Abstract
Background
Lumbar disc herniation is a major cause of radicular pain, but the underlying mechanisms remain largely unknown. Spinal activation of src-family kinases are involved in the development of chronic pain from nerve injury, inflammation, and cancer. In the present study, the role of src-family kinases activation in lumbar disc herniation-induced radicular pain was investigated.
Results
Lumbar disc herniation was induced by implantation of autologous nucleus pulposus, harvest from tail, in lumbar 4/5 spinal nerve roots of rat. Behavior test and electrophysiologic data showed that nucleus pulposus implantation induced persistent mechanical allodynia and thermal hyperalgesia and increased efficiency of synaptic transmission in spinal dorsal horn which underlies central sensitization of pain sensation. Western blotting and immunohistochemistry staining revealed that the expression of phosphorylated src-family kinases was upregulated mainly in spinal microglia of rats with nucleus pulposus. Intrathecal delivery of src-family kinases inhibitor PP2 alleviated pain behaviors, decreased efficiency of spinal synaptic transmission, and reduced phosphorylated src-family kinases expression. Furthermore, we found that the expression of ionized calcium-binding adapter molecule 1 (marker of microglia), tumor necrosis factor-α, interleukin 1 -β in spinal dorsal horn was increased in rats with nucleus pulposus. Therapeutic effect of PP2 may be related to its capacity in reducing the expression of these factors.
Conclusions
These findings suggested that central sensitization was involved in radicular pain from lumbar disc herniation; src-family kinases-mediated inflammatory response may be responsible for central sensitization and chronic pain after lumbar disc herniation.
Keywords: Src-family kinases, central sensitization, long-term potentiation, lumbar disc herniation, radicular pain, microglia, pro-inflammatory cytokines
Introduction
Lumbar disc herniation (LDH) is the most common cause of radicular pain (RP), characterized by allodynia, hyperalgesia, and spontaneous pain. It impairs individuals’ quality of life and work capability and thus has important social and economic implications.1 It has been widely accepted that peripheral sensitization (increased excitation of primary afferent neurons) is involved in RP induced by LDH.2,3 The role and mechanisms of central sensitization (long-term potentiation (LTP) of efficiency of synaptic transmission between primary afferent fibers and pain-processing neurons in spinal dorsal horn) in the generation and maintenance of RP remain unknown.
SFKs are nonreceptor tyrosine kinases that have been implicated in synaptic transmission and plasticity.4,5 Previously, we have reported that SFKs activation in spinal microglia is necessary for the induction of spinal LTP which underlies central sensitization of pain transmission.6 It has been suggested that peripheral nerve injury activates SFKs only in spinal microglia, and SFKs inhibitor PP2 suppresses nerve injury-induced mechanical allodynia,7 suggesting that SFKs in spinal microglia are critical for neuropathic pain induced by nerve injury. However, whether SFKs are activated in rats with RP remains to be determined.
The activation of spinal microglia is crucial for the pathogenesis of chronic pain from nerve injury8,9 and LDH.10,11 In nervous system, activated microglia is the main source of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin 1 -β (IL-1β).12,13 Clinical research and animal experiments proved that cytokine antagonists may exert therapeutic effect on RP.14,15 The causes and concrete mechanisms of microglia activation in RP are still not clear.
In the present study, the role of central sensitization and spinal SFKs on RP from LDH was evaluated in rats with nucleus pulposus (NP) implantation. We found that SFKs activation was involved in mechanical allodynia and central sensitization of rats with NP implantation; the mechanism may be related to spinal microglia activation, and the followed releasing of pro-inflammatory cytokines.
Materials and methods
Animals and reagents
Male Sprague-Dawley rats weighting 200 to 250 g were purchased from Guangdong Laboratory Animal Center. All animal experimental procedures were carried out in accordance with the guideline of National Institutes of Health on animal care and the ethical guidelines.
SFKs inhibitor PP2 (4-amino-5-(4-chlorophenyl)-7-(t-butyl) pyrazolo [3,4-d] pyrimidine, Calbiochem) and its inactive form PP3 (4-amino-7-phenylpyrazolo [3,4-d] pyrimidine, Calbiochem) were first dissolved in dimethyl sulfoxide (DMSO) to make a stock concentration of 50 mM, aliquoted in small volumes, and stored at −80℃. The stock solution was subsequently diluted to 1.2 µg/μl immediately before administration, and the final concentration of DMSO was < 0.5%.
The primary antibodies used in the present study are the following: monoclonal rabbit anti-SFKs (phospho Y418) antibody (Abcam, USA, ab40660), polyclonal rabbit anti-β-actin antibody (Cell Signaling Technology, USA, #4967), monoclonal mouse anti-neuronal specific nuclear protein (NeuN; neuronal marker, Abcam, ab104224), polyclonal goat anti-glial fibrillary acidic protein (GFAP; astrocyte marker, Abcam, ab53554), monoclonal mouse anti-ionized calcium-binding adapter molecule 1 (Iba-1; microglia marker, Abcam, ab15690), polyclonal rabbit anti-TNF-α (Abcam, ab6671), and polyclonal rabbit anti-IL-1β (Abcam, ab9722).
The second antibodies used in the present study are the following: horseradish peroxidase-conjugated immunoglobulin (IgG; Abcam, ab205718, ab6789), Cy3-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch, USA, 111-165-003), fluorescein isothyocyanate (FITC)-conjugated donkey anti-goat IgG (Jackson ImmunoResearch, 705-095-003), and FITC-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch, 715-095-150).
Drug administration
For intrathecal delivery of the drugs, rats were implanted with intrathecal catheters according to the method described previously.16 Briefly, rats were intraperitoneally (i.p.) anesthetized with sodium pentobarbital (40 mg/kg). A sterile polyethylene-10 (PE-10; BD, USA) tube filled with saline was inserted through L5/L6 intervertebral space, and the tip of the tube was placed at the spinal lumbar enlargement level. Any rats with hind limb paralysis or paresis after surgery were excluded. The successful catheterization was confirmed on the next day after catheter implanting by bilateral hind limb paralysis following injection of 2% lidocaine (7 μl) through the catheter within 30 s. For drug administration, the rats were fixed in special fixation box with wakefulness, PP2, PP3, or DMSO solution (vehicle control) was injected from the tips of the catheters, starting 1 h before surgery and once daily (9:00 a.m.) thereafter for seven days. Drugs or vehicle were administered in volumes of 10 μl followed by a flush of 7 μl of saline to ensure drugs delivered into the subarachnoid space.
LDH model
LDH was induced by autologous NP implantation, as described previously.17–19 Under sodium pentobarbital anesthesia (40 mg/kg, i.p.), a midline incision to the spine was made. All surgical procedures were performed on the left side. The paraspinous muscles were dissected free from the left spinous processes to expose the transverse processes. Left L4–L5 laminectomy was made. The facet joint, including articular processes, was carefully removed. The autologous NP (about 10 mg) obtained from coccygeal intervertebral discs was relocated on the lumbar nerve roots after laminectomy without compression. The control (sham) group received the same operation without NP implantation.
Behavioral test
Animals were habituated, and basal pain sensitivity was tested before drug administration or surgery. Mechanical sensitivity was assessed with the up-down method described previously,20 using a set of von Frey hairs with logarithmically incremental stiffness from 0.41 to 15.14 g (0.41, 0.70, 1.20, 2.04, 3.63, 5.50, 8.51, and 15.14 g). The 2.04 g stimulus, in the middle of the series, was applied first. In the event of paw withdrawal absence, the next stronger stimulus was chosen. On the contrary, a weaker stimulus was applied. Each stimulus consisted of a 6 s to 8 s application of the von Frey hair to the sciatic innervated area of hindpaw with a 5-min interval between stimuli. The quick withdrawal or licking of the paw in response to the stimulus was considered as a positive response.
