Abstract
Hodgkin lymphoma is a highly curable malignancy in early and advanced stages. Most patients are diagnosed in their teens or twenties and are expected to live decades beyond their treatment. Therefore, the toxicity of treatment must be balanced with the goal of cure. Thus, treatment has been refined through prognostic models and positron emission tomography-computed tomography (PET-CT)-directed therapy. Stratification by prognostic models defines groups of patients with favorable characteristics who may be treated with less intensive therapy upfront, including fewer cycles of chemotherapy, lower doses of radiation, or omission of radiation altogether. Alternatively, high-risk patients may be assigned to a more aggressive initial approach. The modern use of interim PET-CT allows further tailoring of treatment by response.
Keywords: Hodgkin lymphoma, early unfavorable, early favorable, advanced stage, PET directed, prognosis
Introduction
Patients with classical Hodgkin lymphoma (HL) are generally classified in 1 of 3 groups: early-stage favorable, early-stage unfavorable, or advanced-stage disease. Unfortunately, there is no universal prognostic system for early-stage disease, as each cooperative group uses unique scoring systems for stratifying patients on clinical trials. Therefore, in early-stage disease, the dose of radiation and number of chemotherapy cycles are dependent on which scoring system is used. For initial treatment planning, early-stage disease is categorized by the presence of a bulky mediastinal mass (defined by modern criteria as measuring >10 cm on computed tomography [CT]). Those with bulky masses generally benefit from more cycles of chemotherapy and the addition of consolidative radiation. However, the role of radiation in early-stage disease has become increasingly contentious due to the long-term associated toxicities. Furthermore, patients with early-stage disease who have multiple risk factors were excluded from early-stage trials and are often treated as advanced stage.
Prognostication in advanced-stage patients is defined by the International Prognostic Score (IPS) (Table 3).1 Patients with low-risk IPS (1-3) are treated with 6 cycles of standard ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine), whereas high-risk patients are considered for initial treatment with the more intensive German-derived regimen, escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone).
Table 3.
International Prognostic Score.1
| Risk factor | Points | Points: freedom from progression of disease |
|---|---|---|
| Albumin <4 g/dL | 1 | |
| Hemoglobin <10.5 g/dL | 1 | 0: 84% |
| Male | 1 | 1: 77% |
| Age ⩾45 y | 1 | 2: 67% |
| Stage IV disease | 1 | 3: 60% |
| Leukocytosis (WBC ⩾15 000/mm3) | 1 | 4: 51% |
| Lymphocytopenia (ALC <600/mm3 or <8% of WBC) | 1 | ⩾5: 42% |
Abbreviations: ALC, absolute lymphocyte count; WBC, white blood cell.
Treatment may be further refined through positron emission tomography (PET)-adapted therapy. Achievement of a negative interim PET-CT is highly predictive of long-term progression-free survival (PFS) among patients treated for HL.2–4 Multiple clinical trials assessing PET-adapted treatment for early-stage and advanced-stage HL have demonstrated the advantages of this approach in early detection of those who may benefit from either de-escalation, or intensification of treatment in those responding, or failing to achieve an adequate response, respectively.5–8 Highlighting the complexities of PET-directed approaches are seemingly conflicting results regarding the advantage of changing therapy based on PET-CT results.9 Interim PET is not perfectly predictive of response, particularly in advanced disease where the negative predictive value (NPV) following ABVD ranges from 86% to 95%, but the positive predictive value is as low as 44%.10 The NPV is much stronger in those initially treated with escalated BEACOPP, with NPV generally estimated at greater than 95%.11,12 However, relapses occur in those with interim negative PET-CT, and premature de-escalation of therapy could compromise long-term disease-free survival. Additional issues with interim PET scans include the significant inter-individual reliability, which ranges from 70% to 85%.13,14 Also, the definition of PET negative and PET positive differs between clinical trials. The PET-CT scan results are evaluated on a 5-point Deauville scale, with scores of 4 or 5 indicating uptake that is greater than the liver.13 Practically speaking, patients escalating therapy should interpret a Deauville score of 1 to 3 as negative, whereas those de-escalating therapy should consider a Deauville score of 1 to 2 as negative. Significant debate continues about the interpretation and utility of interim PET-CT in directing therapy for HL.10,15 Nevertheless, PET-CT is recommended for interim staging.
In this review, we will describe the optimal frontline evidence-based therapies for patients with classical Hodgkin lymphoma by stage, prognosis, and interim treatment response.
Early-Stage Hodgkin Lymphoma
Early-stage HL is defined as Ann Arbor stage I to II disease. Long-term prognosis is very favorable with greater than 90% to 95% of patients achieving long-term remissions, depending on additional risk factors.16 Among patients with limited-stage disease, further delineation into favorable or unfavorable risk categories is based on the presence of mediastinal bulk, B symptoms, erythrocyte sedimentation rate (ESR), age, and the number of nodal sites. The large cooperative groups have largely defined classification as “early-favorable” or “early-unfavorable” disease (Table 1). It is also important to note that the classification of nodal sites also differs among each prognostic group (Table 2). Given the discrepancies between the definition of “early-favorable” among these prognostic systems, the practical treatment of early-stage disease is separated by bulky versus nonbulky disease, and further decisions about the number of cycles of therapy and dose of radiation therapy should be based on the appropriate risk group.
Table 1.
Early-stage prognosis in classical Hodgkin lymphoma.
| Risk factor | GHSG | EORTC | NCCN | NCIC |
|---|---|---|---|---|
| Age | ⩾50 | ⩾40 | ||
| Histology | Mixed cellularity or lymphocyte depletion | |||
| ESR and B symptoms | >50 if A, >30 if B | >50 if A, >30 if B | ⩾50 or any B symptoms | >50 or any B symptoms |
| Bulky | MMR > 0.33 | MMR > 0.35 | >10 cm | MMR > 0.33 or >10 cm |
| Nodal sites | >2 | >3 | >3 | >3 |
| Extranodal lesion | Any |
Abbreviations: ESR, erythrocyte sedimentation rate; EORTC, European Organization for the Research and Treatment of Cancer; GHSG, German Hodgkin Study Group; MMR, mediastinal mass ratio; MMT, mediastinal mass/thoracic diameter; NCCN, National Comprehensive Cancer Network, NCIC, National Cancer Institute of Cancer.
Table 2.
Number of lymph node regions by prognostic group.
| Lymph node regions | Ann Arbor | EORTC | GHSG |
|---|---|---|---|
| R cervical/supraclavicular | 1 | 1 | 1a |
| R infraclavicular/subpectoral | 2 | 2b | 1a |
| R axilla | 3 | 2b | 2 |
| L cervical/supraclavicular | 4 | 3 | 3a |
| L infraclavicular/subpectoral | 5 | 4b | 3a |
| L axilla | 6 | 4b | 4 |
| Mediastinum | 7 | 5c | 5c |
| R hilum | 8 | 5c | 5c |
| L hilum | 9 | 5c | 5c |
| Total nodal regions | 9 | 5 | 5 |
Abbreviations: EORTC, European Oncology Research Group; GHSG, German Hodgkin Study Group; L, left; R, right.
