Summary
Women approaching advanced maternal age have extremely poor outcomes with both natural and assisted fertility. Moreover, the incidence of chromosomal abnormalities and birth defects increases with age. As of yet, there is no effective and practical strategy for delaying ovarian aging or improving oocyte quality. We demonstrate that the lifelong consumption of a diet rich in omega-3 fatty acids prolongs murine reproductive function into advanced maternal age, while a diet rich in omega-6 fatty acids is associated with very poor reproductive success at advanced maternal age. Furthermore, even short-term dietary treatment with a diet rich in omega-3 fatty acids initiated at the time of the normal age-related rapid decline in murine reproductive function is associated with improved oocyte quality, while short-term dietary treatment with omega-6 fatty acids results in very poor oocyte quality. Thus, omega-3 fatty acids may provide an effective and practical avenue for delaying ovarian aging and improving oocyte quality at advanced maternal age.
Keywords: advanced maternal age, docosahexaenoic acid, menopause, omega-3 fatty acid, oocyte, reproduction
Introduction
Fertility in women is known to precipitously decline after the age of 35 (Schwartz & Mayaux, 1982), with fecundity being all but lost by the age of 45 (Ventura et al., 2004). With advancements in medical care, a woman’s life expectancy has been prolonged by as much as 30 years over the past century while the age of menopause has changed by a meager 3–4 years during this same time period (Soules & Bremner, 1982). With this, an anomaly has been created in which the reproductive lifespan of women has become strikingly short in the context of overall lifespan, a discrepancy that is more pronounced today than ever before. The modern trend of postponing childbearing in this era of increased longevity, most notable in Western societies, brings the age-related decline in fertility to the forefront of scientific challenges in the field of reproductive medicine (Martin et al., 2010).
Biologically, the age at which menopause occurs is determined by the progressive decline and ultimate depletion of the ovarian oocyte-containing follicle reserve (Hansen, 1986; Faddy et al., 1992; Tilly, 2001) concomitant with the diminishing quality of oocytes evidenced by an increase in chromosomal and spindle abnormalities and mitochondrial dysfunction (Battaglia et al., 1996; Hunt & Hassold, 2008; Selesniemi et al., 2011). These changes significantly contribute to the extremely poor success of natural and assisted fertility attempts for women of advanced reproductive age and to the increased incidence of chromosomal anomalies when conception is successful (Navot et al., 1991b; van Kooij et al., 1996). Similar to humans, laboratory rodents exhibit an age-related decline in ovarian follicle reserve leading to a state of natural infertility approximately halfway through their chronological lifespan (Gosden et al., 1983; Perez et al., 1999; Wu et al., 2005). Aging female mice exhibit many of the physiological changes observed in postmenopausal women, including the loss of cyclic ovarian function, making these animals an ideal in vivo model for the study of ovarian failure. Unfortunately, despite relevant rodent model systems and promising proposed strategies for prolonging the female reproductive lifespan (Perez et al., 1999, 2007; Selesniemi et al., 2008, 2009, 2011; Niikura et al., 2010), an effective and realistic strategy for significantly delaying ovarian aging or improving oocyte quality has yet to be developed.
Changes in the dietary patterns of humans over time may provide insight into novel avenues for delaying ovarian aging. Anthropological and nutritional studies demonstrate a remarkable change in the human diet over the past 100 years, most notably with regard to the type and amount of fat consumed (Eaton & Konner, 1985; Simopoulos, 1991, 2003, 2006, 2009, 2011). These changes are manifested by both an absolute and a relative change in the omega-6 and omega-3 fatty acid consumption. Today, the Western diet provides an omega-6 to omega-3 fatty acid ratio of as high as 25:1, which is in stark contrast to the 1:1 ratio historically consumed by humans (Simopoulos, 2006), creating a nutritional environment that is very different from our ancestors and from which our genetic constitution was selected. This change is particularly relevant given that the shift in dietary habits over the last 100 years is accompanied by a concurrent downward trend in the fertility rates for women over the age of 35 (Baird et al., 2005).
The purpose of this study is twofold: (i) to evaluate the effect of a diet rich in omega-3 fatty acids on murine reproductive function and egg quality and (ii) to determine whether a diet rich in omega-3 fatty acids is safe for long-term consumption. We found that the lifelong consumption of a diet rich in omega-3 fatty acids maintains murine reproductive function at advanced maternal age and that the institution of this diet at the time of the normal rapid decline in murine reproductive function results in a significant improvement in oocyte quality. Additionally, this omega-3-rich diet was found to be safe for long-term consumption over multiple generations without any evidence of essential fatty acid deficiency. These findings have profound implications for both successful natural and assisted reproduction at advanced maternal age.
Results
Reproductive and fertility outcomes in long-term diet studies
We first sought to evaluate the effect of the long-term consumption of a diet rich in either omega-3 or omega-6 fatty acids on reproductive function. To this end, an omega-3 fatty acid-rich diet was designed to mimic the fatty acid composition of cold water fish (Le et al., 2012), with an omega-3 to omega-6 fatty acid ratio of 20:1 provided as docosahexaenoic acid (DHA; 22:6n-3; omega-3 fatty acid) and arachidonic acid (AA; 20:4n-6; omega-6 fatty acid). In contrast, an omega-6 fatty acid-rich diet was designed to mimic the standard Western diet, with fat provided as soybean oil and thus containing an omega-6 to omega-3 fatty acid ratio of approximately 8:1 provided as linoleic acid (LA; 18:2n-6; omega-6 fatty acid) and alpha-linolenic acid (ALA; 18:3n-3; omega-3 fatty acid). A third diet in which all fat was provided as hydrogenated coconut oil (HCO), which is deficient in essential fatty acids, was used as a control for essential fatty acid deficiency. Further details regarding these three isocaloric diets are presented in Table 1.
Table 1.
Composition of experimental diets
| HCO | SOY | DHA | |
|---|---|---|---|
| Casein | 501.2 | 501.2 | 501.2 |
| L-Cystine | 7.2 | 7.2 | 7.2 |
| Sucrose | 400 | 400 | 400 |
| Cornstarch | 1676.5 | 1676.5 | 1676.3 |
| Dyetrose | 589 | 589 | 589 |
| Mineral Mix #210050 | 29.4 | 29.4 | 29.4 |
| Vitamin Mix #310025 | 38.7 | 38.7 | 38.7 |
| Hydrogenated coconut oil | 360 | 0 | 284.4 |
| Soybean oil | 0 | 360 | 0 |
| Docosahexaenoic acid (DHA) | 0 | 0 | 72 |
| Arachidonic acid (AA) | 0 | 0 | 3.6 |
| Total | 3602.0 | 3602.0 | 3601.8 |
All values reported as kcal kg−1 diet.