Thermal hyperalgesia was tested using a plantar test (7370, UgoBasile) according to the method described by Hargreaves et al.21 Briefly, a radiant heat source beneath a glass floor was aimed at the plantar surface of the hindpaws. Three measurements of latency were taken for each hindpaw in each test session. The hindpaw was tested alternately with >5 min intervals between consecutive tests. The three measurements of latency per animal were averaged as the result of per test. The experimenter who conducted the behavioral tests was blinded to all treatments.
Electrophysiologic recording
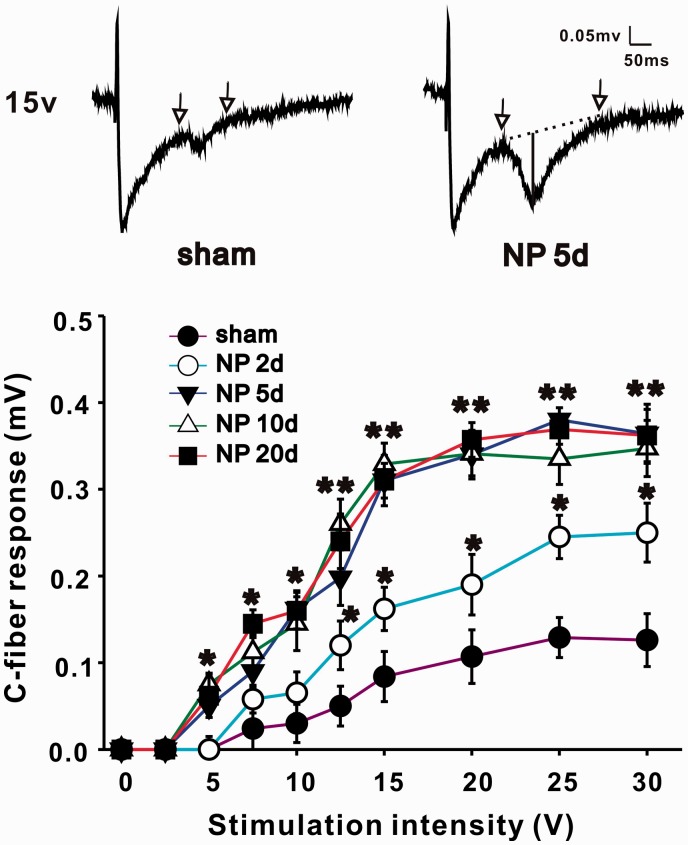
Recording of C-fiber-evoked field potentials in spinal dorsal horn has been described previously.6 Briefly, following electrical stimulation of the left sciatic nerve with a bipolar silver chloride hook-electrode, field potentials were recorded in ipsilateral lumbar enlargement with a glass microelectrode (filled with 0.5 M sodium acetate, impedance 0.5–1 MX). The microelectrode was driven by an electronically controlled microstepping motor (Narishige Scientific Instrument Laboratory) from the spinal surface to a certain depth (100–500 µm). The recording depth was adjusted and confirmed until ideal C-fibers response was recorded. An A/D converter card (ADC-42, PICO) was used to digitize and store data at a sampling rate of 10 kHz. The test stimuli (0.5 ms duration, every 1 min) delivered to the sciatic nerve was used to evoke field potentials in spinal dorsal horn. The stimulus intensity was given from 0 to 30 V in an ascending order (0, 2.5, 5, 7.5, 10, 12.5, 15, 20, 25, and 30 V) manually. Each intensity was repeated five times, and the amplitudes of C-fiber-evoked field potential were recorded and averaged. As sciatic nerves have both A-fibers and C-fibers, the responses of C-fibers were distinguished and confirmed by high threshold (≥7 V) and long latencies (90–130 ms, corresponding to conduction velocities between 0.85 and 1.2 m/s). Amplitude of C-fiber-evoked field potential as shown in original recordings of Figure 2 (vertical line) was determined automatically by parameter extraction software. Baseline, indicated by dotted line, was determined by two highest points within the time range defined manually on either side of C-fiber responses (arrows).
Figure 2.
NP implantation increases C-fiber-evoked field potentials of ipsilateral spinal dorsal horn. In the same stimulus intensity, amplitude of C-fiber-evoked field potentials of NP group (2, 5, 10, and 20 days) is significantly larger than that of sham group (n = 12/group, *P < 0.05, **P < 0.01). At top, two representatively original recordings of sham (left) and NP group 5 days (right) are shown, and traces between two arrows are C-fiber responses. Amplitude of C-fiber-evoked field potential (shown in vertical line of right original recording) is determined automatically by parameter extraction software. Baseline, indicated by dotted line, is determined by two highest points within the time range defined manually on either side of C-fiber responses (arrows). NP: nucleus pulposus.
Western blotting
The dorsal quadrants of L4 to L5 spinal cord on left side were separated and were put into liquid nitrogen immediately, followed by homogenization in 15 mmol/l Tris buffer, pH 7.6 (250 mmol/l sucrose, 1 mM MgCl, 1 mM DTT, 2.5 mM EDTA, 1 m MEGTA, 50 mM NaF, 10 lg/ml leupeptin, 1.25 lg/ml pepstatin, 2.5 lg/ml aprotin, 2 mM sodium pyrophosphate, 0.1 mM NaVO4, 0.5 mM PMSF), and protease inhibitor cocktail (Roche Molecular Biochemicals, Indianapolis, IN). The tissues were sonicated on ice and then centrifuged at 14,000 g for 20 min at 4℃ to isolate the supernatant containing protein samples. The protein samples were stored at −80℃ until assayed.
Proteins were separated by gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA). The blots were blocked with 5% w/v nonfat dry milk in TBST (20 mM Tris-base, pH 7.6, 137 mM NaCl, and 0.1% Tween 20) for 1 h at room temperature and then incubated with monoclonal rabbit anti-SFKs (phospho Y418) antibody (1:1000) or mouse anti-Iba-1 antibody (1:500) or rabbit anti-TNF-α (1:500) antibody or rabbit anti-IL-1β (1:500) antibody overnight at 4℃ with gentle shaking. The blots were washed three times for 15 min each with TBST and then incubated with secondary antibody horseradish peroxidase-conjugated IgG (1:5000) for 1 h at room temperature, then the membrane was washed again, as mentioned earlier. The immune complex was detected by enhanced chemiluminescence kit (Pierce, USA). The band intensities on the membrane were analyzed by densitometry with a computer-assisted imaging analysis system (Kontron IBAS 2.0, Germany). The same membrane was stripped with stripping buffer (67.5 mM Tris pH 6.8, 2% SDS, and 0.7% β-mercaptoethanol) for 30 min at 50℃ and reprobed with polyclonal rabbit anti-β-actin antibody (1:1000), and detected as mentioned earlier. The optical density of p-SFKs or Iba-1 or TNF-α or IL-1β protein was determined by the ratio of the protein signal to the β-actin signal. These ratios were normalized to the control values.
