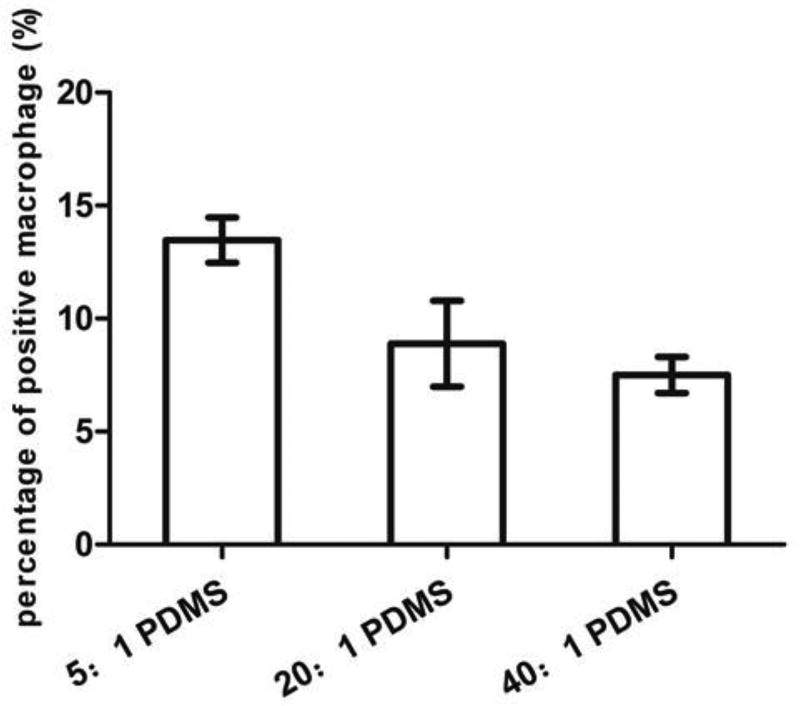
To understand if the biofilm matrix has effect on the observed difference in phagocytosis, we also conducted a test with macrophages attached on different PDMS surfaces first. We then added E. coli cells detached from biofilms on PDMS surfaces with varying stiffness. This allowed us to study the effects of E. coli cell size without the influence of biofilm matrix. As shown in Fig. 5, the number of viable E. coli RP437/pBAD-mPlum cells decreased due to phagocytosis, and the killing effects increased with PDMS stiffness, e.g. (75.0 ± 2.5)% on 5:1 PDMS, (68.3 ± 3.8)% on 20:1 PDMS and (64.4 ± 1.9)% on 40:1 PDMS (r=0.62, p=0.07, Pearson correlation analysis). As shown in Fig. 6, the percentage of positive macrophages was (13.5 ± 1.0)% on 5:1 PDMS, (8.9 ± 1.9)% on 20:1 PDMS and (7.5 ± 0.8)% on 40:1 PDMS (r=0.92, p<0.0001, Pearson correlation analysis). This is consistent with the above results of direct phagocytosis of attached E. coli cells in biofilms, supporting the finding that biofilm cells on stiff PDMS are more susceptible to phagocytosis, both with and without the biofilm matrix.
Fig. 5.
Phagocytosis of detached 5-h E. coli RP437/pBAD-mPlum biofilm cells. The graph shows the percentage of killing after incubating with macrophage U-937 (attached on 5:1, 20:1 or 40:1 PDMS surfaces first) for 90 min in RPMI medium supplemented with 10% FBS at 37°C and 5% CO2 (r=0.62, p=0.07, Pearson correlation analysis).
Fig. 6.
Percentage of positive macrophage cells (associated with E. coli cells) after incubating with detached 5-h E. coli RP437/pBAD-mPlum cells. U937 cells were attached on 5:1, 20:1 or 40:1 PDMS surfaces for 90 min in RPMI medium supplemented with 10% FBS at 37°C and 5% CO2 on 5:1, 20:1 or 40:1 PDMS surfaces (r=0.92, p<0.0001, Pearson correlation analysis).