Prior to evaluating reproductive potential at advanced maternal age, breeding trials were performed to characterize the reproductive potential of animals on these diets during the normal murine female reproductive lifespan. To do this, adult female mice were randomized to one of the three different isocaloric diets (HCO, SOY, DHA). Following 4 weeks of dietary treatment, breeding trials were initiated on this F0 generation with subsequent generations of animals being maintained on the same diet and breeding trials being continued with each subsequent generation as females attained reproductive maturity. The litter size and viability of F1 generation animals (born to F0 dams) in each of the experimental diet groups did not differ (Fig. 1A). Animals on the SOY diet were bred to the F3 generation and animals on the DHA diet were bred to the F6 generation at which time further breeding attempts were terminated. Notably, despite continued attempts at breeding, animals on the HCO diet were not able to successfully reproduce beyond the F1 generation likely secondary to severe essential fatty acid deficiency, defined as a triene/tetraene (T:T) ratio of > 0.2 on the serum fatty acid profile (Table S1). Successive generations of animals on the DHA diet continued to have litter sizes within the expected range with a notable improvement in offspring survival in later generations, from 75% in the F1 generation to 95% in the F5 generation and 100% in the F6 generation (Fig. 1B).
Fig. 1.
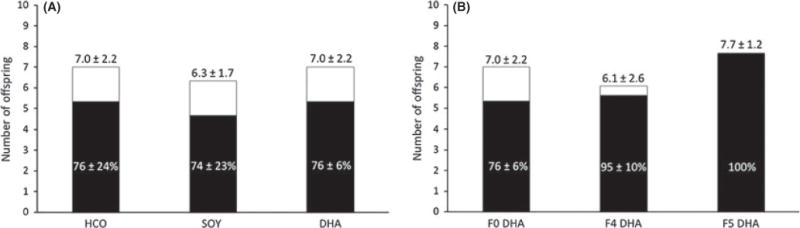
Reproductive and fertility outcomes in long-term diet studies at normal maternal reproductive age (3–6 months). (A) Litter size and viability were not different between F0 generation animals in each of the diet groups (N = 3, 6, 3 litters for SOY, HCO, and DHA groups, respectively). (B) Reproductive function was maintained over six successive generations of animals on the DHA diet with an improvement in viability over successive generations (N = 3, 13, 3 litters for F0, F4, and F5 generations, respectively). Offspring viability assessed at 3 weeks.
Once the ability of animals on the omega-3- and omega-6-rich diets to successfully breed within the normal female reproductive lifespan was confirmed, animals on these diets were then tested for their ability to reproduce at advanced maternal age (> 10 months of age). All animals on the omega-3-rich diet (N = 7) were able to successfully reproduce with an average of 3.3 ± 0.3 litters/animal between 10 and 15 months of age (Fig. 2A). Although the average litter was smaller (4.4 ± 1.9 offspring/litter) for dams at advanced maternal age (> 10 months) compared to younger cohorts of animals (6.0 ± 2.7 offspring/litter) on the same diet (P = 0.10), the overall survival of the offspring born to dams at advanced maternal age was remarkably high at 89%. In stark contrast, none of the 10 aged animals maintained on the omega-6-rich diet had any viable litters (Fig. 2B). As another point of comparison, breeding trials at 10 months of age were also initiated for animals on a standard laboratory rodent chow (N = 7). The reproductive success of these animals also contrasted starkly to those on the omega-3-rich diet, with only two animals having one viable litter each (Fig. 2C). These findings suggest that the remarkable increase in dietary omega-6 fatty acids in the human diet over the last 100 years may actually be detrimental to the reproductive success of women of advanced maternal age (Eaton & Konner, 1985; Simopoulos, 2003, 2006, 2009, 2011).
Fig. 2.
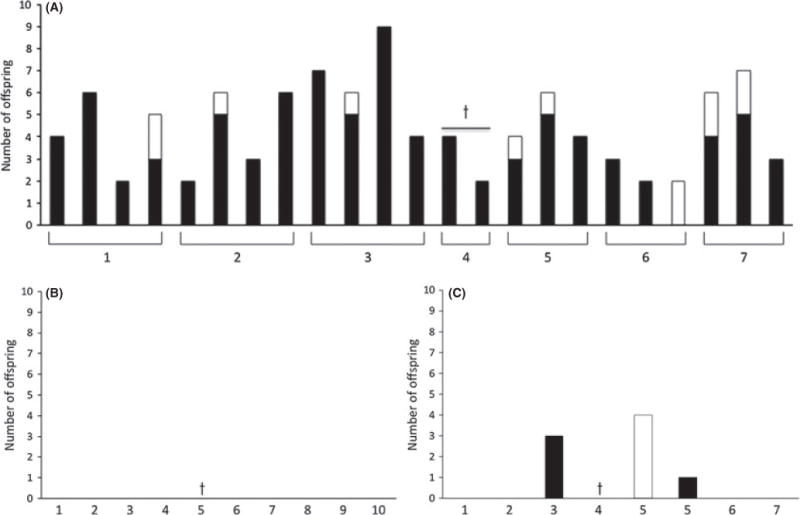
Reproductive and fertility outcomes in long-term diet studies at advanced maternal reproductive age (10–15 months). (A) Reproductive function was maintained in females on the DHA diet at advanced maternal age. (B) Animals on the SOY and (C) CHOW diets had very poor reproductive success at advanced maternal age. Each animal is indicated by a number on the x-axis, and each bar represents one litter. White bars represent total number of offspring, and black bars represent viable offspring. Crosses indicate animals that died or had to be euthanized during the study period. Offspring viability assessed at 2 weeks.
Oocyte quality in acute dietary treatment studies
Understanding that the lifelong consumption of a diet containing a high omega-3 to omega-6 fatty acid ratio is not a very practical strategy for prolonging the natural reproductive lifespan, we next focused on an acute dietary treatment model. As egg quality is recognized as the single most important factor for determining the success of pregnancy for women of advanced reproductive age (Navot et al., 1991a,b), we aimed to determine the effect of acute dietary treatment on oocyte quality at advanced maternal age.
Thirty-six 10-month-old virgin female mice fed a standard laboratory rodent chow (CHOW) until 10 months of age were randomly assigned to each of three different diet groups (N = 12 CHOW, N = 12 SOY, N = 12 DHA). One animal on the SOY diet necessitated euthanasia during week 10 of dietary treatment due to severe dermatitis. The remaining 35 animals survived to complete the 12-week dietary treatment and were euthanized at 13 months of age. There were no differences in the average calories consumed or the average weekly animal body weights between groups (Figure S1). The acute dietary treatment did not result in the development of biochemical essential fatty acid deficiency in any diet group (Table S2). However, even with this relatively short period of dietary treatment, the serum omega-6/omega-3 fatty acid ratio was significantly higher in the DHA diet group compared with both the CHOW and SOY diet groups (Fig. 3A).
Fig. 3.