Immunohistochemistry
Rats were perfused with 300 ml saline followed by 350 ml cold 4% paraformaldehyde (PFA, Sigma) in phosphate buffer (PB, 0.1 M, pH 7.4). The L4/5 spinal cord was removed, postfixed with the same fixative for 3 h, and then replaced with 30% sucrose in PBS for two days at 4℃. Transverse spinal sections (25 µm) were cut in a cryostat (Leica CM1900; −20℃) and processed for immunofluorescence according to the methods as described previously.22
Briefly, all sections were blocked with 3% donkey serum in 0.3% Triton X-100 (Sigma) for 1 h at room temperature and incubated with rabbit anti-rat monoclonal p-SFKs antibody (1:400) overnight at 4℃, followed by incubation with Cy3-conjugated secondary antibody (1:400) for 1 h at room temperature. For double-labeled immunofluorence, spinal sections were incubated with a mixture of the p-SFKs antibody and anti-NeuN (neuronal marker, 1:500; Abcam) or anti-glial fibrillary acidic protein (astrocyte marker, 1:500) or anti-Iba-1 (microglia marker, 1:500), followed by a mixture of FITC- and Cy3-conjugated secondary antibodies (1:400) for 1 h at room temperature. The stained sections were transferred to glass microscope slides, stretched, and arranged using a small paintbrush. Sections were immediately examined with an Olympus IX71 fluorescence microscope (Olympus Optical, Tokyo, Japan), and images were captured with a CCD spot camera.
Statistical analysis
All data were expressed as means ± SEM. For behavioral analysis, the data between groups were compared with nonparametric test (Friedman analysis of variance (ANOVA) for repeated measurements). For the analysis of electrophysiological data, as 10 intensities of stimulus were given (0, 2.5, 5, 7.5, 10, 12.5, 15, 20, 25, and 30 V), the data were divided into 10 groups according to the stimulus intensity. At one certain intensity, the C-fiber responses in different groups were compared by Student’s t test. For analysis of Western blotting, differences between groups were compared by ANOVA followed by Fisher’s protected least significant difference (PLSD) post hoc analysis. The criterion for statistical significance was P < 0.05. Statistical tests were performed with SPSS 13.0 (SPSS, USA).
Results
NP implantation induced mechanical allodynia and thermal hyperalgesia in ipsilateral hindpaws of rats
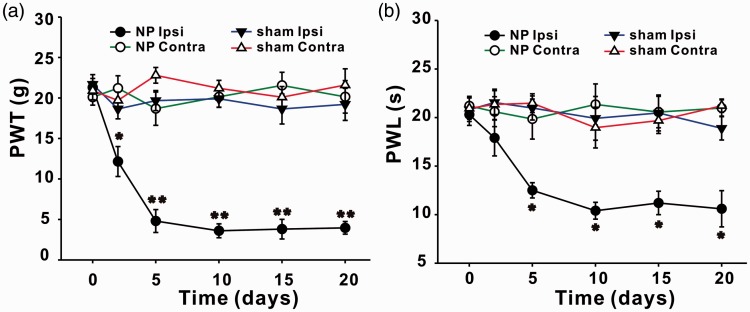
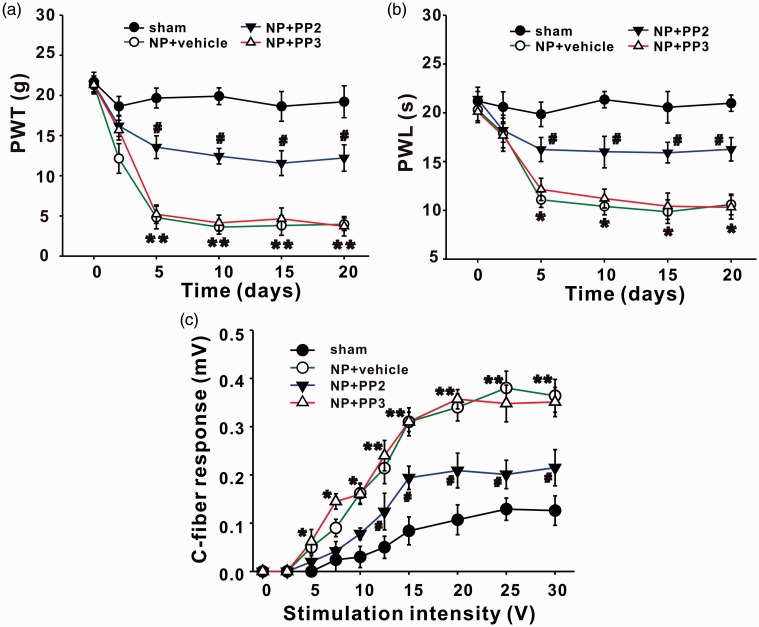
Consistent with the previous study,2,3 rats with implantation of NP displayed mechanical allodynia and thermal hyperalgesia. Compared with sham group, 50% paw withdrawal threshold (PWT) in ipsilateral hindpaws of rats with NP decreased significantly from day 2 (P < 0.05) to day 20 (P < 0.01) after surgery (Figure 1(a)). Paw withdrawal latency (PWL) in ipsilateral hindpaws of rats with NP decreased significantly from day 5 to day 20 (P < 0.05; Figure 1(b)). No significant changes of PWT or PWL on contralateral sides were detected between sham and NP groups (Figure 1).
Figure 1.
NP implantation decreases PWT and PWL of ipsilateral hindpaws of rats. (a) PWT in ipsilateral hindpaws of NP group decrease significantly on day 2, 5, 10, 15, and 20 after surgery compared with sham group (n = 12/group, *P < 0.05, **P < 0.01). PWT in contralateral hindpaws are not different between NP and sham group. (b) PWL in ipsilateral hindpaws of NP group decrease significantly on day 5, 10, 15, and 20 after surgery compared with sham group (n = 12/group, *P < 0.05). PWL in contralateral hindpaws are not different between NP and sham group. NP: nucleus pulposus; PWL: paw withdrawal latency; PWT: paw withdrawal threshold.
C-fiber-evoked field potentials of spinal dorsal horn are increased in rats with NP
Rats that showed mechanical allodynia and thermal hyperalgesia were chosen to perform electrophysiological test, and others without pain behaviors after operation (about 10%) were excluded. Electrical stimulus was delivered to the left sciatic nerve, and C-fiber-evoked field potentials of ipsilateral spinal dorsal horn were recorded. Stimulus intensity was controlled manually with the order described in the former part (Materials and methods section). Differences of C-fiber responses induced by one certain stimulus intensity between sham and NP group (2, 5, 10, and 20 days) were compared by ANOVA. NP implantation (from 2 to 20 days) induced a significant increase in C-fiber responses when the stimulus intensity reached the threshold (*P < 0.05, **P < 0.01, Figure 2). Take one pair of data, for example, when intensity was 15 V, C-fiber responses in sham and NP 5 days group were 0.082 ± 0.021 mV and 0.281 ± 0.018 mV, respectively. The increased responses of C-fiber in NP group indicated the involvement of central sensitization in RP.