To further corroborate the results, we also performed a study where macrophages were attached on the same surfaces of petri dish, followed by the addition of E. coli cells detached from 5-h biofilms on PDMS surfaces with varying stiffness. As shown in Fig. S4, the number of viable E. coli RP437/pBAD-mPlum cells decreased due to phagocytosis and the killing effects increased when the size of E. coli decreased, e.g. (88.8 ± 1.3)%, (80.0 ± 4.0)%, and (77.8 ± 5.6)% of E. coli RP437/pBAD-mPlum cells from 5:1, 20: 1, and 40:1 PDMS, respectively (r=0.80, p=0.0095, Pearson correlation analysis). Although the differences are rather moderate, this result shows that the E. coli cell size does affect phagocytosis and cells attached on stiff surfaces are more susceptible.
Effect of PDMS stiffness on the activity and movement of macrophages
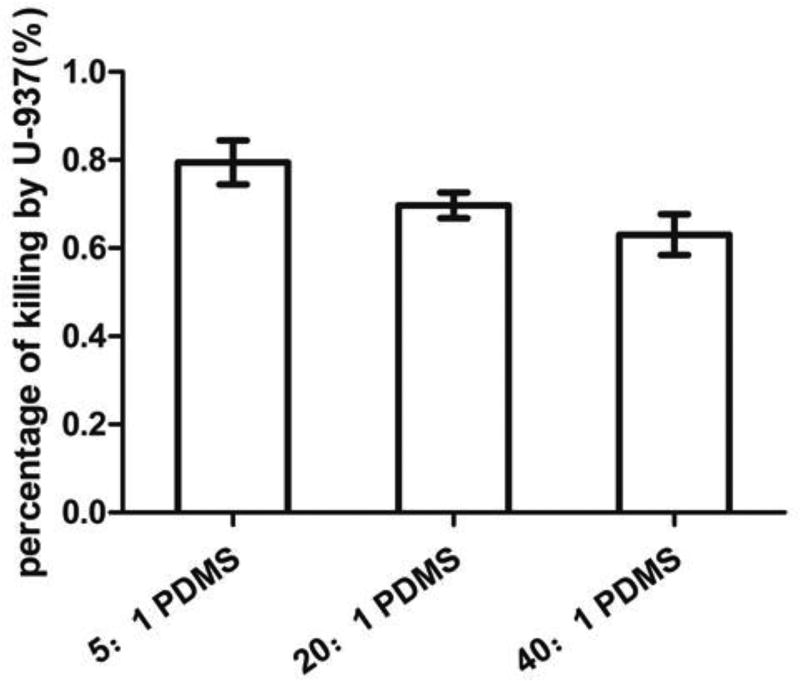
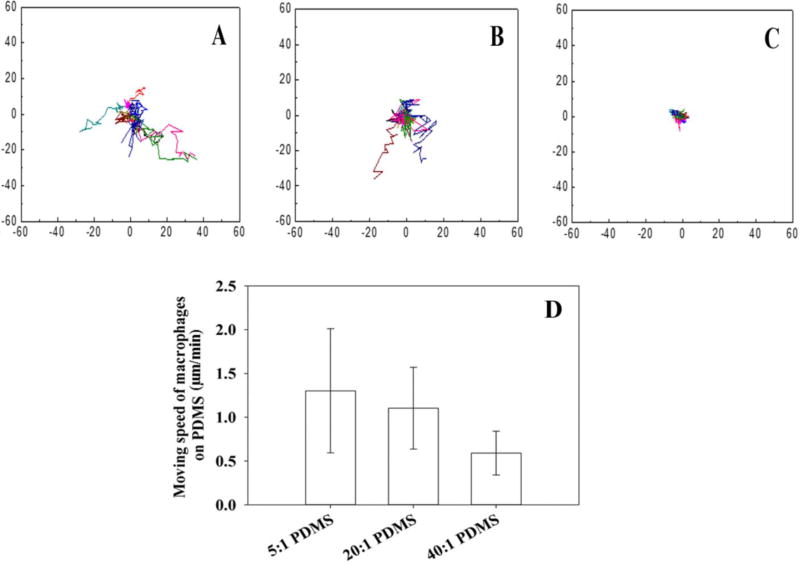
To evaluate the effects of PDMS stiffness on macrophages more specifically, we allowed macrophages to attach on the PDMS surfaces with different stiffness, and then added E. coli cells from an overnight culture (to have the same cell size). As shown in Fig. 7, the number of viable RP437/pBAD-mPlum cells decreased due to phagocytosis, and the killing effects increased when the PDMS surfaces got stiffer, e.g. (79.5 ± 5.0)% on 5:1 PDMS, (69.7 ± 3.0)% on 20:1 PDMS and (63.1 ± 4.7)% on 40:1 PDMS (r=0.89, p=0.0014, Pearson correlation analysis). From the images, we also observed that macrophage cell membrane extension spread longer on stiff PDMS (5:1) surfaces than on soft PDMS (40:1) surfaces (Fig. 3A). Using a custom built chamber, we also monitored E. coli and U937 cells in real time during phagocytosis. By taking images every 3 min for 3 h, the movement of macrophages was tracked. As shown in Fig. 8, it was found that macrophages move faster on the stiff (5:1) PDMS, relative to the soft (40:1) PMDS (0.6 vs 1.3 µm/min, p=0.005, one-way ANOVA adjusted by Tukey test).