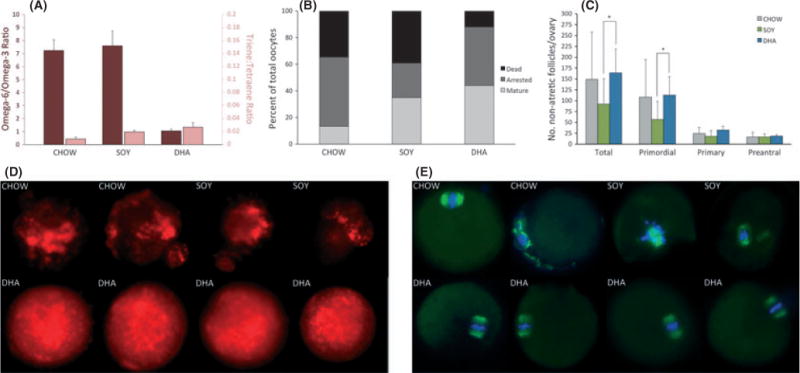
Fatty acid profiles, ovarian follicle counts, and oocyte quality in acute dietary treatment studies. (A) Serum omega-6/omega-3 fatty acid (dark red bars) and triene/tetraene ratios (light red bars) of animals in each acute dietary treatment group. The serum omega-6/omega-3 fatty acid ratio was more than 7-fold lower in the DHA group compared to the CHOW (P = 0.008) and SOY (P = 0.008) groups, and no animal in any diet group had evidence of biochemical essential fatty acid deficiency (triene/tetraene ratio > 0.2) (N = 5, 5, 5 animals for CHOW, SOY, and DHA groups, respectively). (B) Oocyte characterization demonstrates that a larger percentage of oocytes from animals in the DHA group were fully mature and fewer were atretic compared to the CHOW and SOY groups (N = 53, 23, and 25 oocytes for CHOW, SOY, and DHA groups, respectively). (C) Ovarian follicle counts demonstrate a greater number of total (P = 0.04) and primordial follicles (P = 0.04) in ovaries from animals following acute treatment with the DHA diet compared to the SOY diet (N = 6, 6, and 7 animals for CHOW, SOY, and DHA groups, respectively). (D) Representative mitochondrial staining of oocytes obtained from animals in each of the acute dietary treatment groups. Mitochondria appeared normal in 6/6 (100%) mature oocytes from animals in the DHA group, compared with 0/4 (0%) and 1/3 (33%) mature oocytes in the CHOW and SOY diet groups, respectively (P = 0.006). (E) Representative tubulin (spindle apparatus, green) and DNA (blue) staining of oocytes obtained from animals in each of the acute dietary treatment groups. Meiotic spindles appeared normal in 4/5 (80%) mature oocytes from animals in the DHA group as compared with 2/3 (66%) and 0/5 (0%) mature oocytes from animals in the CHOW and SOY groups, respectively (P = 0.03). All bars indicate mean ± SD.
Oocyte yield following hormonal stimulation in addition to the oocyte maturational status and quality was evaluated for the 35 female mice that survived to 13 months of age. A total of 53 oocytes were collected from CHOW-fed animals compared with 23 and 25 for the SOY and DHA diet groups, respectively. On evaluation of oocyte maturational status, a greater percentage of the oocytes harvested from animals on the DHA diet (44%) were found to be fully mature (MII stage), representing the fertilization-competent egg pool, compared with oocytes from animals on the CHOW (13%) and SOY (35%) diets (P = 0.01). Additionally, only 12% of oocytes from animals on the DHA diet were atretic as compared with 39% and 35% of oocytes from animals on the SOY and CHOW diets, respectively (P = 0.09) (Fig. 3B). Similarly, ovarian follicle counts demonstrated that the number of primordial and total nonatretic follicles were significantly lower in ovaries from animals on the SOY diet compared to animals on the DHA diet (Fig. 3C).
The quality of the fully mature (MII stage) oocytes collected from animals in each of the three diet groups was evaluated. Fully mature oocytes were selected for this analysis because age-related defects in oocytes are clearly evident at this maturation stage and because these oocytes represent the fertilization-competent egg pool. Oocyte quality was evaluated by assessing mitochondrial staining pattern and spindle integrity with individual oocytes randomly assigned to each of these endpoints. Mitochondrial aggregation has been linked to the decline in coyote quality with advanced maternal age, and a uniform cytoplasmic distribution of mitochondria without aggregation is indicative of a good quality oocyte (Tarin et al., 2001). Confocal analysis of the mitochondria revealed that although mitochondria had a uniform cytoplasmic distribution pattern in 6/6 (100%) MII oocytes from animals on the DHA diet, there was extensive mitochondrial aggregation in oocytes from animals in the other diet groups with 1/3 (33%) MII oocytes from animals on the SOY diet and 0/4 (0%) MII oocytes from animals on the CHOW diet being classified as normal (P = 0.006) (Fig. 3D). Similarly, confocal analysis of a-tubulin and DNA distribution revealed that meiotic spindles in 4/5 (80%) MII oocytes collected from DHA animals were regular in shape and size with distinct microtubule morphology. In contrast, 0/5 (0%) and 2/3 (66%) MII oocytes from SOY- and CHOW-fed animals, respectively, had normal meiotic spindles (P = 0.03) (Fig. 3E).
Safety evaluation
The omega-3 fatty acid-rich diet associated with the beneficial reproductive effects in this study provided 2% of total calories in the form of the omega-3 fatty acid DHA. Importantly, this diet does not contain any of the traditional essential fatty acids, ALA and LA (Burr & Burr, 1973), but rather contains downstream molecules in the omega-3 and omega-6 fatty acid pathways (DHA and AA). As such, we aimed to determine whether animals maintained on our omega-3-rich diet developed any biochemical or clinical evidence of essential fatty acid deficiency. Clinically, essential fatty acid deficiency results in compromised growth, reproduction, and lactation (Burr & Burr, 1973). As we have already confirmed the ability of animals on the omega-3-rich diet to reproduce and lactate successfully over multiple generations, we now focused on growth patterns as an additional clinical indicator of essential fatty acid deficiency and on serum fatty acid profiles to evaluate for biochemical essential fatty acid deficiency. Lastly, histologic evaluation of all major organ systems was performed.
Fatty acid profiles
No animals in either the SOY (F1 or F2 generation) or the DHA (F1, F2, or F5 generation) diet groups had any evidence of biochemical essential fatty acid deficiency. In contrast, all animals on the HCO diet had evidence of biochemical essential fatty acid deficiency with consistently elevated serum T/T ratios (Fig. 4A). These findings can be confirmed by the mead acid (20:3n-9; omega-9 fatty acid), as there is a relative overproduction of mead acid in the setting of essential fatty acid deficiency. Mead acid accounted for 9.37 ± 1.00% of the total fatty acid content in the F1 HCO group as compared with only 0.12 ± 0.04% in the F1 SOY and 0.02 ± 0.04% in the F1 DHA group.
Fig. 4.