Expression of p-SFKs was upregulated mainly in microglia of ipsilateral spinal dorsal horn of rats with NP
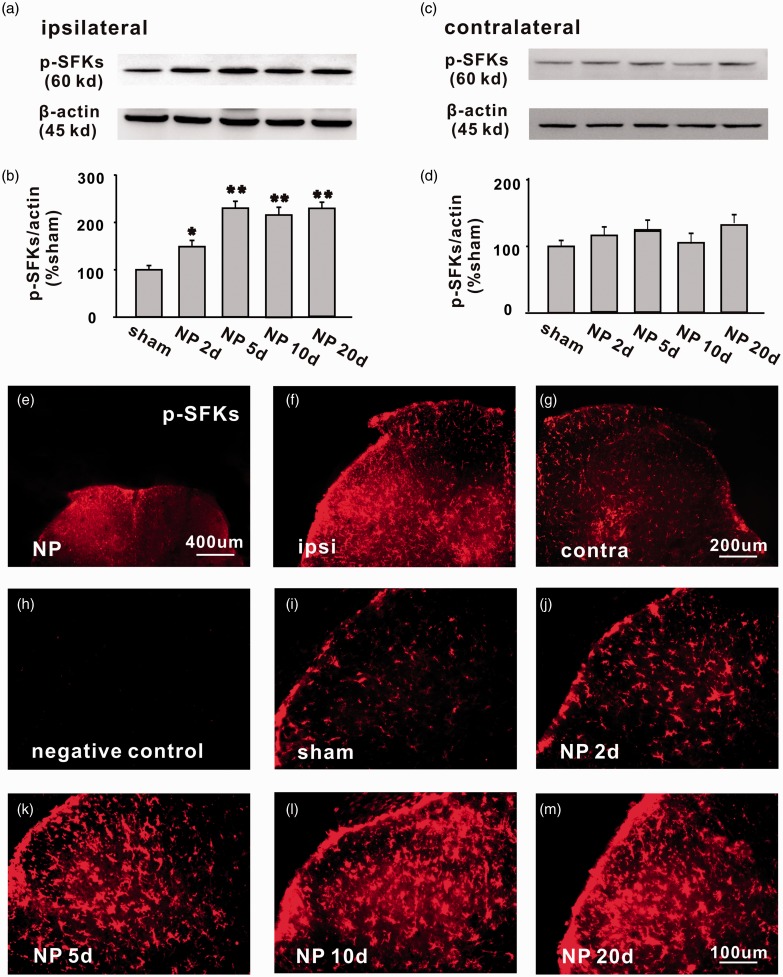
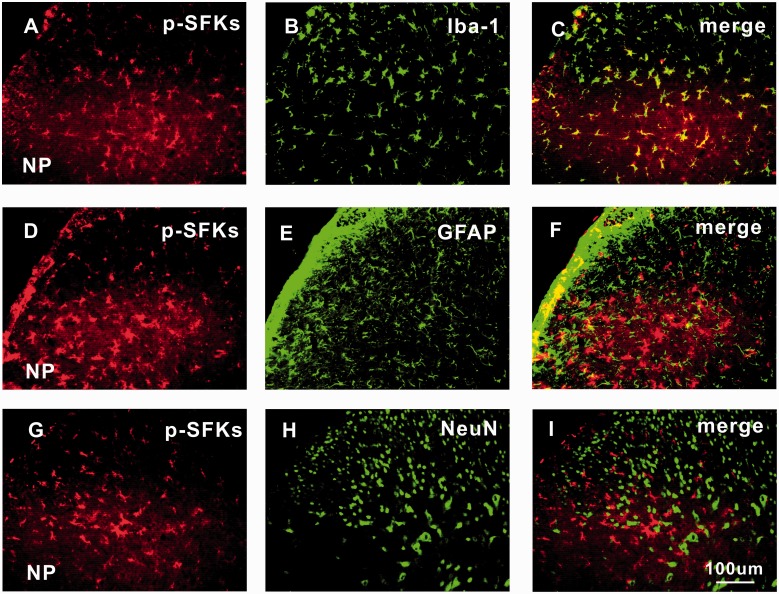
The expression of p-SFKs in bilateral spinal dorsal horn was detected by Western blotting analysis. The protein level of p-SFKs in ipsilateral spinal dorsal horn was significantly higher in NP group than that in the sham group, starting from day 2 (*P < 0.05) to day 20 (**P < 0.01) after surgery (Figure 3(a) and (b)). No significant difference of p-SFKs was detected in contralateral spinal dorsal horn between the two groups (Figure 3(c) and (d)). Similar changes of p-SFKs were also observed in the spinal dorsal horn by immunohistochemistry (Figure 3(e) to (m)). No signals were detected in the negative control groups, in which only the secondary antibody but not the primary antibody was added to the spinal sections (Figure 3(h)). Immunofluorescence double staining showed that the p-SFKs were co-localized mainly with microglia (Figure 4(a) to (c)), very few with astrocytes (Figure 4(d) to (f)) but not with neurons (Figure 4(g) to (i)).
Figure 3.
The expression of p-SFKs is significantly increased in ipsilateral spinal dorsal horn of rats with NP from day 2 to day 20 after surgery. (a, c) The bands respectively show the expression of p-SFKs and β-actin in ipsilateral and contralateral L4/5 spinal dorsal horn after surgery. (b, d) The histograms respectively show the quantification of p-SFKs normalized by β-actin in ipsilateral and contralateral side (n = 6/group, *P < 0.05, **P < 0.01). (e) Representative immunofluorescence staining (50×) shows the expression of p-SFKs in bilateral spinal dorsal horn of rats with NP. (f, g) Representative immunofluorescence staining (100×) shows the expression of p-SFKs in ipsilateral and contralateral spinal dorsal horn of rats with NP. (h) The photograph shows that no signal was detected in negative control experiments, in which only secondary antibody but no primary antibody was added (n = 2). (i)–(m) representative immnofluorenscence staining (200×) shows the expression of p-SFKs in spinal dorsal horn of sham, NP 2, 5, 10, and 20 days group (n = 6/group). Scale bars (e) = 400 µm; Scale bars (f, g) = 200 µm; Scale bars (h–m) = 100 µm. NP: nucleus pulposus; p-SFK: phosphorylated src-family kinase.
Figure 4.
p-SFKs are expressed mainly in spinal microglia, very few in astrocytes but not in neurons of rats with NP. Double immunofluorescence staining (200×) in ipsilateral L4/5 spinal dorsal horn between p-SFKs (red: (a), (d), and (g)) and Iba-1 (a microglia marker, green: (b)); GFAP (an astrocyte marker, green: (e)); and NeuN (a neuronal marker, green: (h)) shows that p-SFKs are located mainly in microglia (c), very few in astrocytes (f) but not in neurons (i) of spinal dorsal horn five days after surgery. Scale bars (a)–(i) = 100 µm (n = 6/group). NeuN: neuronal specific nuclear protein; GFAP: glial fibrillary acidic protein; Iba-1: ionized calcium-binding adapter molecule 1; NP: nucleus pulposus; p-SFK: phosphorylated src-family kinase.