Cervical/supraclavicular and infraclavicular/subpectoral regions are combined into one nodal region in the GHSG, whereas each count as a separate lymph node group in the EORTC.
Infraclavicular/subpectoral and axillary nodal regions are combined in EORTC.
Mediastinal and bilateral hilar nodes are combined into one region in the EORTC and GHSG.
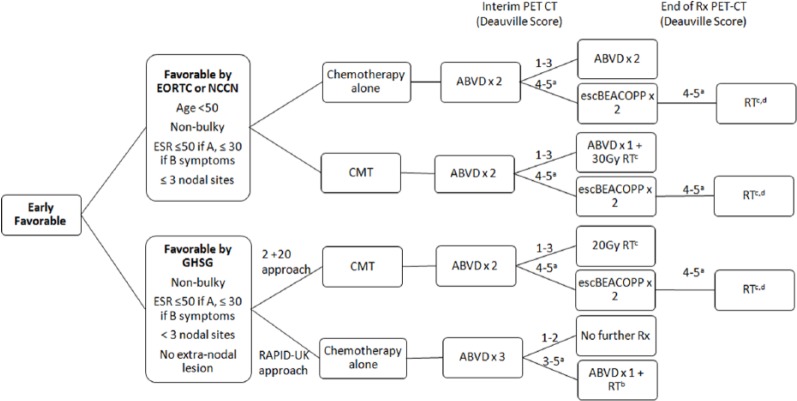
Early-favorable stage HL
Early-favorable disease includes patients with a limited number of lymph nodal regions, nonbulky disease, low ESR, and no B symptoms (Figure 1). The choice of therapy depends on risk factors beyond bulk, as well as response on interim PET-CT. These options include the following: 2 cycles of ABVD and 20 Gy of involved site radiation therapy (ISRT), 3 to 4 cycles of ABVD and 30 Gy of ISRT, or 3 to 4 cycles of ABVD alone.
Figure 1.
Treatment Schema: Early Favorable Hodgkin Lymphoma.
aPatients without response should have a biopsy. If biopsy is positive, consider transitioning to a salvage regimen.
bThis approach is based on the RAPID-UK study, but not a preferred approach. Patients with Deauville score of 4 to 5 should have a biopsy and consider transitioning to escalated BEACOPP if negative or a salvage regimen and autologous transplant if biopsy is positive.
cRadiation with smaller fields, including involved site irradiation (ISRT) or involved nodal irradiation (INRT).
dRadiation dose varies based on PET response and bulky versus nonbulky disease.
GHSG indicates German Hodgkin Study Group; EORTC, European Organization for the Research and Treatment of Cancer; NCCN, National Comprehensive Cancer Network; CMT, combination modality therapy; RT, radiation therapy; escBEACOPP, escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone; ABVD, doxorubicin, bleomycin, vinblastine, dacarbazine; Rx, treatment.
Combination modality therapy
Patients with favorable disease based on the German Hodgkin Study Group (GHSG) criteria are candidates for treatment with 2 cycles of ABVD followed by 20 Gy of ISRT. It is important to remember that the GHSG criterion for early-favorable disease differs from other prognostic scoring systems, largely by their limitation to patients with only 1 to 2 sites of disease. The “2 + 20” approach arose from data in the HD10 trial demonstrating noninferiority compared with other more intense treatment regimens. The HD10 study compared 4 treatment strategies in patients with early-favorable HL by GHSG criteria: 2 or 4 cycles of ABVD combined with either 30 or 20 Gy of involved field radiation therapy (IFRT).17 The 4 regimens were found to be equally effective, but with increased rates of infection and hematologic toxicity in patients receiving 4 versus 2 cycles of ABVD. The 5-year freedom from treatment failure rate was 91%, and overall survival (OS) was 97% in patients treated with 2 cycles of ABVD and 20 Gy of radiation. The difference in 5-year failure-free survival (FFS) in the most intensive compared with the least intensive strategy was only 1.6%.18
Patients with stage IA to IIA nonbulky disease who are favorable by European Organization for the Research and Treatment of Cancer (EORTC), National Comprehensive Cancer Network (NCCN), or National Cancer Institute of Cancer criteria, but unfavorable by the GHSG may be treated with combination modality therapy (CMT) with ABVD for 3 cycles followed by 30 Gy of ISRT or 3 to 4 cycles of ABVD alone in a PET-directed manner. Combination modality therapy is supported by several clinical trials that demonstrate improved PFS.9,17,19 However, the long-term survival is inferior to treatment with chemotherapy alone owing to an increased rate of death from causes other than HL, including second malignancies and cardiac death.16,18,19
Chemotherapy alone
Treatment with 4 to 6 cycles of ABVD alone is supported by the HD6 trial, which demonstrated superior long-term survival in patients with stage IA to IIA nonbulky disease treated with chemotherapy alone compared with CMT.16 The experimental arm was randomized to ABVD alone with interim CT scans at 2 and 4 cycles. Those who achieved a complete response (CR) after 2 cycles of therapy received 4 cycles of ABVD total, whereas those with partial response or an unconfirmed CR (CRu) received 6. The radiation arm received an outdated technique, subtotal nodal radiation therapy, with or without ABVD depending on the additional risk factors. The 12-year PFS was 87% in the chemotherapy-alone arm compared with 92% in the RT arm (P = .05); however, the 12-year OS was superior in ABVD alone; 94% compared with 87% (P = .04). Although the use of radiation initially seemed to decrease the rate of Hodgkin-related deaths, after longer follow-up, there were increased rates of death in the radiation arm (24 versus 12 patients). These included deaths due to second cancers (10 versus 4), causes other than HL (10 versus 2), secondary malignancies (10 versus 2), and cardiac events (26 versus 16). This trial had a median follow-up of 12 years and therefore captured more radiation-induced late toxicities than prior studies. Second malignancies began 5 years after treatment and continued to arise for decades, highlighting the importance of extended follow-up beyond 5 to 10 years. Further support for chemotherapy alone in these patients was provided by individual data comparisons of patients included on the GHSG HD10 and HD11 and Canadian HD6 trials. These trials demonstrated superior 8-year PFS in those treated with CMT compared with ABVD alone (93% versus 87%), but the same OS at 8 years (95% in all groups).20 It is likely that beyond 10 years, OS in the chemotherapy alone arm will supersede that seen in the CMT, as rates of radiation complications accumulate over time.