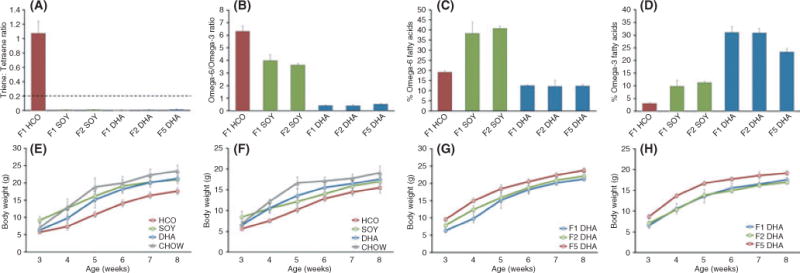
Evaluation of safety of the omega-3-rich diet with fatty acid profiles and growth. (A–D) Serum fatty acid profiles over multiple generations (N = 4, 5, 5, 5, 4, 15 for F1 HCO, F1 SOY, F2 SOY, F1 DHA, F2 DHA, and F5 DHA groups, respectively). (A) Serum triene/tetraene ratios demonstrate that no animals on the SOY or DHA diet had evidence of biochemical essential fatty acid deficiency (triene/tetraene ratio > 0.2, horizontal dashed line). (B) Serum omega-6/omega-3 fatty acid ratios and the total percent of fatty acid attributable to (C) omega-6 and (D) omega-3 fatty acids differed significantly between groups. (E–H) Growth data. Weekly average body weight of representative F1 generation (E) males and (F) females from week 3 (wean) to week 8 of life. Weekly body weights did not differ between animals on the CHOW, SOY, and DHA diets but were consistently lower for animals on the HCO diet (N = 5, 11, 7, 5 male and N = 5, 6, 9, 5 female animals for CHOW, HCO, SOY, and DHA diet groups, respectively). Weekly average body weight of representative F1, F2, and F5 generation (G) males and (H) females from week 3 (wean) to week 8 of life. Animals on the DHA diet continued to demonstrate normal growth despite lifelong treatment with this diet over multiple generations (N = 5, 8, 6 male and N = 5, 5, 6 female animals for F1, F2, and F5 DHA groups, respectively). All data represented as mean ± SD.
The dietary treatments did significantly change the fatty acid profiles of the serum resulting in a lower omega-6/omega-3 fatty acid ratio in the DHA group compared with the SOY and HCO groups (Fig. 4B–D). Interestingly, the serum omega-6/omega-3 fatty acid ratio in the SOY diet group (3.98 ± 0.48 for F1 and 3.62 ± 0.12 for F2) was very similar to the ratio reported for humans consuming a typical Western diet (4.72 ± 0.19) (Ambring et al., 2006), indicating that this experimental diet does effectively mimic the serum omega-6 and omega-3 fatty acid distribution seen in Western societies.
Growth
Animal weights were monitored for F1 generation animals on each of the experimental diets (HCO, SOY, and DHA) and compared to age-matched animals on a standard laboratory rodent chow to provide a point of reference. There were no differences in the growth patterns of the SOY and DHA animals from weaning to adulthood when compared with the standard laboratory rodent chow-fed animals. Animals on the HCO diet had retarded growth evidenced by consistently lower weekly weights than animals on the other diets, a difference that was more pronounced for males than for females (Fig. 4E, F). Successive generations of animals on the DHA diet were monitored to ensure normal growth patterns in later generations of animals, and among these, no differences were noted in the growth of F2 and F5 generation animals (Fig. 4G, H) compared with the F1 generation. This suggests that even the lifelong consumption of this omega-3 fatty acid-rich diet over multiple generations is not associated with any detrimental effect of growth.
Histology
Haemotoxylin- and eosin-stained slides of the brain, heart, lung, liver, kidney, spleen, and femur from a total of 15 adult F5 generation animals were reviewed by a rodent pathologist. These were compared to haemotoxylin- and eosin-stained slides of the same organs from 5 age-matched animals on a standard rodent chow. No abnormalities were noted on review of the brain, heart, liver, kidney, and femur specimens. Three of the 15 lung samples from animals on the DHA diet had mild emphysematous changes, potentially a result of trauma during the harvest and preservation. Additionally, four animals on the DHA diet had mild extramedullary hematopoiesis in the spleen, a nonspecific finding in the laboratory mouse.
Discussion
Humans are enjoying increasing age-related longevity as a result of the incredible advances made over the last century in medicine and public health. Unfortunately, the female reproductive axis has not been so fortunate. Without any feasible strategies to delay the age-associated decline in fertility, the female reproductive axis continues to age very rapidly compared to other organ systems resulting in the cessation of normal ovarian function relatively early in life (Richardson et al., 1987; Faddy et al., 1992). Additionally, pregnancies that are successful in older women are plagued by a much higher incidence of chromosomal abnormalities. One of the most widely known consequences of pregnancy at advanced maternal age is the dramatic rise in the incidence of trisomy 21, which affects 30% of all clinical pregnancies in women in their forties compared with only 2% of clinical pregnancies in women in their twenties (Hassold & Chiu, 1985; Hassold & Hunt, 2009). Thus, strategies focused on prolonging the reproductive lifespan of women must address the deterioration in egg quality that is known to occur with age, a factor recognized as the single most important determinant of the success of pregnancies in women of advanced reproductive age (Navot et al., 1991a,b). Here, we provide evidence that dietary omega-3 fatty acids not only prolong the reproductive lifespan but also result in a remarkable improvement in egg quality in a murine model. These findings have profound potential implications for both successful natural and assisted reproduction at advanced maternal age.
Armed with the knowledge that the shift in human dietary habits over the last 100 years toward a very high omega-6 to omega-3 fatty acid ratio is accompanied by a concurrent downward trend in the fertility rates for women over the age of 35 in Western societies (Baird et al., 2005), we sought to determine the impact of a diet rich in omega-3 fatty acids on reproductive success at advanced maternal age. Based on the results of the current study, we now have evidence that mice on an omega-3 fatty acid-rich diet are able to successfully reproduce well beyond the normal expected reproductive lifespan for these animals. Although the average litter was slightly smaller (4.4 ± 1.9 offspring/litter) for dams at advanced maternal age (> 10 months) on the omega-3-rich diet compared to younger cohorts of animals (6.0 ± 2.7 offspring/litter) on the same diet, the survival of the offspring born to dams at advanced maternal age was remarkably high at 89%. In stark contrast, aged animals (> 10 months) maintained on a standard laboratory rodent chow or an omega-6 fatty acid-rich diet (designed to mimic the typical Western diet) had extremely poor reproductive success. These are striking findings and suggest that if this holds true in the human, the increase in dietary omega-6 fatty acids in the human diet over the last 100 years may actually be detrimental to the reproductive success of women of advanced maternal age.