SFKs inhibitor PP2 alleviated pain behaviors, decreased C-fiber-evoked field potentials, and reduced p-SFKs expression of rats with NP
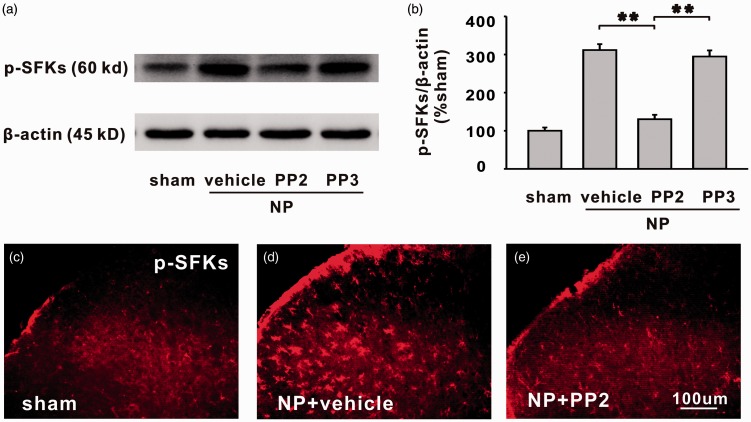
Then, whether the SFKs were involved in LDH-induced mechanical allodynia, thermal hyperalgesia, and central sensitization were tested. PP2, a specific inhibitor of SFKs was intrathecally injected, starting from 1 h before surgery and once daily thereafter for seven days. We found that compared with its inactivated form PP3 and vehicle, PP2 significantly increased PWT and PWL which were both decreased by NP from day 2 to day 20 (Figure 5(a) and (b), closed triangle; NP + vehicle vs. NP + PP2, *P < 0.05, **P < 0.01; NP + PP3 vs. NP + PP2, #P < 0.05). At the end of the experiments (day 20 after NP implantation), rats were recruited for electrophysiologic recording and molecular biological testing. Data showed that PP2 significantly decreased C-fiber response that was elevated by NP (Figure 5(c), closed triangle; NP + vehicle vs. NP + PP2, *P < 0.05, **P < 0.01; NP + PP3 vs. NP + PP2, #P < 0.05), and the increased expression of p-SFKs by NP implantation was significantly reduced by PP2 but not by vehicle or PP3 (Figure 6(a) to (e), **P < 0.01).
Figure 5.
The decrease in PWT and PWL of ipsilateral hindpaws and the increase in amplitude of C-fiber-evoked field potentials of spinal dorsal horn in NP group are reversed by SFKs inhibitor PP2 but not by vehicle or PP3. (a, b) Compared with vehicle or PP3, intrathecal delivery of PP2 significantly increases the PWT and PWL of ipsilateral hindpaws in NP group (n = 6/group, NP + vehicle vs. NP + PP2, *P < 0.05, **P < 0.01; NP + PP3 vs. NP + PP2, #P < 0.05). (c) PP2 significantly decreases the amplitude of C-fiber-evoked field potentials of spinal dorsal horn in NP group (n = 12/group, NP + vehicle vs. NP + PP2, *P < 0.05, **P < 0.01; NP + PP3 vs. NP + PP2, #P < 0.05). NP: nucleus pulposus; PWL: paw withdrawal latency; PWT: paw withdrawal threshold.
Figure 6.
The increased expression of p-SFKs in spinal dorsal horn of NP group is moderated by SFKs inhibitor PP2 but not by vehicle or PP3. (a) The bands show the expression of p-SFKs and β-actin in ipsilateral L4/5 spinal dorsal horn in different groups. (b) The histograms show the quantification of p-SFKs normalized by β-actin. Compared with vehicle or PP3, PP2 significantly decreased expression of p-SFKs (n = 6/group, **P < 0.01). (c)–(e) Representative immnofluorescence staining (200×) shows the expression of p-SFKs in sham group, NP + vehicle group, and NP + PP2 group. Scale bars (C–E) = 100 µm (n = 6/group). NP: nucleus pulposus; p-SFK: phosphorylated src-family kinase.
SFKs inhibitor PP2 obstructed microglia activation in spinal dorsal horn of rats with NP
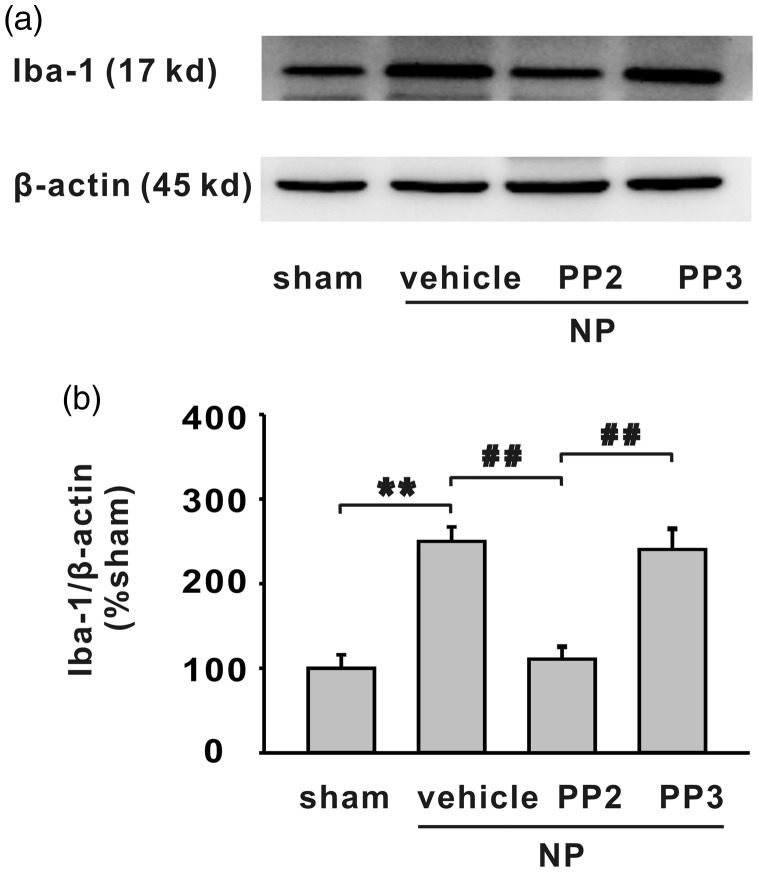
We further examined the activation of microglia in NP group and the role of PP2 on microglia activation. Western blotting data showed that Iba-1 was significantly increased in ipsilateral side of spinal dorsal horn of rats with NP (Figure 7(a) and (b), **P < 0.01). Compared with vehicle or PP3, SFKs inhibitor PP2 significantly decreased the expression of Iba-1 in ipsilateral spinal dorsal horn of rats with NP (Figure 7(a) and (b), **P < 0.01).
Figure 7.
The increased expression of Iba-1 in spinal dorsal horn by NP implantation is alleviated by SFKs inhibitor PP2 but not vehicle or PP3. (a) The bands show the expression of Iba-1 and β-actin in ipsilateral L4/5 spinal dorsal horn in different groups. (b) The histograms show the quantification of Iba-1 normalized by β-actin. Compared with sham group, NP implantation significantly increased expression of Iba-1 (n = 6/group, **P < 0.01). Compared with vehicle or PP3, PP2 significantly decreased expression of Iba-1 (n = 6/group, ##P < 0.01). Iba-1: ionized calcium-binding adapter molecule 1; NP: nucleus pulposus.
SFKs inhibitor PP2 alleviated the upregulation of TNF-α and IL-1β in spinal dorsal horn of rats with NP
In several pathological states, activated microglia may release pro-inflammatory cytokines such as TNF-α and IL-1β.23–25 Both cytokines were demonstrated to be involved in chronic pain and central sensitization. 6,22,26–28 Therefore, it was possible that the activated spinal microglia in LDH model may release TNF-α and IL-1β; inhibition of SFKs may decrease the expression of these cytokines.