Fig. 7.
Effects of PDMS stiffness on the activity of macrophage U937 cells. The graph shows the killing of E. coli RP437/pBAD-mPlum from overnight cultures. U937 cells were attached on 5:1, 20:1 or 40:1 PDMS surfaces in RPMI medium supplemented with 10% FBS at 37°C and 5% CO2 (r=0.89, p=0.0014, Pearson correlation analysis).
Fig. 8.
Tracking U937 cells during phagocytosis of E. coli RP437/pBAD-mPlum on PDMS surfaces with different levels of stiffness. Plots A–C show the moving tracks of U937 cells on stiff (5:1; A), medium (20:1; B), and soft (40:1; C) PDMS surfaces. The moving speeds of U937 cells are shown in figure D (5:1 PDMS vs 40:1 PDMS, p=0.005, one-way ANOVA adjusted by Tukey test). The cells were tracked for 3 h. In plots A–C, all cell tracks are shown with the same starting point for the convenience in comparison.
Discussion
Recently, we reported that the early stage biofilm cells on stiff PDMS (5:1) surfaces have lower aspect ratio, grow slower, and are less susceptible to antibiotics compares to those on soft (40:1) PDMS surfaces (Song and Ren 2014) This finding shows that material stiffness has profound impacts on the physiology of attached bacterial cells. To understand if such effects play a role in the interaction between bacteria and host cells, we conducted this study to investigate phagocytosis of early stage biofilm cells on soft and stiff surfaces. Previously, Champion et al. (2009) showed that the IgG opsonized poly (lactide-co-glycolide) (PLGA) particle with low aspect ratio (AR) are more easily internalized by macrophages. Because early stage E. coli biofilm cells on stiff PDMS exhibited low AR, we hypothesized that early stage E. coli biofilm cells on stiff PDMS will be uptaken more than those on soft PDMS. The obtained results support this hypothesis since more E. coli biofilm cells on stiff PDMS were killed by macrophages than those on soft surfaces. Further experiment revealed that E. coli cell size does affect phagocytosis. In addition to size and shape, the type and abundance of surfaces appendages and proteins may also differ between cells on stiff and soft surface. These may also play a role in phagocytosis and thus deserve further study. This is part of our ongoing work.
In addition to biofilm cells, we found that macrophages on stiff PDMS surfaces were more active than on soft PDMS surfaces. The macrophages on stiff PDMS surfaces moved faster, showed longer movement tracks, and the cells were more spread than those on soft PDMS surfaces. These results are consistent with the report of Blakney et al. (2012), that the macrophages on stiff PEG hydrogel (840 kPa) are more spread than those on soft PEG hydrogel (130 kPa); and the report of Adlerz et al. (2016), which showed that the human monocyte-derived macrophages moves faster on stiff polyacrylamide hydrogel (280 kPa) than soft polyacrylamide hydrogel (3 kPa), although the materials and the ranges of stiffness are different from our study. While previous works on the effects of surface stiffness on macrophages focus on hydrogels, our results show that macrophages are also more active in the presence of E. coli biofilm cells on hydrophobic PDMS surfaces.