PET-directed therapy
Practitioners should first determine whether patients are eligible for 2 cycles of ABVD + 20 Gy of radiation. Otherwise, after discussing the short-term and long-term risks associated with radiation therapy, a chemotherapy alone or CMT approach should be planned. Given the long-term survival benefit discussed above, treatment with ABVD alone is favored over CMT in patients with favorable disease who do not meet criteria for “2 + 20.” Interim PET-CT is performed after 2 cycles of therapy. Once a CR is reached, further imaging may be performed with CT only.
Chemotherapy-alone planned
Those planning for a chemotherapy-alone approach would be eligible for treatment with 3 to 4 cycles of ABVD with interim PET staging. The minimum amount of therapy (3 cycles) is supported by the RAPID-UK study.21 If planning this approach, patients would be treated with 3 cycles of ABVD, followed by interim PET-CT. Patients with a negative PET (defined as a Deauville score of 1-2) would require no further treatment. Those with a Deauville score of 3 to 5 may proceed to 1 additional cycle of ABVD and 30-Gy ISRT. In this study, those treated with only 3 cycles of ABVD had exceptional PFS at 3 years; 94.6% in patients receiving 4 cycles of ABVD + 30 Gy of IFRT compared with 90.8% for those receiving 3 cycles ABVD alone. Despite impressive results with limited chemotherapy, the RAPID study had several limitations, including short follow-up and the incorporation of mostly very favorable patients (2/3 favorable by GHSG criteria). Therefore, we would recommend only using this treatment approach in patients who are favorable by the stringent GHSG criteria to prevent undertreatment in the less favorable patients who were underrepresented in this trial.
In all other patients, PET-CT scan should be performed after 2 cycles of ABVD (during the last 2 weeks of the second cycle). Patients achieving a CR after 2 cycles may proceed to 2 additional cycles of therapy to complete a total of 4 cycles.16 Patients with a Deauville score of 4 or 5 may be transitioned to 2 cycles of escalated BEACOPP for 2 cycles. Positron emission tomography scan may be repeated after escalated BEACOPP therapy, and radiation omitted if a CR is achieved (Deauville 1-3).
Combination modality therapy planned
Patients who are planning CMT should be treated with ABVD for 2 cycles followed by an interim PET-CT. Those who are favorable by the GHSG criteria with a Deauville score of 1 to 3 receive 20 Gy of radiation therapy. Patients with more than 2 sites of disease, but otherwise favorable disease by EORTC or NCCN criteria who achieve a CR after 2 cycles of ABVD (Deauville score of 1-3) may proceed to 1 to 2 additional cycles of ABVD and 30 Gy of ISRT.22 Those with an incomplete response (Deauville score of 4-5) should proceed to biopsy and transitioned to escalated BEACOPP as above. The H10 trial (EORTC/LYSA [Lymphoma Study Association]/FIL [Fondazione Italiana Linfomi])9 assessed PET intensification in early-stage disease. Although the initial study was terminated early due to a prespecified early stopping rule, updated results have recently been published22 demonstrating that patients with a positive PET2 had improved outcomes (PFS) from intensification to escalated BEACOPP chemotherapy followed and involved nodal radiation therapy (INRT) compared with standard ABVD and INRT, with a 13% overall improvement in 5-year PFS (5-year PFS of 91% versus 77% in the BEACOPP versus ABVD arms, respectively). Although treatment intensification clearly improved PFS, it did not affect OS at 5-year follow-up.
Early-unfavorable stage HL
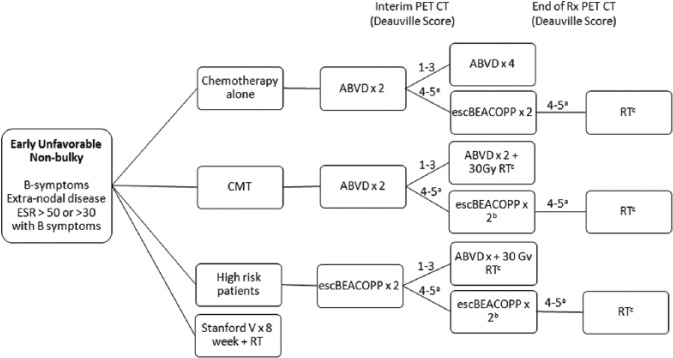
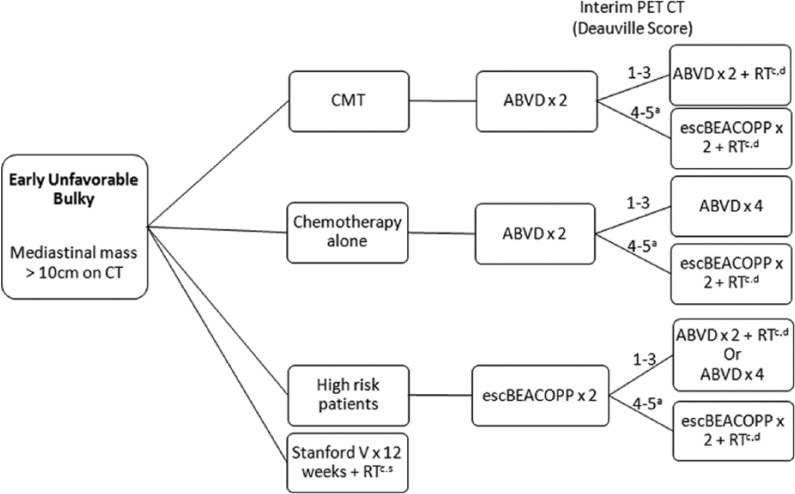
Patients with bulky mediastinal masses, B symptoms, elevated ESR, or multiple nodal sites are generally classified as early-unfavorable HL (Figures 2 and 3). Patients are further classified as unfavorable bulky or nonbulky. About 20% to 25% of patients with early-stage HL have bulky mediastinal masses. Traditionally, mediastinal bulk was defined as a mediastinal mass measuring more than one-third of the thoracic diameter on a posterior-anterior chest radiograph. The modern definition uses a chest CT scan and defines bulk as a mass greater than 10 cm.23 Patients with large mediastinal masses are at higher risk for relapse, and the standard of care in this population is CMT with chemotherapy and radiation. However, the optimal regimen and number of cycles of chemotherapy are debated.
Figure 2.
Treatment Schema: Early Unfavorable (non-bulky) Hodgkin Lymphoma.
aPatients without response should have a biopsy. If biopsy is positive, consider transitioning to a salvage regimen.
bReimage after 2 cycles of escBEACOPP to ensure response; if no response, consider changing to a salvage regimen.
cRadiation techniques with smaller fields including involved site irradiation (ISRT) or involved nodal irradiation (INRT) are preferred.
ABVD indicates doxorubicin, bleomycin, vinblastine, dacarbazine; CMT: combination modality therapy; escBEACOPP: escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone; ESR, erythrocyte sedimentation rate; PET-CT, positron emission tomography-computed tomography; RT: radiation therapy; Rx: treatment.