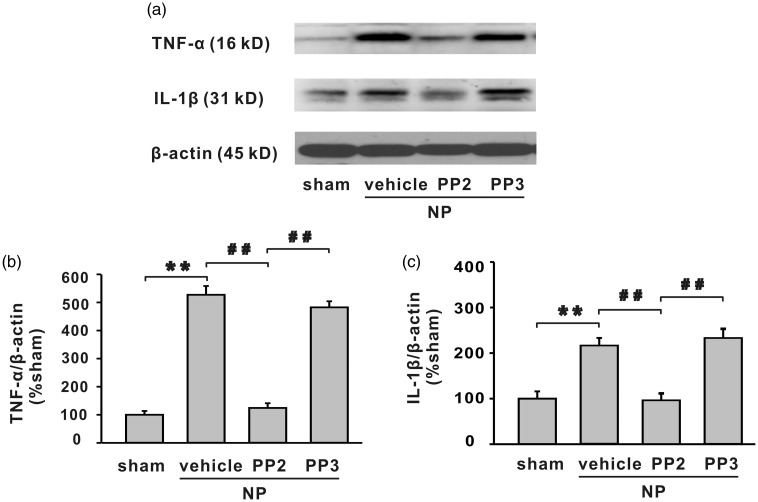
Western blotting analysis demonstrated that NP implantation significantly increased protein level of TNF-α and IL-1β in spinal dorsal horn (Figure 8, **P < 0.01), and SFKs inhibitor PP2 decreased the upregulation of the cytokines (Figure 8, ##P < 0.01).
Figure 8.
The increased expression of TNF-α and IL-1β in spinal dorsal horn by NP implantation is alleviated by SFKs inhibitor PP2 but not vehicle or PP3. (a) The bands show the expression of TNF-α, IL-1β, and β-actin in ipsilateral L4/5 spinal dorsal horn in different groups. (b, c) The histograms respectively show the quantification of TNF-α and IL-1β normalized by β-actin. NP implantation significantly increased protein level of TNF-α and IL-1β in spinal dorsal horn (**P < 0.01). Compared with vehicle or PP3, PP2 significantly decreased expression of TNF-α and IL-1β (n = 6/group, ##P < 0.01). IL-1β: interleukin 1 beta; NP: nucleus pulposus; TNF-α: tumour necrosis factor-α.
Discussion
The present study demonstrated that autologous NP implantation to the spinal nerve produced unilateral prolonged mechanical allodynia and thermal hyperalgesia accompanied by increased efficiency of synaptic transmission in spinal dorsal horn. The behavioral and electrophysiological changes were correlated with increased expression of p-SFKs in microglia of ipsilateral spinal dorsal horn. Intrathecal delivery of SFKs inhibitor PP2 alleviated NP-induced mechanical allodynia and thermal hyperalgesia, decreased synaptic transmission efficiency, and reduced p-SFKs expression. Importantly, PP2 significantly inhibited the expression of Iba-1, TNF-α, and IL-1β in spinal dorsal horn. These results suggest that SFKs-dependent central sensitization, microglia activation, and the followed releasing of pro-inflammatory cytokines may be involved in NP-induced RP.
It is widely accepted that the mechanism of neuropathic pain includes two aspects: (1) peripheral sensitization: the altered expression, trafficking, and function of ion channels in primary afferent neurons which lead to high excitability of the neurons and increased production of pain signals and (2) central sensitization: the LTP of the efficiency of synaptic transmission between primary afferent neurons and the postsynaptic neurons in spinal dorsal horn which further exaggerate pain signals.29–32 Spinal LTP may be induced by electrical primary afferent fibers, inflammation, and injury of peripheral nerves.33–35 The role and mechanism of peripheral sensitization in RP from LDH were extensively studied.2,3,36 Anzai et al.37 reported that responses of pain-processing neurons in superficial spinal dorsal horn were enhanced in rats with NP implantation, suggesting that central sensitization was involved in the process.37 However, the mechanism is still unclear. Here, we compared the amplitude of C-fiber-evoked field potentials in different stimulus intensity between NP group (2, 5, 10, and 20 days) and sham group. When the intensity reached the threshold, C-fiber responses of NP group was significantly higher than that of sham group which further supported the involvement of central sensitization in RP.
We have reported that the activation of SFKs in spinal dorsal horn was required for the induction of LTP of C-fiber-evoked field potentials by electrical stimulation of sciatic nerve, indicating the importance of SFKs in central sensitization of pain transmission.6 It is possible that the increased efficiency of pain-related synaptic transmission in rats with NP may be related to SFKs activation. As expected, p-SFKs expression in ipsilateral spinal dorsal horn was significantly increased in rats with NP implantation with a time course in accordance with the generation and maintenance of pain sensation (from day 2 to day 20 after surgery). SFKs inhibitor PP2 significantly decreased C-fiber-evoked field potentials and alleviated pain symptoms. The findings supported our hypothesis that spinal SFKs activation contributes to RP by inducing central sensitization.
It has been demonstrated that spinal activation of SFKs is involved in the development of chronic pain from peripheral nerve injury,7 inflammation,38 and cancer.39 In the present study, in a rat model of LDH, the importance of spinal SFKs in RP from LDH was examined. Behavior test showed that autologous NP implantation produced an ipsilateral mechanical allodynia and thermal hyperalgesia, characterized by decreased PWT and PWL which are similar to previous reports.17,18 The detailed findings that increased expression of p-SFKs was only found on ipsilateral but not on contralateral side of spinal dorsal horn further confirmed the role of SFKs activation on RP. All of the evidence suggests that the activation of SFKs may be a common mechanism for neuropathic pain from a different pathogenesis. As spinal SFKs mainly expressed in microglia, it is possible that the kinases may affect the function of spinal microglia.
Microglia are the resident immune cells mediating a number of pathological events in central nervous system. The morphological features of microglial activation include cell body hypertrophy with thickened and retracted processes, increased cell number, and increased staining of microglial markers, such as CD11b and Iba-1.40,41 SFKs have been implicated as upstream molecules activated early after surface receptor activation leading to activation or inhibition of the immune response.42 SFKs are also considered important activators of the Toll-like receptor family, playing key roles in regulating cytokine expression.43,44 In accordance with previous study,10,11 we found that microglia were obviously activated in ipsilateral spinal dorsal horn of rats with NP implantation; moreover, the expression of TNF-α and IL-1β in spinal dorsal horn was increased in rats with NP. Intrathecal delivery of SFKs inhibitor PP2 attenuated the upregulation of Iba-1, TNF-α, and IL-1β, indicating the mechanism of SFKs in RP. These results suggested that in rats with RP, SFKs phosphorylation may lead to microglia activation and the followed releasing of TNF-α and IL-1β.
It is widely proved that pro-inflammatory cytokines, especially TNF-α and IL-1β, play crucial role in chronic pain and central sensitization. TNF-α and IL-1β were upregulated in dorsal root ganglia and spinal dorsal horn of rats with chronic pain from peripheral nerve injury,22,27 diabetes,45 and chemotherapeutics,46,47 and the cytokines antagonists had positive effect on relieving chronic pain. In addition, TNF-α and IL-1β were proved to be sufficient and necessary to induce spinal LTP, which manifests central sensitization of pain transmission.6,26,48–50 Clinically, TNF-α level in serum of patients with RP was significantly increased,51 and epidural administration of TNF-α inhibitor etanercept alleviated pain symptoms for patients with LDH and spinal canal stenosis.14,52 In animal model of LDH from NP implantation, spinal expression of TNF-α and IL-1β was increased.53 TNF-α neutralizing antibody, TNF-α inhibitor infliximab ,or IL-1β antagonist attenuated mechanical allodynia of rats with NP implantation or nerve root compression.15,54,55 These data suggested the importance of TNF-α and IL-1β in RP. Now, our present data demonstrated that the upregulation of TNF-α and IL-1β might be related to the SFKs and microglia activation in spinal dorsal horn. Drugs targeting SFKs or microglia activation may be helpful for alleviating RP from LDH. However, a recent clinical study showed that serum levels of TNF-α, IL-6, and IL-8 in patients with RP were significantly higher than that of healthy subjects.56 Expressions of IL-6 in spinal dorsal horn and dorsal root ganglia were increased in a rat model of RP.57 Therefore, it is possible that IL-6 and IL-8 may also be involved in the process, and whether they are downstream molecules of SFKs activation still needs to be proved.