However, even in light of the recent increasing consumption of fish oil supplements in Western societies, the lifelong consumption of a diet containing a very high omega-3 to omega-6 fatty acid ratio is not a feasible strategy for prolonging the natural reproductive lifespan. A more clinically relevant strategy would include dietary changes that women who desire to delay childbearing could initiate at the time of, or immediately prior to, the presumed time of the natural decline in reproductive fertility. Our data in a murine model suggest that the institution of a diet rich in omega-3 fatty acids around the time of the expected rapid decline in natural fertility results in a remarkable improvement in oocyte quality as measured by mitochondrial dynamics and the structure of the spindle apparatus. These findings are particularly important as egg quality is recognized as the single most important factor for determining the success of pregnancy for women of advanced reproductive age (Navot et al., 1991a,b). With advanced age, the meiotic cell cycle of the egg becomes prone to errors of chromosomal segregation, which results in a much higher proportion of aneuploidy in oocytes ovulated by older women (Hunt, 1998; Hassold & Hunt, 2009). Strategies to improve the quality of oocytes in aged animal models are limited. Chronic antioxidant treatment has been shown to counteract the negative effects of female aging on oocyte quality (Tarin et al., 2002a); however, this treatment has not been shown to improve reproductive success, and the clinical application is not feasible due to very significant negative effects on ovarian and uterine function (Tarin et al., 2002b). Adult onset caloric restriction has been shown to sustain the function of the murine female reproductive axis into advanced chronological age with one half of calorically restricted animals remaining fertile for 6 months beyond the time at which control animals experienced a loss of fertility with a 73% survival for pups born to these dams at advanced maternal age (Selesniemi et al., 2008). This caloric restriction strategy has also been shown to improve oocyte quality (Selesniemi et al., 2011), although the clinical application remains limited due to the expected deleterious health effects associated with the very severe caloric restriction necessary to obtain these beneficial effects. Our data suggest that dietary omega-3 fatty acids may protect against the age-related decline in oocyte quality thus providing an avenue for women of advanced reproductive age to successfully conceive and deliver viable offspring. In addition, the potential for improving oocyte quality and thus decreasing the aneuploidy rate in women of advanced reproductive age may have profound implications for chromosomal disorders such as Down’s Syndrome.
Certainly, it must be recognized that the omega-3 fatty acid-rich diet associated with the beneficial reproductive effects in this study provided 2% of total calories in the form of the omega-3 fatty acid DHA. While this is not achievable by a single daily dietary fish oil supplement, it does represent a level of supplementation that can be clinically achieved. Prior studies do suggest that even very high doses of the omega-3 fatty acids DHA and eicosapentaenoic acid (EPA; 20:5n-3) are well tolerated and can be safely administered to both pediatric and adult patients (Lloyd-Still et al., 2006; Sorgi et al., 2007; Gura et al., 2008). Our data focus on DHA as the omega-3 fatty acid of interest, and although it remains unclear whether other omega-3 fatty acids would have a similar effect, we provide strong evidence that DHA certainly does have a positive impact on the reproductive function of the female mouse. Additionally, it is well recognized that the beneficial effects of dietary omega-3 fatty acids are determined by both the ratio of omega-3 to omega-6 fatty acids in the diet and the absolute doses of these fatty acids (Simopoulos, 2002). Thus, the parallel clinical application of our work would require pharmacologic doses of omega-3 fatty acids in addition to limiting the omega-6 fatty acid content in the diet.
In addition to the beneficial effects of omega-3 fatty acids on murine reproductive function, we provide evidence that DHA and AA alone may be a sufficient source of fat for the maintenance of life and the prevention of essential fatty acid deficiency. About 80 years ago, ALA and LA were determined by Burr and Burr to be the essential fatty acids necessary for healthy skin and successful growth, reproduction, and lactation (Burr & Burr, 1973). DHA and AA are downstream molecules in the omega-3 and omega-6 fatty acid pathways, respectively, that have been identified as critical metabolites with important roles in numerous physiological and biochemical processes. Our results demonstrate that a diet containing a 20:1 ratio of DHA/AA is safe for long-term consumption with no appreciable adverse health effects in a murine model. Animals on this diet for over six generations consistently had very low T/T ratios and mead acid levels, suggesting the absence of biochemical essential fatty acid deficiency. More importantly, these animals had no evidence of clinical essential fatty acid deficiency with maintenance of skin health and normal growth, reproduction, and lactation.
The precise mechanisms via which the omega-3 fatty acid DHA has a positive impact on the female reproductive axis remains to be fully elucidated. This study provides the first step in this pursuit by clearly demonstrating that the consumption of a diet rich in omega-3 fatty acids does in fact improve reproductive success at advanced maternal age. We also provide evidence that oocyte quality is improved in animals consuming an omega-3-rich diet; however, the specific molecular mechanisms via which this improvement is mediated are yet to be determined. Not only should future studies be conducted to better understand the effect of dietary omega-3 fatty acids on oocyte quality but also to better understand the potential effects on the uterus/endometrium that may also have a positive impact on reproductive function.
In summary, this study has uncovered a striking beneficial effect of a diet rich in omega-3 fatty acids on murine reproductive success and oocyte quality at ages normally associated with poor reproductive parameters. A diet rich in omega-3 fatty acids, comprising 2.1% of total calories provided as a 20:1 ratio of DHA/AA, was found to be safe for consumption over several generations and with a remarkable improvement in natural fertility at advanced age. Most clinically relevant are the findings suggesting that the acute dietary treatment of animals during the time of the naturally occurring steep decline in reproductive potential results in improved oocyte quality as measured by the structure of the spindle apparatus and mitochondrial dynamics. If these murine data are translatable to humans, the intake of a diet rich in omega-3 fatty acids and limited in the omega-6 fatty acid content may delay the natural decline in oocyte quality that occurs with age, thus potentially allowing for continued successful reproduction and decreased aneuploidy rates.
Experimental procedures
All animal husbandry and experimental procedures were reviewed and approved by the institutional animal care and use committee of Children’s Hospital Boston. All animals were housed on paper chip bedding in a barrier room with regulated temperature (21 °C ± 1.6 °C), humidity (45%±10%), and an alternating 12-h light and dark cycle with ad libitum access to water and study diets.
Long-term diet studies (reproduction and fertility)
Animals
Virgin C57BL/6 adult female mice and adult male C57BL/6 mice were obtained from Jackson Laboratories (#000664; Jackson Laboratories, Bar Harbor, ME, USA). Male fertility was confirmed prior to breeding trials, and no males older than the age of 6 months were used for any breeding trials.
Feeding regimen
Adult female animals were randomized to one of three different diet groups each containing 10% of total calories in the form of fat provided as soybean oil (SOY; #110990, Dyets Inc., Bethlehem, PA, USA), hydrogenated coconut oil (HCO; #102328, Dyets Inc., Bethlehem, PA, USA), or a 20:1 ratio of DHA/AA (DHA; #102536, Dyets Inc.). The detailed composition of each diet is shown in Table 1.