Figure 3.
Treatment Schema: Early Unfavorable (bulky) Hodgkin Lymphoma.
aPatients without response should have a biopsy. If biopsy is positive, consider transitioning to a salvage regimen.
bReimage after 2 cycles of escBEACOPP to ensure response; if no response, consider changing to a salvage regimen.
cRadiation techniques with smaller fields including involved site irradiation (ISRT) or involved nodal irradiation (INRT) are preferred.
dBulky disease sites may receive 30 to 36 Gy of RT.
ABVD indicates doxorubicin, bleomycin, vinblastine, dacarbazine; CMT: combination modality therapy; escBEACOPP: escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone; PET-CT, positron emission tomography-computed tomography; RT: radiation therapy; Rx: treatment.
Combination modality therapy
The most commonly used initial regimen in early-unfavorable HL is ABVD × 4 to 6 cycles followed by ISRT to 30 Gy.24,25 This treatment results in OS exceeding 90% and FFS ranging from 80% to 85%.26,27 Attempts to improve outcomes through alternative chemotherapy regimens such as Stanford V and escalated BEACOPP have failed to affected OS, largely owing to the increased rate of treatment-related mortality with escalated BEACOPP, and highly effective salvage therapy in those who relapse following standard ABVD.25,28,29 The HD14 study assessed more than 1600 patients with early-unfavorable HL by the GHSG criteria, including 18.7% of patients with bulky mediastinal masses.30 Patients were treated with ABVD for 4 cycles followed by radiation to 30 Gy (Arm A) or a 2 + 2 strategy of 2 cycles of escalated BEACOPP followed by 2 cycles of ABVD and 30 Gy of radiation (Arm B). There was no difference in OS, although there was a significant 6.2% advantage in PFS favoring the escalated BEACOPP arm at 5 years. Improvements in PFS were offset by higher toxicity in the escalated BEACOPP arm, including 4 treatment-related deaths.
Chemotherapy alone
Although CMT is considered the standard of care in patients with bulky mediastinal masses, 2 retrospective analyses have suggested that it may be safe to omit radiation therapy in patients with unfavorable disease, regardless of bulk, following a negative PET-CT at the end of ABVD treatment.31,32 Outcomes in patients with bulky disease who achieved PET negativity did not differ from those without bulky disease. Unlike early-favorable patients in whom 4 cycles of ABVD alone may be adequate therapy, we recommend that those with an elevated ESR, B symptoms, or bulky mediastinal masses be treated with 6 cycles of ABVD if radiation is omitted.
Of note, patients at the highest risk for relapses are those with multiple adverse factors including bulky mediastinal masses plus B symptoms, >4 sites of disease, and extranodal disease. These patients were excluded from trials in early-stage disease and may be considered for initial therapy according to the algorithm for advanced-stage disease.30
PET-directed therapy
Patients should be categorized based on the presence or absence of bulk (mediastinal mass >10 cm on CT) and determined whether a CMT approach is planned. Those with nonbulky unfavorable disease are considered for omission of radiation therapy, whereas those with bulky disease should generally complete radiation at the end of chemotherapy. All patients should have an interim PET-CT after 4 cycles of therapy to assess response and those with a Deauville score of 5 should have a biopsy documenting disease prior to changing therapy.
Nonbulky disease
Patients with nonbulky disease planned for a chemotherapy alone approach should be treated with ABVD for 2 cycles followed by an interim PET-CT (Figure 2). Patients with a Deauville score of 1 to 3 may complete 4 additional cycles of therapy for a total of 6 cycles of ABVD.
Guidelines support CMT or ABVD alone33; however, the role of radiation therapy in nonbulky disease continues to be fiercely debated.34 Radiation improves local disease control and PFS by up to 5% to 7%.9 However, it compromises long-term OS beyond 10 years due to increased rates of secondary malignancies that begin at 5 years and continue to occur 20 to 40 years beyond treatment, as well as death from other causes.16,18,35 Combination modality therapy trials included outdated forms of radiation therapy such as IFRT and subtotal nodal irradiation that could have theoretically increased this risk. Current guidelines recommend smaller radiation fields such as ISRT and INRT based on prospective data demonstrating significantly increased doses of radiation to healthy tissue and higher rates of second malignancies in patients treated with the larger fields of IFRT.36 However, available data do not demonstrate the impact of radiation which diminishes the use of smaller radiation fields or lower doses in the current era.17,35
Those planned for CMT with a Deauville score of 1 to 3 on interim PET may proceed to 30 Gy of RT (4 doses of ABVD + 30 Gy RT). Patients who have a Deauville score of 4 to 5 may proceed to ABVD for 2 cycles followed by 30 Gy of RT (total of 6 cycles of ABVD).21 However, this approach is not preferred. We recommend these patients transition to escalated BEACOPP for 2 cycles followed by RT to 30 Gy based on data from EORTC/LYSA/FIL H-10 study.22,37 Patients who are PET negative after escalated BEACOPP may be considered for omission of radiation therapy, acknowledging that there will be an increased risk of relapse in the short term, although likely no impact on long-term survival.
Bulky disease
Patients with stage II bulky disease are generally recommended for initial treatment with ABVD followed by PET-CT after 2 cycles of therapy (Figure 3). Those with a Deauville score of 1 to 3 may proceed to 2 additional cycles of ABVD and 30 Gy of ISRT or to 4 additional cycles of ABVD chemotherapy. Support for 4 to 6 cycles in this setting comes from a subgroup analysis of the HD11 trial, which compared patients with bulky disease treated with 4 versus 6 cycles of ABVD with 30-Gy IFRT and noted no difference in PFS or OS.26 Overall, this approach results in long-term tumor control in approximately 80% of patients.19
Patients who have a Deauville score of 4 to 5 may proceed to ABVD for 2 to 4 more cycles followed by radiation (total of 6 cycles of ABVD).21 However, we would recommend transitioning to escalated BEACOPP for 2 cycles followed by radiation therapy.7,38
Some practitioners suggest initial treatment with escalated BEACOPP in high-risk stage II patients. Most studies looking at BEACOPP compared with ABVD frontline have failed to show benefit in stage II patients.39 If BEACOPP is chosen in this setting, it should be given for 2 cycles followed by interim PET and de-escalation to ABVD for 4 remaining cycles if interim PET-CT is scored as a Deauville 1 to 3.40 If CMT is planned, another approach would be 2 cycles of escalated BEACOPP followed by interim PET and completion of 2 cycles of ABVD and 30 Gy of ISRT in PET-negative patients based on the HD14 data in early-unfavorable disease.10
An alternative regimen for bulky disease is the Stanford V regimen (with mechlorethamine, doxorubicin, vincristine, bleomycin, vinblastine, etoposide, and prednisone)25 followed by 36 Gy of ISRT. This regimen was noninferior to 6 to 8 cycles ABVD and radiation regarding PFS and OS (5-year FFS: 85% versus 79% in ABVD and Stanford V, respectively, hazard ratio [HR]: 0.68, 95% confidence interval [CI]: 0.37-1.25, P = .22; 5-year OS: 96% versus 92%, HR: 0.49, 95% CI: 0.16-1.47, P = .19). Although Stanford V is still an option, it is less commonly used outside academic medical centers and additionally has the increased concern for infertility due to the use of mechlorethamine and etoposide.