By testing phagocytosis in complementary experiments, we found that the higher activity of phagocytosis of biofilm cells on stiff PDMS is likely attributed to two factors: small cells of E. coli and higher motility of macrophages. As shown in Figure S4, macrophages in petri dishes internalized the relatively short E. coli cells detached from stiff (5:1) PDMS faster than the longer ones detached from soft (40:1) PDMS. The results in Figure 5, 6, and 8 showed that macrophages are also more active on stiff PDMS than soft ones. Based on these findings, we speculate that bacterial infections associated with implanted biomaterials can be better controlled by optimizing material stiffness. Since PDMS is commonly used in medical devices, it is plausible to test this hypothesis.
Beyond the different physiology of biofilm cells on soft and stiff PDMS surfaces, why and how the macrophages respond to them differently are also interesting. Phagocytosis is highly depended on surface receptors. For example, the IgG opsonized polymeric particles used in previous studies are recognized by Fc receptors of macrophage (Champion and Mitragotri 2006; 2009) but the recognition of bacterial pathogens by macrophages is based on complement receptors. What receptors are used by macrophage cells to internalize biofilm cells, and how material stiffness affects the receptor binding and internalization rate are important questions for further investigation. In addition, because macrophages produce secretory mediators after binding to bacterial pathogens, it will be interesting to investigate the amount of secretory mediators, such as IL-1 and TNF-α, involved in the phagocytosis of biofilm cells on soft and stiff PDMS surfaces, and the contribution of secretory mediators to the higher phagocytosis rate of biofilm cells on stiff surfaces.
In summary, we demonstrated in this study that the stiffness of substrate material can affect the phagocytosis of attached bacterial cells. Specifically, increase in the stiffness of PDMS (40:1, 20:1 and 5:1 PDMS surfaces were studied) led to higher efficiency of phagocytosis of E. coli biofilm cells. The effects were attributed to higher motility of macrophages and smaller size of bacterial cells on stiff surfaces compared to soft ones. Collectively, the results from this study indicate that material stiffness is an important factor that affects the interaction between bacteria and host cells; and thus, it may be possible to develop better biomaterials by tailing the mechanical properties to reduce fouling and/or to promote the antimicrobial activities of host immune cells.
Supplementary Material
Acknowledgments
We thank Dr. John S. Parkinson at the University of Utah for providing the strain E. coli RP437, Dr. Roger Tsien at the University of California San Diego for sharing pBAD-mPlum, and Dr. Jeremy Gilbert at Clemson University for providing human macrophage monocytes cell line U-937. We also thank Grace Altimus at Syracuse University for helping with the flow cytometry analysis and Dr. Gary Winslow in the Department of Microbiology and Immunology at SUNY Upstate Medical University for critical review of the manuscript.
Funding
This study was funded by the U.S. National Institute of Health (IR21EY025750-01A1) and a support to Y.Z. from China Scholarship Council (No.201508110227).
Footnotes
Electronic supplementary material
The online version of this article contains supplementary material, which is available to authorized users.
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethical standards
This article does not contain any study with human participants or animals performed by any of the authors.
References
- Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Ann Rev Immuno. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- Adlerz KM, Aranda-Espinoza H, Hayenga HN. Substrate elasticity regulates the behavior of human monocyte-derived macrophages. Eur Biophy J. 2016;45:301–309. doi: 10.1007/s00249-015-1096-8. [DOI] [PubMed] [Google Scholar]