The initial animals were termed the F0 generation, and these animals remained on their respective diets for 4 weeks prior to the initiation of breeding trials. Subsequent generations of animals were maintained on the same diet as their mother for their entire lifetime. Males were rotated between cages such that no male consumed any particular diet for longer than 1 week.
Breeding trials
After 4 weeks of dietary treatment, breeding trials were initiated with F0 animals in each of the diet groups. The offspring were termed the F1 generation. After reaching reproductive maturity, the F1 animals were bred to generate an F2 generation, and subsequent breeding trials were continued to the F3 generation for the SOY animals and the F6 generation for the DHA animals. Animals on the HCO diet were unable to successfully breed beyond the F1 generation. The total number of offspring delivered per litter and the number of offspring delivered that were viable (survived to wean) were recorded separately for each pregnancy. Offspring that did not survive were either found dead at birth or died very shortly thereafter. All viable offspring were allowed to remain with the dam until wean (postpartum day 21), at which time the offspring were removed from the cages to allow for subsequent mating attempts with the dam. All male offspring were euthanized, and a subset of randomly selected females from each generation was kept for further breeding.
Breeding trials at advanced murine reproductive age, defined as age > 10 months, were continued on a subset of F2 and F3 female animals in the SOY (N = 10) and DHA (N = 7) diet groups. To provide a comparison, breeding trials were concomitantly initiated on age-matched female animals on a standard laboratory rodent chow (CHOW; National Institute on Aging, Bethesda, MD, USA). Breeding trials were conducted exactly as described above except that viable offspring were allowed to remain with the dam until postpartum day 14, at which time the offspring were euthanized and subsequent mating attempts with the dam were continued. Breeding trials were continued in this fashion until the dam reached 15 months of age.
Acute dietary treatment studies (oocyte quality)
Animals
Virgin female C57BL/6 mice were obtained from the National Institute on Aging (NIA, Bethesda, MD, USA) at the age of 10 months. These animals were fed the NIH-31 standard laboratory rodent chow from birth to time of purchase.
Feeding regimen
Female animals were randomized to one of three different diet groups (N = 12/group): CHOW, SOY, and DHA. The amount of diet consumed and the growth of each animal were monitored on a weekly basis. All animals received the experimental diet until euthanasia at 13 months of age, equating to 12 weeks of dietary treatment.
Fatty acid profiles
Serum fatty acid profiles were performed on five representative serum samples from animals in each of the diet groups. Total fatty acids were extracted as per the modified Folch method (Folch et al., 1957). Fatty acid analysis was performed on a Hewlett-Packard 6890N gas chromatograph (GMI Inc., Ramsey, MN, USA) coupled to an HP-5975B mass spectrometer equipped with Supelcowax SP-10 capillary column (GMI Inc.). Fatty acid concentrations (nmol mL−1 serum) were calculated by proportional comparison of peak areas to the area of the 17:0 internal standard.
Oocyte retrieval
Mice (N = 12 CHOW, N = 11 SOY, N = 12 DHA) were superovulated with an intraperitoneal injection of pregnant mare serum gonadotropin (PMSG, 10IU; Sigma-Aldrich, St. Louis, MO, USA) followed by human chorionic gonadotropin (hCG, 10IU; Sigma-Aldrich) 48 h later. Oocytes were collected from oviducts 15–16 h after hCG injection by puncturing the oviducts with an insulin syringe. Retrieved oocytes were denuded of cumulus cells by a brief incubation in 80 IU mL−1 of hyaluronidase (Sigma-Aldrich), followed by three washes with human tubal fluid (HTF) (Irvine Scientific, Santa Ana, CA, USA) supplemented with 0.4% BSA (fraction V, fatty acid free; Sigma-Aldrich). Oocytes were counted and classified using a Hoffman light microscope as mature metaphase II (MII; presence of first polar body in perivitelline space), maturation arrested (germinal vesicle breakdown with no polar body extrusion, or germinal vesicle intact), or dead (condensed, fragmented cytoplasm). Oocytes from the three diet groups were analyzed in parallel.
Mitochondrial analysis
A subset of mature (MII) oocytes collected from each diet group were denuded of adherent somatic (cumulus) cells and incubated in HTF medium supplemented with 0.4% BSA and 200 nM MitoTracker Red CMRox (Life Technologies, Grand Island, NY, USA) for 60 min at 37 °C. Oocytes were washed and incubated in acidified Tyrode solution (Irvine Scientific), washed, fixed, and washed again followed by an incubation in phosphate-buffered saline (PBS; Sigma-Aldrich) containing 0.5% BSA, 0.05% Tween-20 (Sigma-Aldrich), and 0.1% Triton X-100 (Sigma-Aldrich) for 1 h. Oocytes were then mounted using Vectashield (Vector Laboratories, Burlingame, CA, USA) and analyzed by confocal microscopy by two independent trained observers. Oocytes with a uniform cytoplasmic distribution of active mitochondria were scored as normal.
DNA and spindle apparatus analysis
A subset of mature (MII) oocytes collected from each diet group were washed in PBS containing 0.5% BSA and briefly incubated in acidified Tyrode solution to soften and remove the zona pellucida. The oocytes were then washed and fixed in 2.0% neutral-buffered paraformaldehyde containing 0.5% BSA. Permeabilization and blocking were performed by incubating the oocytes in mouse blocking solution (Vector Laboratories) supplemented with 0.5% BSA, 0.1% Triton X, 0.05% Tween-20, and 5% normal goat serum (Vector Laboratories). Oocytes were washed and incubated overnight in a 1:200 dilution of mouse anti-a-tubulin antibody (Sigma-Aldrich) in PBS containing 0.5% BSA, washed and incubated with a 1:250 dilution of goat antimouse IgG conjugated with Alexa Fluor-488 (Life Technologies). Following washing, oocytes were mounted using Vectashield containing propidium iodide (Vector Laboratories) and analyzed by confocal microscopy. For the spindle analysis, oocytes with barrel-shaped bipolar spindles having distinct and well-organized microtubule fibers, along with tightly aligned chromosomes on the metaphase plate, were scored as normal. Oocytes from the three groups were analyzed in parallel.
Ovarian follicle counts
Ovaries were fixed, paraffin embedded, serially sectioned (8 lm), and aligned in order on glass microscope slides. The sections were then stained with haemotoxylin and picric methyl blue and analyzed for the number of nonatretic primordial, primary, and small preantral follicles in every other section with a random start, as previously described (Morita et al., 1999). Only those follicles containing an oocyte with a clearly visible nucleus were scored. Given that this procedure samples one half of the entire ovarian volume, the total number of follicles per ovary was then estimated by multiplying the cumulative counts for each ovary by a correction factor of 2. All counts were performed by a blinded study investigator.
Safety evaluation
Fatty acid profiles
Serum fatty acid profiles were performed on serum samples collected from randomly chosen animals. Representative samples were chosen to represent different generations of animals on the HCO, SOY, and DHA diets. Serum was collected from the following representative animals: F1 HCO (n = 4), F1 SOY (n = 5), F2 SOY (N = 5), F1 DHA (N = 5), F2 DHA (N = 4), F5 DHA (N = 15), and fatty acid extraction and analysis were performed as described above.