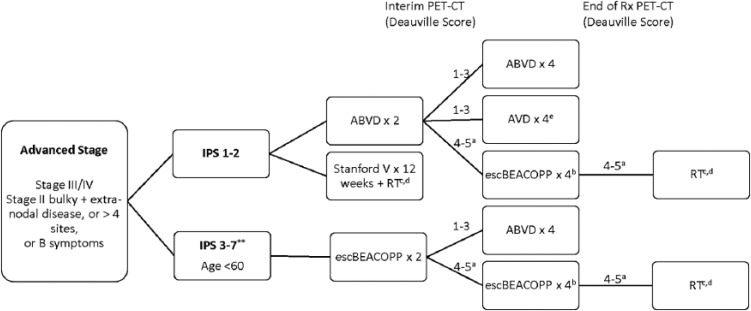
Advanced-Stage Disease
Advanced-stage disease generally refers to patients with Ann Arbor stage III/IV disease (Figure 4); however, patients with high-risk stage II disease are also frequently included. In contrast to early-stage disease where the long-term cure exceeds 90%, only approximately 65% to 75% of patients with advanced-stage Hodgkin will remain disease free at 10 years.28,39,41 The IPS score defines prognosis by incorporating the following risk factors: albumin level, hemoglobin, sex, age >45 years, stage IV, and the presence of leukocytosis or lymphocytosis (Table 3).1 Patients with an IPS greater than or equal to 3 were found to have inferior treatment outcomes and identified as potentially requiring more intensive therapy.1
Figure 4.
Treatment Schema: Advanced Stage Hodgkin Lymphoma.
aPatients with concern for progression should have a biopsy. If biopsy is positive, consider transitioning to a salvage regimen.
bInterim PET-CT after 2 cycles of escBEACOPP to ensure response. If no response, consider transitioning to salvage regimen.
cRadiation techniques with smaller fields including involved site irradiation (ISRT) or involved nodal irradiation (INRT) are preferred.
dRadiation to initially bulky site or with Deauville score of 4 to 5 after completion of chemotherapy.
eConsider omitting bleomycin for elderly patients, or those with pulmonary comorbidities, or patients at risk for bleomycin lung toxicity.
ABVD indicates doxorubicin, bleomycin, vinblastine, dacarbazine; escBEACOPP, escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone; IPS, International Prognostic Score; PET-CT, positron emission tomography-computed tomography; RT, radiation therapy; Rx, treatment.
**Consideration for escalated BEACOPP in patients <age 60 years with high-risk disease. Also may treat with ABVD.
Guidelines generally recommend treatment with chemotherapy alone, starting with either ABVD or escalated BEACOPP depending on the IPS risk score. Escalated BEACOPP differs from ABVD by incorporating elevated doses of etoposide, doxorubicin, and cyclophosphamide. Multiple head-to-head, randomized comparisons of these 2 regimens have found overall similar long-term survival at approximately 75% to 85% at 10 years, improved PFS with BEACOPP (65%-70% in ABVD at 10 years compared with 75%-80% with escBEACOPP)39,41 but increased rates of infertility,42 grade 4 infections, hospitalizations for neutropenia, and secondary hematologic malignancies.39,43 The controversy continues, however, as one large meta-analysis failed to identify an OS advantage with the more aggressive regimen,44 whereas the second demonstrated a significant improvement in OS to the tune of 5% to 10% at 5 years in patients who were treated with 6 cycles of escalated BEACOPP initially.45 Patients with stage II disease were included in the former meta-analysis but excluded from the latter, potentially accounting for some of this discrepancy. In addition, there is ongoing debate about the role radiation in advanced-stage disease with bulky mediastinal masses. In general, it may be omitted in those able to achieve PET negativity at the end of treatment.46 Stanford V for 12 weeks followed by ISRT is also an accepted approach in low-risk advanced-stage disease.47 This regimen showed no statistically significant difference compared with 6 to 8 cycles ABVD (overall response rate of 73% for ABVD and 69% for Stanford V and FFS of 74% for ABVD and 71% for Stanford V (P = .32). However, this regimen is not commonly used outside of Stanford for the reasons previously listed.
Treatment by IPS risk score
Patients with an IPS score of 1 of 3 are generally treated with 6 cycles of ABVD. Other options for therapy in patients with low-risk (IPS <3) disease include the Stanford V regimen for 12 weeks.47 This regimen also results in similar efficacy and minimal toxicity compared with ABVD. Those with an IPS score of ⩾3 and age <60 years may consider initial treatment with either ABVD or escalated BEACOPP. However, a recent large randomized clinical trial comparing high-risk patients with an IPS score ⩾3 and stage III/IV disease treated with ABVD × 8 versus escalated BEACOPP × 4 followed by baseline BEACOPP × 4 demonstrated no difference in event-free survival or OS at 8 years.28
PET-adapted therapy
Practitioners should first decide whether patients are to be treated initially with ABVD or escalated BEACOPP. Interim PET-CT is performed after 2 cycles of therapy (regardless of which regimen is initiated). Once a CR is reached, further imaging may be performed with CT only.
Starting with ABVD
Patients planned for initial therapy with ABVD who achieve a CR on interim PET (Deauville score of 1-3) may continue to complete a total of 6 cycles of therapy. Alternatively, some patients may de-escalate therapy to AVD (omitting bleomycin) for the remaining 4 cycles. A large phase III clinical trial demonstrated near equivalency when de-escalating to AVD after negative interim PET, with 3-year PFS and OS of 85.7% and 97.2%, respectively, for ABVD × 6 compared with 84.4% and 97.6%, respectively in ABVD followed by AVD.7
Patients with a Deauville score of 4 to 5 on interim PET after 2 cycles of therapy can be considered for intensification to escalated BEACOPP. Positron emission tomography should then be repeated after 3 cycles of escalated BEACOPP.7,38 Those with a Deauville score of 1 to 3 may continue with 1 additional cycle of escalated BEACOPP. This technique was supported by large phase II and phase III studies.5,6 The US intergroup study of response-adapted therapy in stage III/IV patients demonstrated a 2-year PFS of 64% for PET2-positive patients, which was favorable compared with the expected 2-year PFS of 15% to 30% in patients who continue ABVD.6 Furthermore, the Europeans performed a large multinational study of high-risk stage II through stage IV patients and found that those with PET positivity after 2 cycles who transitioned to escalated BEACOPP had a 3-year PFS of 68%, OS of 88%, and more than 74% of patients achieved PET negativity.7 In this trial, patients with a negative PET at the completion of therapy could omit radiation therapy.