- Anwar H, Dasgupta M, Costerton J. Testing the susceptibility of bacteria in biofilms to antibacterial agents. Antimicrob Agents Ch. 1990;34:2043–2046. doi: 10.1128/aac.34.11.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakney AK, Swartzlander MD, Bryant SJ. The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly (ethylene glycol)-based hydrogels. J Biomed Mater Res A. 2012;100:1375–1386. doi: 10.1002/jbm.a.34104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryers JD. Medical biofilms. Biotechnol Bioeng. 2008;100:1–18. doi: 10.1002/bit.21838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion JA, Mitragotri S. Role of target geometry in phagocytosis. P Natl Acad Sci USA. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion JA, Mitragotri S. Shape induced inhibition of phagocytosis of polymer particles. Pharm Res. 2009;26:244–249. doi: 10.1007/s11095-008-9626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Nace GW, Irwin PL. A 6× 6 drop plate method for simultaneous colony counting and MPN enumeration of Campylobacter jejuni, Listeria monocytogenes, and Escherichia coli. J Microbiol Meth. 2003;55:475–479. doi: 10.1016/S0167-7012(03)00194-5. [DOI] [PubMed] [Google Scholar]
- Costerton JW, Cheng K, Geesey GG, Ladd TI, Nickel JC, Dasgupta M, Marrie TJ. Bacterial biofilms in nature and disease. Ann Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- Dubois-Brissonnet F, Trotier E, Briandet R. The biofilm lifestyle involves an increase in bacterial membrane saturated fatty acids. Front Microbiol. 2016;7:1673. doi: 10.2289/fmicb.2016.01673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi N, Mitragotri S. Macrophages recognize size and shape of their targets. PloS one. 2010;5(4):e10051. doi: 10.1371/journal.pone.0010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans ND, Minelli C, Gentleman E, LaPointe V, Patankar SN, Kallivretaki M, Chen X, Roberts CJ, Stevens MM. Substrate stiffness affects early differentiation events in embryonic stem cells. Eur Cell Mater. 2009;18:1–13. doi: 10.1201/9781420080124.ch1. [DOI] [PubMed] [Google Scholar]
- Fuard D, Tzvetkova-Chevolleau T, Decossas S, Tracqui P, Schiavone P. Optimization of poly-di-methyl-siloxane (PDMS) substrates for studying cellular adhesion and motility. Microelectron Eng. 2008;85:1289–1293. doi: 10.1016/j.mee.2008.02.004. [DOI] [Google Scholar]
- Hou S, Burton EA, Simon KA, Blodgett D, Luk YY, Ren D. Inhibition of Escherichia coli biofilm formation by self-assembled monolayers of functional alkanethiols on gold. Appl Environ Microbiol. 2007;83:4300–4307. doi: 10.1128/AEM.02633-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S, Gu H, Smith C, Ren D. Microtopographic patterns affect Escherichia coli biofilm formation on poly (dimethylsiloxane) surfaces. Langmuir. 2011;27:2686–2691. doi: 10.1021/la1046194. [DOI] [PubMed] [Google Scholar]
- Khoury AE, Lam K, Ellis B, Costerton JW. Prevention and control of bacterial infections associated with medical devices. ASAIO journal. 1992;38:M174–M178. doi: 10.1097/00002480-199207000-00013. [DOI] [PubMed] [Google Scholar]
- Martens E, Demain AL. The antibiotic resistance crisis, with a focus on the United States. J Antibiotics. 2017;70:520–526. doi: 10.1038/ja.2017.30. [DOI] [PubMed] [Google Scholar]
- Olsen I. Biofilm-specific antibiotic tolerance and resistance. Eur J Clin Microbiol. 2015;34:877–886. doi: 10.1007/s10096-015-2323-z. [DOI] [PubMed] [Google Scholar]
- Parkinson JS, Houts SE. Isolation and behavior of Escherichia coli deletion mutants lacking chemotaxis functions. J Bacteriol. 1982;151:106–113. doi: 10.1128/jb.151.1.106-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song F, Koo H, Ren D. Effects of material properties on bacterial adhesion and biofilm formation. J Dent Res. 2015;94(8):1027–34. doi: 10.1177/0022034515587690. [DOI] [PubMed] [Google Scholar]
- Song F, Ren D. Stiffness of cross-linked poly (dimethylsiloxane) affects bacterial adhesion and antibiotic susceptibility of attached cells. Langmuir. 2014;30:10354–10362. doi: 10.1021/la502029f. [DOI] [PubMed] [Google Scholar]
- Tauber AI. Metchnikoff and the phagocytosis theory. Nat Rev Mol Cell Bio. 2003;4:897–901. doi: 10.1038/nrm1244. [DOI] [PubMed] [Google Scholar]
- Underhill DM, Goodridge HS. Information processing during phagocytosis. Nat Rev Immuno. 2012;12:492–502. doi: 10.1038/nri3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergidis P, Patel R. Novel approaches to the diagnosis, prevention, and treatment of medical device-associated infections. Infect Di Clin N Am. 2012;26:173–186. doi: 10.1016/j.idc.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Dissertation. University of South Florida; 2011. Polydimethylsiloxane mechanical properties measured by macroscopic compression and nanoindentation techniques. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.