Growth
The growth of representative litters born to dams on each of the diets in the long-term diet arm of the study was monitored with serial weights obtained from wean to adulthood. Representative and randomly chosen F1 litters in the SOY and HCO groups and F1, F2, and F5 litters in the DHA group were monitored. Similarly, the weights of litters born to dams on a standard laboratory chow (CHOW) were also monitored to provide an additional point of reference.
Histologic analysis
Fifteen adult animals from the F5 generation on the DHA diet were euthanized for histologic analysis of the organ systems. Brain, heart, lung, liver, kidney, spleen, and long bone (femur) from each of these animals were harvested, fixed in 10% formalin, embedded in paraffin, and stained with haemotoxylin and eosin. Comparison samples were obtained from 5 age-matched C57BL/6 mice on a standard laboratory rodent chow. All slides were reviewed by a rodent pathologist and were classified as either normal or abnormal based on the histologic appearance. Details regarding any notable abnormalities were recorded.
Statistical analysis
All continuous variables presented as mean ± standard deviation (SD). Continuous variables were analyzed with the Student’s t-test or, when the data were not normally distributed, the Mann–Whitney U-test. Continuous variables from more than three independent groups were analyzed with the Kruskal–Wallis one-way analysis of variance. Categorical variables were analyzed with the chi-square test. Significance was assessed using a two-sided 5% alpha level. All statistical analyses were performed with the GRAPHPAD Prism software (version 4.0; San Diego, CA, USA).
Supplementary Material
Table S1 Serum fatty acid profiles of F1 generation animals on the HCO, SOY, and DHA diets.
Table S2 Serum fatty acid profiles of aged animals following acute dietary treatment with the CHOW, SOY, or DHA diet.
Fig. S1 Animal growth and diet consumption in acute dietary treatment.
Acknowledgments
Financial disclosure
This research was supported by the Children’s Hospital Boston Surgical Research Foundation, the Children’s Hospital Boston Vascular Biology Program Research Funds and the Massachusetts General Hospital Vincent Memorial Research Funds. D.N. was supported by grant T32DK007754-13, H.D.L. and E.M.F. were supported by the Joshua Ryan Rappaport Fellowship.
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
Author contributions
D.N. designed and performed experiments, collected and analyzed data, and wrote the manuscript; H.D.L. designed and performed experiments and collected and analyzed data; E.F., S.C., D.W., Y.A.W., A.H.P., and L.G. performed experiments and collected data; S.J.R. performed histologic evaluation; J.L.T designed experiments and interpreted data; B.R.R. and M.P. designed experiments, interpreted data, and oversaw experiments. All authors have read and approved the manuscript.
References
- Ambring A, Johansson M, Axelsen M, Gan L, Strandvik B, Friberg P. Mediterranean-inspired diet lowers the ratio of serum phospholipid n-6 to n-3 fatty acids, the number of leukocytes and platelets, and vascular endothelial growth factor in healthy subjects. Am J Clin Nutr. 2006;83:575–581. doi: 10.1093/ajcn.83.3.575. [DOI] [PubMed] [Google Scholar]
- Baird DT, Collins J, Egozcue J, Evers LH, Gianaroli L, Leridon H, Sunde A, Templeton A, Van Steirteghem A, Cohen J, Crosignani PG, Devroey P, Diedrich K, Fauser BC, Fraser L, Glasier A, Liebaers I, Mautone G, Penney G, Tarlatzis B. Fertility and ageing. Hum Reprod Update. 2005;11:261–276. doi: 10.1093/humupd/dmi006. [DOI] [PubMed] [Google Scholar]
- Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod. 1996;11:2217–2222. doi: 10.1093/oxfordjournals.humrep.a019080. [DOI] [PubMed] [Google Scholar]
- Burr GO, Burr MM. Nutrition classics from The Journal of Biological Chemistry 82:345–67, 1929 A new deficiency disease produced by the rigid exclusion of fat from the diet. Nutr Rev. 1973;31:248–249. doi: 10.1111/j.1753-4887.1973.tb06008.x. [DOI] [PubMed] [Google Scholar]
- Eaton SB, Konner M. Paleolithic nutrition A consideration of its nature and current implications. N Engl J Med. 1985;312:283–289. doi: 10.1056/NEJM198501313120505. [DOI] [PubMed] [Google Scholar]
- Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7:1342–1346. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Gosden RG, Laing SC, Felicio LS, Nelson JF, Finch CE. Imminent oocyte exhaustion and reduced follicular recruitment mark the transition to acyclicity in aging C57BL/6J mice. Biol Reprod. 1983;28:255–260. doi: 10.1095/biolreprod28.2.255. [DOI] [PubMed] [Google Scholar]
- Gura KM, Lee S, Valim C, Zhou J, Kim S, Modi BP, Arsenault DA, Strijbosch RA, Lopes S, Duggan C, Puder M. Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics. 2008;121:e678–e686. doi: 10.1542/peds.2007-2248. [DOI] [PubMed] [Google Scholar]
- Hansen JP. Older maternal age and pregnancy outcome: a review of the literature. Obstet Gynecol Surv. 1986;41:726–742. doi: 10.1097/00006254-198611000-00024. [DOI] [PubMed] [Google Scholar]
- Hassold T, Chiu D. Maternal age-specific rates of numerical chromosome abnormalities with special reference to trisomy. Hum Genet. 1985;70:11–17. doi: 10.1007/BF00389450. [DOI] [PubMed] [Google Scholar]
- Hassold T, Hunt P. Maternal age and chromosomally abnormal pregnancies: what we know and what we wish we knew. Curr Opin Pediatr. 2009;21:703–708. doi: 10.1097/MOP.0b013e328332c6ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PA. The control of mammalian female meiosis: factors that influence chromosome segregation. J Assist Reprod Genet. 1998;15:246–252. doi: 10.1023/A:1022580024402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PA, Hassold TJ. Human female meiosis: what makes a good egg go bad? Trends Genet. 2008;24:86–93. doi: 10.1016/j.tig.2007.11.010. [DOI] [PubMed] [Google Scholar]
- van Kooij RJ, Looman CW, Habbema JD, Dorland M, te Velde ER. Age-dependent decrease in embryo implantation rate after in vitro fertilization. Fertil Steril. 1996;66:769–775. doi: 10.1016/s0015-0282(16)58634-8. [DOI] [PubMed] [Google Scholar]