Starting with escalated BEACOPP
Patients planned for initial therapy with escalated BEACOPP should have interim restaging with a PET-CT after 2 cycles of therapy. Options for those who are responding well to initial therapy (Deauville score of 1-3) include either de-escalation to ABVD for 4 additional cycles40 or de-escalation to baseline BEACOPP.48 In general, de-escalation to ABVD is favored. High-risk patients may require 6 cycles of escBEACOPP if interim PET-CT remains positive (Deauville 4-5).
Consolidative radiation therapy
Patients with a residual mass >2 cm after therapy should have a PET-CT to confirm disease response. Those with a negative PET require no further consolidation, whereas those with a positive PET should receive consolidative radiation therapy.46 A prospective analysis in British Columbia found that patients with bulky versus nonbulky disease at diagnosis and a negative PET scan at the end of treatment had no difference in the 3-year time to progression (TTP) in those with bulky versus nonbulky disease at diagnosis (86% versus 91%, P = .71). However, patients with a positive PET scan had a far inferior outcome (3-year TTP: 55% versus 89%, P = .00001). This was also demonstrated by German HD15 trial.49
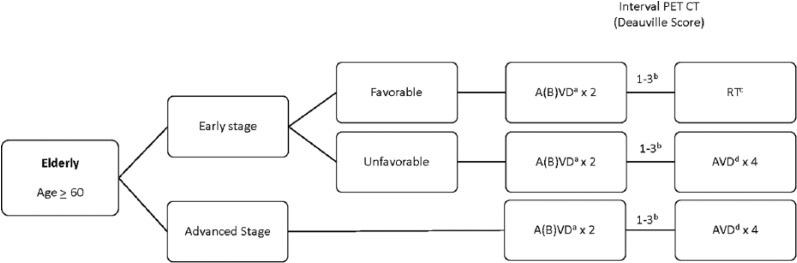
Elderly
Elderly patients are defined as age ⩾60 years and represent between 15% and 30% of all HL cases (Figure 5). These patients have a universally poor prognosis, with 5-year OS rates ranging from 40% to 55%.50 The standard of care in this population remains ABVD chemotherapy. However, up to one-third of patients will have bleomycin lung toxicity (BLT) compared with approximately 5% for younger patients; furthermore, the risk of death from BLT is also higher, with up to 25% mortality related to BLT.51–53 Trials attempting to improve on standard of care with ABVD have been disappointing overall.54,55 Other attempts to minimize toxicity have included elimination of bleomycin from ABVD. Among elderly patients with early-favorable HL, elimination of bleomycin resulted in decreased local control; however, long-term survival was not compromised, with OS rates exceeding 98%.56 These data suggest that an upfront regimen of AVD may be considered, particularly in patients at high risk for BLT. Alternatively, trials in younger patients have demonstrated that bleomycin may be omitted after 2 cycles in those achieving a response without compromising efficacy.56 To address the balance the toxicity with efficacy, the GHSG compared elderly favorable patients treated with either 2 cycles of ABVD or AVD each followed by IFRT compared with 4 cycles of ABVD.54 Grade III/IV events were much higher in patients receiving 4 cycles of therapy (65% overall) as were BLT. Given the lack of data in this group, the standard of care should be with 2 cycles of AVD or ABVD followed by IFRT in early-favorable HL. Patients with advanced disease should be considered for 2 cycles of ABVD, followed by 2 to 4 cycles of AVD or AVD for a total of 4 to 6 cycles depending on tolerability.51,56
Figure 5.
Treatment Schema: Elderly Hodgkin Lymphoma.
aBleomycin may be omitted from initially in patients at risk for bleomycin lung toxicity.
bRe-image after 2 cycles to assess response. Patients without response should have a biopsy. If biopsy is positive, consider transitioning to a salvage regimen with safety and efficacy in the elderly such as brentuximab vedotin or PD-1 inhibitors.
Use of BEACOPP generally not recommended in this population.
cRadiation techniques with smaller fields including involved site irradiation (ISRT) or involved nodal irradiation (INRT) are preferred.
dPatients who receive bleomycin initially and achieve a CR after 2 cycles, may omit bleomycin from future cycles.
Conclusions
Treatment of Hodgkin lymphoma has become increasingly complex with the use of prognostic systems and interim PET-directed therapy. As we begin to gather more information on the long-term consequences of our treatments, it has become clear that the most aggressive therapies do not benefit all patients in the long run. By carefully selecting patients who require more or less aggressive therapy from the beginning and then tailoring treatment to response, we hope to strike the balance between treatment efficacy and toxicity.
Footnotes
Peer review:Seven peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1137 words, excluding any confidential comments to the academic editor.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: PBA wrote the first draft of the manuscript. LIG contributed to the writing of the manuscript. PBA and LIG agree with manuscript results and conclusions, jointly developed the structure and arguments for the paper, made critical revisions and approved final version, and reviewed and approved the final manuscript.