Conclusions
The present study firstly demonstrated that spinal SFKs-dependent central sensitization may be involved in LDH-induced RP. The mechanism may be related to the activation of microglia and releasing of pro-inflammatory cytokines. Targeting SFKs might represent a new strategy for treating RP from LDH.
Authors’ Contributions
Yangliang Huang, Shaoyu Liu, Yi Zhong, and Xianguo Liu were responsible for designing and executing the entire research project. Yangliang Huang, Yongyong Li, Yi Zhong, Xiongxiong Zhong, Yuming Hu, and Pan Liu carried out all experiments and statistical analysis. Yuanshu Zhao and Zhen Deng helped provide material and facilities. Yangliang Huang and Yongyong Li contributed equally to this work. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grant from the National Natural Science Foundation of China (No. 81600968), Medical Scientific Research Foundation of Guangdong Province of China (Nos. A2017101 and A2017319).
References
- 1.Waddell G. Low back pain: a twentieth century health care enigma. Spine 1996; 21: 2820–2825. [DOI] [PubMed] [Google Scholar]
- 2.Yan J, Zou K, Liu X, et al. Hyperexcitability and sensitization of sodium channels of dorsal root ganglion neurons in a rat model of lumber disc herniation. Eur Spine J 2016; 25: 177–185. [DOI] [PubMed] [Google Scholar]
- 3.Mukai M, Sakuma Y, Suzuki M, et al. Evaluation of behavior and expression of NaV1.7 in dorsal root ganglia after sciatic nerve compression and application of nucleus pulposus in rats. Eur Spine J 2014; 23: 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalia LV, Gingrich JR, Salter MW. Src in synaptic transmission and plasticity. Oncogene 2004; 23: 8007–8016. [DOI] [PubMed] [Google Scholar]
- 5.Ohnishi H, Murata Y, Okazawa H, et al. Src family kinases: modulators of neurotransmitter receptor function and behavior. Trends Neurosci 2011; 34: 629–637. [DOI] [PubMed] [Google Scholar]
- 6.Zhong Y, Zhou L, Ren W, et al. The direction of synaptic plasticity mediated by C-fibers in spinal dorsal horn is decided by Src-family kinases in microglia: the role of tumor necrosis factor-alpha. Brain Behav Immun 2010; 24: 874–880. [DOI] [PubMed] [Google Scholar]
- 7.Katsura H, Obata K, Mizushima T, et al. Activation of Src-family kinases in spinal microglia contributes to mechanical hypersensitivity after nerve injury. J Neurosci 2006; 26: 8680–8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci 2005; 28: 101–107. [DOI] [PubMed] [Google Scholar]
- 9.Inoue K, Tsuda M. Microglia and neuropathic pain. Glia 2009; 57: 1469–1479. [DOI] [PubMed] [Google Scholar]
- 10.Cho HK, Cho YW, Kim EH, et al. Changes in pain behavior and glial activation in the spinal dorsal horn after pulsed radiofrequency current administration to the dorsal root ganglion in a rat model of lumbar disc herniation: laboratory investigation. J Neurosurg Spine 2013; 19: 256–263. [DOI] [PubMed] [Google Scholar]
- 11.Cho HK, Ahn SH, Kim SY, et al. Changes in the expressions of Iba1 and calcitonin gene-related peptide in adjacent lumbar spinal segments after lumbar disc herniation in a rat model. Korean Med Sci 2015; 30: 1902–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inoue K. The function of microglia through purinergic receptors: neuropathic pain and cytokine release. Pharmacol Ther 2006; 109: 10–26. [DOI] [PubMed] [Google Scholar]
- 13.Tsuda M, Beggs S, Salter MW, et al. Microglia and intractable chronic pain. Glia 2013; 61: 55–61. [DOI] [PubMed] [Google Scholar]
- 14.Freeman BJ, Ludbrook GL, Hall S, et al. Randomized, double-blind, placebo-controlled, trial of transforaminal epidural etanercept for the treatment of symptomatic lumbar disc herniation. Spine 2013; 38: 1986–1994. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki N, Kikuchi S, Konno S, et al. Anti-TNF-alpha antibody reduces pain-behavioral changes induced by epidural application of nucleus pulposus in a rat model depending on the timing of administration. Spine 2007; 32: 413–416. [DOI] [PubMed] [Google Scholar]
- 16.Xu J, Wang W, Zhong XX, et al. EXPRESS: Methylcobalamin ameliorates neuropathic pain induced by vincristine in rats: effect on loss of peripheral nerve fibers and imbalance of cytokines in the spinal dorsal horn. Mol Pain 2016; 12: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miao G, Liu Z, Wei S, et al. Lipoxin A4 attenuates radicular pain possibly by inhibiting spinal ERK, JNK and NF-κB/p65 and cytokine signals, but not p38, in a rat model of non-compressive lumbar disc herniation. Neuroscience 2015; 300: 10–18. [DOI] [PubMed] [Google Scholar]
- 18.Wang Q, Zhu H, Zou K, et al. Sensitization of P2X3 receptors by cystathionine β-synthetase mediates persistent pain hypersensitivity in a rat model of lumbar disc herniation. Mol Pain 2015; 11: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho HK, Kang JH, Kim SY, et al. Changes in neuroglial activity in multiple spinal segments after caudal epidural pulsed radiofrequency in a rat model of lumbar disc herniation. Pain Physician 2016; 19: E1197–E1209. [PubMed] [Google Scholar]
- 20.Chaplan S, Bach F, Pogrel J, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 21.Hargreaves K, Dubner R, Brown F, et al. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988; 32: 77–88. [DOI] [PubMed] [Google Scholar]
- 22.Gui WS, Wei X, Mai CL, et al. Interleukin-1β overproduction is a common cause for neuropathic pain, memory deficit, and depression following peripheral nerve injury in rodents. Mol Pain 2016; 12: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong XY, Liu L, Yang QW. Functions and mechanisms of microglia/ macrophages in neuroinflammation and neurogenesis after stroke. Prog Neurobiol 2016; 142: 23–44. [DOI] [PubMed] [Google Scholar]
- 24.Tiwari V, Guan Y, Raja SN. Modulating the delicate glial-neuronal interactions in neuropathic pain: promises and potential caveats. Neurosci Biobehav Rev 2014; 45: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D, Couture R, Hong Y. Activated microglia in the spinal cord underlies diabetic neuropathic pain. Eur J Pharmacol 2014; 728: 59–66. [DOI] [PubMed] [Google Scholar]
- 26.Liu YL, Zhou LJ, Hu NW, et al. Tumor necrosis factor-alpha induces long-term potentiation of C-fiber evoked field potentials in spinal dorsal horn in rats with nerve injury: the role of NF-kappa B, JNK and p38 MAPK. Neuropharmacology 2007; 52: 708–715. [DOI] [PubMed] [Google Scholar]
- 27.Xu JT, Xin WJ, Zang Y, et al. The role of tumor necrosis factor-alpha in the neuropathic pain induced by Lumbar 5 ventral root transection in rat. Pain 2006; 123: 306–321. [DOI] [PubMed] [Google Scholar]