- Le HD, Meisel JA, de Meijer VE, Fallon EM, Gura KM, Nose V, Bistrian BR, Puder M. Docosahexaenoic acid and arachidonic acid prevent essential fatty acid deficiency and hepatic steatosis. J Parenter Enteral Nutr. 2012;36:431–441. doi: 10.1177/0148607111414580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Still JD, Powers CA, Hoffman DR, Boyd-Trull K, Lester LA, Benisek DC, Arterburn LM. Bioavailability and safety of a high dose of docosahexaenoic acid triacylglycerol of algal origin in cystic fibrosis patients: a randomized, controlled study. Nutrition. 2006;22:36–46. doi: 10.1016/j.nut.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Mathews TJ, Kirmeyer S, Osterman MJ. Births: final data for 2007. Natl Vital Stat Rep. 2010;58:1–85. [PubMed] [Google Scholar]
- Morita Y, Perez GI, Maravei DV, Tilly KI, Tilly JL. Targeted expression of Bcl-2 in mouse oocytes inhibits ovarian follicle atresia and prevents spontaneous and chemotherapy-induced oocyte apoptosis in vitro. Mol Endocrinol. 1999;13:841–850. doi: 10.1210/mend.13.6.0306. [DOI] [PubMed] [Google Scholar]
- Navot D, Bergh PA, Williams M, Garrisi GJ, Guzman I, Sandler B, Fox J, Schreiner-Engel P, Hofmann GE, Grunfeld L. An insight into early reproductive processes through the in vivo model of ovum donation. J Clin Endocrinol Metab. 1991a;72:408–414. doi: 10.1210/jcem-72-2-408. [DOI] [PubMed] [Google Scholar]
- Navot D, Bergh PA, Williams MA, Garrisi GJ, Guzman I, Sandler B, Grunfeld L. Poor oocyte quality rather than implantation failure as a cause of age-related decline in female fertility. Lancet. 1991b;337:1375–1377. doi: 10.1016/0140-6736(91)93060-m. [DOI] [PubMed] [Google Scholar]
- Niikura Y, Niikura T, Wang N, Satirapod C, Tilly JL. Systemic signals in aged males exert potent rejuvenating effects on the ovarian follicle reserve in mammalian females. Aging (Albany NY) 2010;2:999–1003. doi: 10.18632/aging.100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez GI, Robles R, Knudson CM, Flaws JA, Korsmeyer SJ, Tilly JL. Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat Genet. 1999;21:200–203. doi: 10.1038/5985. [DOI] [PubMed] [Google Scholar]
- Perez GI, Jurisicova A, Wise L, Lipina T, Kanisek M, Bechard A, Takai Y, Hunt P, Roder J, Grynpas M, Tilly JL. Absence of the proapoptotic Bax protein extends fertility and alleviates age-related health complications in female mice. Proc Natl Acad Sci USA. 2007;104:5229–5234. doi: 10.1073/pnas.0608557104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson SJ, Senikas V, Nelson JF. Follicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab. 1987;65:1231–1237. doi: 10.1210/jcem-65-6-1231. [DOI] [PubMed] [Google Scholar]
- Schwartz D, Mayaux MJ. Female fecundity as a function of age: results of artificial insemination in 2193 nulliparous women with azoospermic husbands. Federation CECOS N Engl J Med. 1982;306:404–406. doi: 10.1056/NEJM198202183060706. [DOI] [PubMed] [Google Scholar]
- Selesniemi K, Lee HJ, Tilly JL. Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell. 2008;7:622–629. doi: 10.1111/j.1474-9726.2008.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selesniemi K, Lee HJ, Niikura T, Tilly JL. Young adult donor bone marrow infusions into female mice postpone age-related reproductive failure and improve offspring survival. Aging (Albany NY) 2009;1:49–57. doi: 10.18632/aging.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selesniemi K, Lee HJ, Muhlhauser A, Tilly JL. Prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc Natl Acad Sci USA. 2011;108:12319–12324. doi: 10.1073/pnas.1018793108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulos AP. Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr. 1991;54:438–463. doi: 10.1093/ajcn/54.3.438. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56:365–379. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. Importance of the ratio of omega-6/omega-3 essential fatty acids: evolutionary aspects. World Rev Nutr Diet. 2003;92:1–22. doi: 10.1159/000073788. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother. 2006;60:502–507. doi: 10.1016/j.biopha.2006.07.080. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. Evolutionary aspects of the dietary omega-6:omega-3 fatty acid ratio: medical implications. World Rev Nutr Diet. 2009;100:1–21. doi: 10.1159/000235706. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. Importance of the omega-6/omega-3 balance in health and disease: evolutionary aspects of diet. World Rev Nutr Diet. 2011;102:10–21. doi: 10.1159/000327785. [DOI] [PubMed] [Google Scholar]
- Sorgi PJ, Hallowell EM, Hutchins HL, Sears B. Effects of an open-label pilot study with high-dose EPA/DHA concentrates on plasma phospholipids and behavior in children with attention deficit hyperactivity disorder. Nutr J. 2007;6:16. doi: 10.1186/1475-2891-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soules MR, Bremner WJ. The menopause and climacteric: endocrinologic basis and associated symptomatology. J Am Geriatr Soc. 1982;30:547–561. doi: 10.1111/j.1532-5415.1982.tb05661.x. [DOI] [PubMed] [Google Scholar]
- Tarin JJ, Perez-Albala S, Cano A. Cellular and morphological traits of oocytes retrieved from aging mice after exogenous ovarian stimulation. Biol Reprod. 2001;65:141–150. doi: 10.1095/biolreprod65.1.141. [DOI] [PubMed] [Google Scholar]
- Tarin JJ, Perez-Albala S, Cano A. Oral antioxidants counteract the negative effects of female aging on oocyte quantity and quality in the mouse. Mol Reprod Dev. 2002a;61:385–397. doi: 10.1002/mrd.10041. [DOI] [PubMed] [Google Scholar]
- Tarin JJ, Perez-Albala S, Pertusa JF, Cano A. Oral administration of pharmacological doses of vitamins C and E reduces reproductive fitness and impairs the ovarian and uterine functions of female mice. Theriogenology. 2002b;57:1539–1550. doi: 10.1016/s0093-691x(02)00636-2. [DOI] [PubMed] [Google Scholar]
- Tilly JL. Commuting the death sentence: how oocytes strive to survive. Nat Rev Mol Cell Biol. 2001;2:838–848. doi: 10.1038/35099086. [DOI] [PubMed] [Google Scholar]
- Ventura SJ, Abma JC, Mosher WD, Henshaw S. Estimated pregnancy rates for the United States, 1990–2000: an update. Natl Vital Stat Rep. 2004;52:1–9. [PubMed] [Google Scholar]
- Wu JM, Zelinski MB, Ingram DK, Ottinger MA. Ovarian aging and menopause: current theories, hypotheses, and research models. Exp Biol Med (Maywood) 2005;230:818–828. doi: 10.1177/153537020523001106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Serum fatty acid profiles of F1 generation animals on the HCO, SOY, and DHA diets.
Table S2 Serum fatty acid profiles of aged animals following acute dietary treatment with the CHOW, SOY, or DHA diet.
Fig. S1 Animal growth and diet consumption in acute dietary treatment.