Disclosures and Ethics: As a requirement of publication, author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality, and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1. Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N Engl J Med. 1998;339:1506–1514. [DOI] [PubMed] [Google Scholar]
- 2. Rigacci L, Puccini B, Zinzani PL, et al. The prognostic value of positron emission tomography performed after two courses (INTERIM-PET) of standard therapy on treatment outcome in early stage Hodgkin lymphoma: a multicentric study by the fondazione italiana linfomi (FIL). Am J Hematol. 2015;90:499–503. [DOI] [PubMed] [Google Scholar]
- 3. Gallamini A, Patti C, Viviani S, et al. Early chemotherapy intensification with BEACOPP in advanced-stage Hodgkin lymphoma patients with a interim-PET positive after two ABVD courses. Br J Haematol. 2011;152:551–560. [DOI] [PubMed] [Google Scholar]
- 4. Ciammella P, Filippi AR, Simontacchi G, et al. Post-ABVD/pre-radiotherapy (18)F-FDG-PET provides additional prognostic information for early-stage Hodgkin lymphoma: a retrospective analysis on 165 patients. Br J Radiol. 2016;89:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zinzani PL, Broccoli A, Gioia DM, et al. Interim positron emission tomography response-adapted therapy in advanced-stage Hodgkin lymphoma: final results of the phase II part of the HD0801 study. J Clin Oncol. 2016;34:1376–1385. [DOI] [PubMed] [Google Scholar]
- 6. Press OW, Li H, Schoder H, et al. US intergroup trial of response-adapted therapy for stage III to IV Hodgkin lymphoma using early interim fluorodeoxyglucose-positron emission tomography imaging: southwest oncology group S0816. J Clin Oncol. 2016;34:2020–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnson P, Federico M, Kirkwood A, et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N Engl J Med. 2016;374:2419–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Radford J, Illidge T, Counsell N, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med. 2015;372:1598–1607. [DOI] [PubMed] [Google Scholar]
- 9. Raemaekers JM, Andre MP, Federico M, et al. Omitting radiotherapy in early positron emission tomography-negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol. 2014;32:1188–1194. [DOI] [PubMed] [Google Scholar]
- 10. Mesguich C, Cazeau AL, Bouabdallah K, et al. Hodgkin lymphoma: a negative interim-PET cannot circumvent the need for end-of-treatment-PET evaluation. Br J Haematol. 2016;175:652–660. [DOI] [PubMed] [Google Scholar]
- 11. Kedmi M, Apel A, Davidson T, et al. High-risk, advanced-stage Hodgkin lymphoma: the impact of combined escalated BEACOPP and ABVD treatment in patients who rapidly achieve metabolic complete remission on interim FDG-PET/CT scan. Acta Haematol. 2016;135:156–161. [DOI] [PubMed] [Google Scholar]
- 12. Markova J, Kahraman D, Kobe C, et al. Role of [18F]-fluoro-2-deoxy-D-glucose positron emission tomography in early and late therapy assessment of patients with advanced Hodgkin lymphoma treated with bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine and prednisone. Leuk Lymphoma. 2012;53:64–70. [DOI] [PubMed] [Google Scholar]
- 13. Biggi A, Gallamini A, Chauvie S, et al. International validation study for interim PET in ABVD-treated, advanced-stage Hodgkin lymphoma: interpretation criteria and concordance rate among reviewers. J Nucl Med. 2013;54:683–690. [DOI] [PubMed] [Google Scholar]
- 14. Barrington SF, Kirkwood AA, Franceschetto A, et al. PET-CT for staging and early response: results from the Response-Adapted Therapy in Advanced Hodgkin Lymphoma study. Blood. 2016;127:1531–1538. [DOI] [PubMed] [Google Scholar]
- 15. Evens AM, Kostakoglu L. The role of FDG-PET in defining prognosis of Hodgkin lymphoma for early-stage disease. Blood. 2014;124:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meyer RM, Gospodarowicz MK, Connors JM, et al. ABVD alone versus radiation-based therapy in limited-stage Hodgkin’s lymphoma. N Engl J Med. 2012;366:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Engert A, Plutschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med. 2010;363:640–652. [DOI] [PubMed] [Google Scholar]
- 18. Hay AE, Klimm B, Chen BE, et al. Treatment of stage I-II a non-bulky Hodgkin’s lymphoma (HL): an individual patient-data comparison of German Hodgkin study group (GHSG) HD10 and HD11 combined-modality therapy (CMT) and NCIC clinical trials group (NCIC CTG) HD.6 ABVD Alone. Blood. 2015;120:548. [Google Scholar]
- 19. Eich HT, Diehl V, Gorgen H, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol. 2010;28:4199–4206. [DOI] [PubMed] [Google Scholar]
- 20. Hay AE, Klimm B, Chen BE, et al. An individual patient-data comparison of combined modality therapy and ABVD alone for patients with limited-stage Hodgkin lymphoma. Ann Oncol. 2013;24:3065–3069. [DOI] [PubMed] [Google Scholar]
- 21. Radford J, Illidge T, Barrington S. PET-directed therapy for Hodgkin’s lymphoma. N Engl J Med. 2015;373:392. [DOI] [PubMed] [Google Scholar]
- 22. Andre MP, Girinsky T, Federico M, et al. Early positron emission tomography response-adapted treatment in stage I and II Hodgkin lymphoma: final results of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol. 2017;35:1786–1794. [DOI] [PubMed] [Google Scholar]
- 23. Bradley AJ, Carrington BM, Lawrance JA, Ryder WD, Radford JA. Assessment and significance of mediastinal bulk in Hodgkin’s disease: comparison between computed tomography and chest radiography. J Clin Oncol. 1999;17:2493–2498. [DOI] [PubMed] [Google Scholar]
- 24. Engert A, Schiller P, Josting A, et al. Involved-field radiotherapy is equally effective and less toxic compared with extended-field radiotherapy after four cycles of chemotherapy in patients with early-stage unfavorable Hodgkin’s lymphoma: results of the HD8 trial of the German Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2003;21:3601–3608. [DOI] [PubMed] [Google Scholar]
- 25. Advani RH, Hong F, Fisher RI, et al. Randomized phase III trial comparing ABVD plus radiotherapy with the Stanford V regimen in patients with stages I or II locally extensive, bulky mediastinal Hodgkin lymphoma: a subset analysis of the North American intergroup E2496 trial. J Clin Oncol. 2015;33:1936–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gunther JR, Fanale MA, Reddy JP, et al. Treatment of early-stage unfavorable Hodgkin lymphoma: efficacy and toxicity of 4 versus 6 cycles of ABVD chemotherapy with radiation. Int J Radiat Oncol Biol Phys. 2016;96:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klimm B, Goergen H, Fuchs M, et al. Impact of risk factors on outcomes in early-stage Hodgkin’s lymphoma: an analysis of international staging definitions. Ann Oncol. 2013;24:3070–3076. [DOI] [PubMed] [Google Scholar]
- 28. Carde P, Karrasch M, Fortpied C, et al. Eight cycles of ABVD versus four cycles of BEACOPPescalated plus four cycles of BEACOPPbaseline in stage III to IV, international prognostic score >/= 3, high-risk Hodgkin lymphoma: first results of the phase III EORTC 20012 intergroup trial. J Clin Oncol. 2016;34:2028–2036. [DOI] [PubMed] [Google Scholar]