- 28.Zhong Y, Zhou LJ, Ren WJ, et al. Interleukin-1β induces long-term potentiation of C-fiber evoked field potentials in spinal dorsal horn in rats with neuropathic pain. Open Pain J 2009; 2: 18–23. [Google Scholar]
- 29.Woolf CJ. The pathophysiology of peripheral neuropathic pain – abnormal peripheral input and abnormal central processing. Acta Neurochir Suppl 1993; 58: 125–130. [DOI] [PubMed] [Google Scholar]
- 30.Tibbs GR, Posson DJ, Goldstein PA. Voltage-gated ion channels in the PNS: novel therapies for neuropathic pain? Trends Pharmacol Sci 2016; 37: 522–542. [DOI] [PubMed] [Google Scholar]
- 31.Liu XG, Zhou LJ. Long-term potentiation at spinal C-fiber synapses: a target for pathological pain. Curr Pharm Des 2015; 21: 895–905. [DOI] [PubMed] [Google Scholar]
- 32.Ruscheweyh R, Wilder-Smith O, Drdla R, et al. Long-term potentiation in spinal nociceptive pathways as a novel target for pain therapy. Mol Pain 2011; 7: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandkühler J, Liu X. Induction of long-term potentiation at spinal synapses by noxious stimulation or nerve injury. Eur J Neurosci 1998; 10: 2476–2480. [DOI] [PubMed] [Google Scholar]
- 34.Zhou LJ, Ren WJ, Zhong Y, et al. Limited BDNF contributes to the failure of injury to skin afferents to produce a neuropathic pain condition. Pain 2010; 148: 148–157. [DOI] [PubMed] [Google Scholar]
- 35.Hathway GJ, Vega-Avelaira D, Moss A, et al. Brief, low frequency stimulation of rat peripheral C-fibers evokes prolonged microglial-induced central sensitization in adults but not in neonates. Pain 2009; 144: 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takebayashi T, Cavanaugh J, Cüneyt Ozaktay A, et al. Effect of nucleus pulposus on the neural activity of dorsal root ganglion. Spine 2001; 26: 940–945. [DOI] [PubMed] [Google Scholar]
- 37.Anzai H, Hamba M, Onda A, et al. Epidural application of nucleus pulposus enhances nociresponses of rat dorsal horn neurons. Spine 2002; 27: E50–E55. [DOI] [PubMed] [Google Scholar]
- 38.Tan Y, Li K, Chen X, et al. Activation of Src family kinases in spinal microglia contributes to formalin-induced persistent pain state through p38 pathway. J Pain 2012; 13: 1008–1015. [DOI] [PubMed] [Google Scholar]
- 39.De Felice M, Lambert D, Holen I, et al. Effects of Src-kinase inhibition in cancer-induced bone pain. Mol Pain 2016; 12: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsuda M, Inoue K. Neuron-microglia interaction by purinergic signaling in neuropathic pain following neurodegeneration. Neuropharmacology 2016; 104: 76–81. [DOI] [PubMed] [Google Scholar]
- 41.Popioled-Barczyk K, Mika J. Targeting the microglia signaling pathways: new insights in the modulation of neuropathic pain. Curr Med Chem 2016; 23: 2908–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lowell CA. Src-family and Syk kinases in activating and inhibitory pathways in innate immune cells: signaling cross talk. Cold Spring Harb Perspect Biol 2011; 3: a002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Page TH, Smolinska M, Gillespie J, et al. Tyrosine kinases and inflammatory signalling. Curr Mol Med 2009; 9: 69–85. [DOI] [PubMed] [Google Scholar]
- 44.Thakur KK, Saini J, Mahajan K, et al. Therapeutic implications of Toll-like receptors in peripheral neuropathic pain. Pharmacol Res 2017; 115: 224–232. [DOI] [PubMed] [Google Scholar]
- 45.Huang Y, Zang Y, Zhou L, et al. The role of TNF-alpha/NF-kappa B pathway on the up-regulation of voltage-gated sodium channel Nav1.7 in DRG neurons of rats with diabetic neuropathy. Neurochem Int 2014; 75: 112–119. [DOI] [PubMed] [Google Scholar]
- 46.Li ZY, Zhang YP, Zhang J, et al. The possible involvement of JNK activation in the spinal dorsal horn in bortezomib-induced allodynia: the role of TNF-α and IL-1β. J Anesth 2016; 30: 55–63. [DOI] [PubMed] [Google Scholar]
- 47.Zhang J, Su YM, Li D, et al. TNF-α-mediated JNK activation in the dorsal root ganglion neurons contribute to bortezomib-induced peripheral neuropathy. Brain Behav Immun 2014; 38: 185–191. [DOI] [PubMed] [Google Scholar]
- 48.Gruber-Schoffnegger D, Drdla-Schutting R, Hönigsperger C, et al. Induction of thermal hyperalgesia and synaptic long-term potentiation in the spinal cord lamina I by TNF-α and IL-1β is mediated by glia cells. J Neurosci 2013; 33: 6540–6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chirila AM, Brown TE, Bishop RA, et al. Long-term potentiation of glycinergic synapses triggered by interleukin 1β. Proc Natl Acad Sci U S A 2014; 111: 8263–8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taves S, Berta T, Chen G, et al. Microglia and spinal cord synaptic plasticity in persistent pain. Neural Plast 2013; 2013: 753656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kraychete DC, Sakata RK, Issy AM, et al. Serum cytokine levels in patients with chronic low back pain due to herniated disc: analytical cross-sectional study. Sao Paulo Med J 2010; 128: 259–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohtori S, Miyagi M, Eguchi Y, et al. Epidural administration of spinal nerves with the tumor necrosis factor-alpha inhibitor, etanercept, compared with dexamethasone for treatment of sciatica in patients with lumbar spinal stenosis: a prospective randomized study. Spine 2012; 37: 439–444. [DOI] [PubMed] [Google Scholar]
- 53.Liu ZH, Miao GS, Wang JN, et al. Resolvin D1 inhibits mechanical hypersensitivity in sciatica by modulating the expression of nuclear factor-κB, phospho-extracellular signal-regulated kinase, and pro- and antiinflammatory cytokines in the spinal cord and dorsal root ganglion. Anesthesiology 2016; 124: 934–944. [DOI] [PubMed] [Google Scholar]
- 54.Olmarker K, Nutu M, Størkson R. Changes in spontaneous behavior in rats exposed to experimental disc herniation are blocked by selective TNF-alpha inhibition. Spine 2003; 28: 1635–1641. [DOI] [PubMed] [Google Scholar]
- 55.Rothman SM, Winkelstein BA. Cytokine antagonism reduces pain and modulates spinal astrocytic reactivity after cervical nerve root compression. Ann Biomed Eng 2010; 38: 2563–2576. [DOI] [PubMed] [Google Scholar]
- 56.Wang K, Bao JP, Yang S, et al. A cohort study comparing the serum levels of pro- or anti-inflammatory cytokines in patients with lumbar radicular pain and healthy subjects. Eur Spine J 2016; 25: 1428–1434. [DOI] [PubMed] [Google Scholar]
- 57.Sun YE, Peng L, Sun X, et al. Intrathecal injection of spironolactone attenuates radicular pain by inhibition of spinal microglia activation in a rat model. PLoS One 2012; 7: e39897. [DOI] [PMC free article] [PubMed] [Google Scholar]