- 29. Ansell SM. Hodgkin lymphoma: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol. 2014;89:771–779. [DOI] [PubMed] [Google Scholar]
- 30. von Tresckow B, Plutschow A, Fuchs M, et al. Dose-intensification in early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD14 trial. J Clin Oncol. 2012;30:907–913. [DOI] [PubMed] [Google Scholar]
- 31. Nguyen VT, Pophali PA, Tsai JP, et al. Early stage, bulky Hodgkin lymphoma patients have a favorable outcome when treated with or without consolidative radiotherapy: potential role of PET scan in treatment planning [published online ahead of print July 13, 2016]. Br J Haematol. doi: 10.1111/bjh.14236. [DOI] [PubMed] [Google Scholar]
- 32. Savage KJ, Connors JM, Villa DR, et al. Advanced stage classical Hodgkin lymphoma patients with a negative PET-scan following treatment with ABVD have excellent outcomes without the need for consolidative radiotherapy regardless of disease bulk at presentation. Blood. 2015;126:579. [Google Scholar]
- 33. Dhakal S, Advani R, Ballas LK, et al. ACR appropriateness criteria(R) Hodgkin lymphoma-favorable prognosis stage I and II. Am J Clin Oncol. 2016;39:535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meyer RM, Hoppe RT. Point/counterpoint: early-stage Hodgkin lymphoma and the role of radiation therapy. Blood. 2012;120:4488–4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schaapveld M, Aleman BM, van Eggermond AM, et al. Second cancer risk up to 40 years after treatment for Hodgkin’s lymphoma. N Engl J Med. 2015;373:2499–2511. [DOI] [PubMed] [Google Scholar]
- 36. Murray L, Sethugavalar B, Robertshaw H, et al. Involved node, site, field and residual volume radiotherapy for lymphoma: a comparison of organ at risk dosimetry and second malignancy risks. Clin Oncol. 2015;27:401–410. [DOI] [PubMed] [Google Scholar]
- 37. Early FDG-PET adapted treatment improves the outcome of early FDG-PET-positive patients with stages I/II Hodgkin lymphoma (HL): final results of the randomized intergroup EORTC/LYSA/FIL H10 trial. Clin Adv Hematol Oncol. 2015;13:16–17. [Google Scholar]
- 38. Ganesan P, Rajendranath R, Kannan K, et al. Phase II study of interim PET-CT-guided response-adapted therapy in advanced Hodgkin’s lymphoma. Ann Oncol. 2015;26:1170–1174. [DOI] [PubMed] [Google Scholar]
- 39. Merli F, Luminari S, Gobbi PG, et al. Long-term results of the HD2000 trial comparing ABVD versus BEACOPP versus COPP-EBV-CAD in untreated patients with advanced Hodgkin lymphoma: a study by fondazione italiana linfomi. J Clin Oncol. 2016;34:1175–1181. [DOI] [PubMed] [Google Scholar]
- 40. Avigdor A, Bulvik S, Levi I, et al. Two cycles of escalated BEACOPP followed by four cycles of ABVD utilizing early-interim PET/CT scan is an effective regimen for advanced high-risk Hodgkin’s lymphoma. Ann Oncol. 2010;21:126–132. [DOI] [PubMed] [Google Scholar]
- 41. Engert A, Diehl V, Franklin J, et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin’s lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol. 2009;27:4548–4554. [DOI] [PubMed] [Google Scholar]
- 42. Behringer K, Breuer K, Reineke T, et al. Secondary amenorrhea after Hodgkin’s lymphoma is influenced by age at treatment, stage of disease, chemotherapy regimen, and the use of oral contraceptives during therapy: a report from the German Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2005;23:7555–7564. [DOI] [PubMed] [Google Scholar]
- 43. Behringer K, Diehl V. Twenty-five years clinical trials of the German Hodgkin Study Group (GHSG). Eur J Haematol Suppl. 2005;66:21–25. [DOI] [PubMed] [Google Scholar]
- 44. Bauer K, Skoetz N, Monsef I, Engert A, Brillant C. Comparison of chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for patients with early unfavourable or advanced stage Hodgkin lymphoma. Cochrane Database Syst Rev. 2011;8:CD007941. [DOI] [PubMed] [Google Scholar]
- 45. Skoetz N, Trelle S, Rancea M, et al. Effect of initial treatment strategy on survival of patients with advanced-stage Hodgkin’s lymphoma: a systematic review and network meta-analysis. Lancet Oncol. 2013;14:943–952. [DOI] [PubMed] [Google Scholar]
- 46. Savage KJ, Connors JM, Klasa RJ, et al. The use of FDG-PET to guide consolidative radiotherapy in patients with advanced-stage Hodgkin lymphoma with residual abnormalities on CT scan following ABVD chemotherapy. J Clin Oncol. 2011;29(15_suppl):8034–8034. [Google Scholar]
- 47. Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J Clin Oncol. 2013;31:684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Borchmann P, Haverkamp H, Diehl V, et al. Eight cycles of escalated-dose BEACOPP compared with four cycles of escalated-dose BEACOPP followed by four cycles of baseline-dose BEACOPP with or without radiotherapy in patients with advanced-stage Hodgkin’s lymphoma: final analysis of the HD12 trial of the German Hodgkin Study Group. J Clin Oncol. 2011;29:4234–4242. [DOI] [PubMed] [Google Scholar]
- 49. Engert A, Haverkamp H, Kobe C, et al. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet. 2012;379:1791–1799. [DOI] [PubMed] [Google Scholar]
- 50. Evens AM, Hong F, Gordon LI, et al. Efficacy and tolerability of ABVD and Stanford V for elderly advanced-stage Hodgkin lymphoma (HL): analysis from the phase III randomized U.S. Intergroup Trial E2496. J Clin Oncol. 2011;29:8035. [Google Scholar]
- 51. Boll B, Goergen H, Behringer K, et al. Bleomycin in older early-stage favorable Hodgkin lymphoma patients: analysis of the German Hodgkin Study Group (GHSG) HD10 and HD13 trials. Blood. 2016;127:2189–2192. [DOI] [PubMed] [Google Scholar]
- 52. Evens AM, Hong F, Gordon LI, et al. The efficacy and tolerability of adriamycin, bleomycin, vinblastine, dacarbazine and Stanford V in older Hodgkin lymphoma patients: a comprehensive analysis from the North American intergroup trial E2496. Br J Haematol. 2013;161:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Evens AM, Helenowski I, Ramsdale E, et al. A retrospective multicenter analysis of elderly Hodgkin lymphoma: outcomes and prognostic factors in the modern era. Blood. 2012;119:692–695. [DOI] [PubMed] [Google Scholar]
- 54. Zallio F, Tamiazzo S, Monagheddu C, et al. Reduced intensity VEPEMB regimen compared with standard ABVD in elderly Hodgkin lymphoma patients: results from a randomized trial on behalf of the Fondazione Italiana Linfomi (FIL). Br J Haematol. 2016;172:879–888. [DOI] [PubMed] [Google Scholar]
- 55. Ballova V, Ruffer JU, Haverkamp H, et al. A prospectively randomized trial carried out by the German Hodgkin Study Group (GHSG) for elderly patients with advanced Hodgkin’s disease comparing BEACOPP baseline and COPP-ABVD (study HD9elderly). Ann Oncol. 2005;16:124–131. [DOI] [PubMed] [Google Scholar]
- 56. Behringer K, Goergen H, Hitz F, et al. Omission of dacarbazine or bleomycin, or both, from the ABVD regimen in treatment of early-stage favourable Hodgkin’s lymphoma (GHSG HD13): an open-label, randomised, non-inferiority trial. Lancet. 2015;385:1418–1427. [DOI] [PubMed] [Google Scholar]